Abstract
Pancreas transplantation in type 1 diabetes patients could result in (re)activation of allo- and autoreactive T lymphocytes. Anti-thymocyte globulin (ATG) induction treatment is a successful, but broadly reactive anti-lymphocyte therapy used in pancreas and islet transplantation. A more selective alternative is daclizumab, a monoclonal antibody directed against the interleukin-2 receptor (CD25) on activated lymphocytes. We tested the hypothesis that daclizumab is more selective and has less immunological side effects than ATG. Thirty-nine simultaneous pancreas–kidney transplantation patients with type 1 diabetes were randomized for induction therapy with ATG or daclizumab. Auto- and recall immunity was measured cross-sectionally by lymphocyte stimulation tests with a series of auto- and recall antigens in 35 successfully transplanted patients. T cell autoimmunity to islets was low in both groups, except for a marginal but significantly higher reactivity against glutamic acid decarboxylase (GAD)65 in daclizumab-treated patients. The memory responses to recall antigens were significantly higher in the daclizumab-treated group compared to ATG-treated patients, specifically against purified protein derivative (PPD) (anti-bacterial immunity), Haemophilus influenzae virus matrix protein-1 (anti-viral immunity) and p53 [anti-tumour (auto)immunity]. These data imply that daclizumab is more specifically affecting diabetes-related immune responses than ATG. The autoimmunity is affected effectively after daclizumab induction, while memory responses towards bacterial, viral and tumour antigens are preserved.
Keywords: antibodies, diabetes mellitus, immunotherapy, pancreas, transplantation
Introduction
Type I diabetes mellitus is characterized by T cell-mediated autoimmune destruction of insulin-producing β cells [1–8]. Replacement of these β cells can be achieved by pancreas transplantation. The majority of pancreas transplantations are performed in patients with end-stage renal failure. Simultaneous pancreas–kidney transplantation is an established treatment and is the most frequent kind of pancreas transplantation performed in the case of end-stage renal failure. During recent decades the experience and success with this therapeutic option has increased [9–12]. The immunological goal is to avoid alloreactivity and recurrence of autoimmunity. Different immunosuppressive treatment and induction protocols were developed. Anti-thymocyte globulin (ATG) proved to be a useful and powerful drug for induction and anti-rejection therapy in pancreas and islet transplantation [13, 14]. ATG is obtained from the serum of immunized rabbits or horses. It is a polyclonal anti-T cell therapy and depletes different subsets of the T lymphocyte repertoire [15]. Although successful, ATG treatment is a broad-spectrum and inconsistent anti-lymphocyte therapy [16]. The general depletion of allo- and autoreactive T lymphocytes is conceivably accompanied by the depletion of recall immunity. The loss of recall immunity affects the immune defence against viruses and bacteria, and immune surveillance for tumour genesis. To overcome this disadvantage, monoclonal immunoglobulins directed against specific T cell subsets have been developed. Daclizumab is a monoclonal antibody directed against the low-affinity interleukin (IL)-2 receptor alpha chain (CD25). Activated lymphocytes express the IL-2 receptor on their surface. As a consequence, daclizumab targets the activated lymphocytes [17, 18]. Allo- and autoreactive T lymphocytes can become (re-)activated during pancreas transplantation due to the introduction of donor tissue and new β cells. These lymphocytes are believed to be affected by daclizumab induction therapy. Daclizumab has been shown to be effective in reducing allograft rejection in renal transplantation and has proved its value in the treatment of T cell-mediated autoimmune disorders such as non-infectious intermediate and posterior uveitis and human T cell leukaemia virus 1 (HTLV-I)-associated myelopathy [19–24]. The so-called Edmonton protocol for successful islet transplantation in type 1 diabetic patients includes daclizumab induction therapy [25]. Daclizumab is also considered in combination with mycophenolate mofetil (MMF) as intervention therapy in new-onset type 1 diabetes patients (http://www.diabetestrialnet.org) [26].
Both allo- and autoreactivity are present after simultaneous pancreas–kidney transplantation. The recall immunity, on the other hand, is supposed to be at a resting memory state during the transplant period and should therefore be relatively spared for the effect of daclizumab. As a consequence, daclizumab could serve theoretically as a good alternative for the polyclonal induction therapy with ATG, provided that it affects recurrent islet autoimmunity.
We tested the effect of daclizumab induction therapy on autoreactive lymphocytes compared to ATG induction therapy in a cross-sectional study in type 1 diabetes patients transplanted successfully with a combined kidney and pancreas.
Patients and methods
Patients
Between October 1999 and May 2002, 39 simultaneous pancreas–kidney transplantations were performed in long-term type I diabetic patients with end-stage renal failure at the LUMC, Leiden. Patients were randomized to receive either a single dose of ATG (Fresenius) [9 mg/kg body weight, intravenously (i.v.)] [27] during transplantation (n = 19) or five consecutive doses of daclizumab (1 mg/kg body weight, i.v.) administered every 2 weeks, starting at the moment of transplantation (n = 20). Maintenance immunosuppression consisted of cyclosporin A, mycophenolate mofetil and prednisone in all patients. Rejection episodes were treated with steroids (Solumedrol), or in case of steroid resistance with a second antibody therapy with ATG from a different source (Merieux). No significant difference was found for recipient age, male–female ratio, body mass index and duration of diabetes in both the ATG and daclizumab treatment groups (Table 1). The 3-year patient, pancreas graft and kidney graft survival rates for ATG- versus daclizumab-treated patients were 100% and 90% (patient survival), 84·2% and 94·7% (pancreas graft survival) and 94·7% and 94·7% (kidney graft survival). None of these clinical parameters were statistically different between the two treatment groups. Additional clinical details on this cohort have been described elsewhere [28].
Table 1.
Patient characteristics. Values represent means ± standard deviation.
| Characteristics | ATG | Daclizumab | P-value |
|---|---|---|---|
| Recipient age (years) | 44·1 ± 8·3 | 40·3 ± 7·4 | 0·14 |
| Recipient sex (M/F) | 10/9 | 14/6 | 0·33 |
| Body mass index (kg/m2) | 23·9 ± 3·3 | 23·2 ± 2·7 | 0·76 |
| Duration diabetes (years) | 29·2 ± 8·3 | 26·9 ± 6·5 | 0·35 |
ATG: anti-thymocyte globulin. For additional details, please see [28].
Auto- and recall immunity were cross-sectionally measured by lymphocyte stimulation tests with different auto- and recall antigens in 35 successfully transplanted patients (ATG: n = 16; daclizumab: n = 19). Approval by the local ethical committee with informed consent from participants was obtained for this study.
Lymphocyte proliferation test
The lymphocyte stimulation test was performed cross-sectionally after a successful combined pancreas and kidney transplantation. Heparinized blood was drawn between 10 and 50 months after transplantation [mean 25·4 months (±10) for daclizumab-treated patients and 26·5 months (±10) for ATG-treated patients]. Peripheral blood mononuclear cells (PBMC) were isolated from freshly drawn heparinized blood and tested as described previously [3]. In short, 150 000 PBMC were cultured in tissue-coated, round-bottomed 96-well plates (Costar, Cambridge, MA, USA) in Iscove's modified Dulbecco's medium with 2 mmol/l glutamine (Gibco, Paisley, Scotland, UK) supplemented with 10% human type AB pool serum in the presence of antigen, recombinant IL-2 or medium alone in 150 μl at 37°C, 5% CO2. After 5 days, RPMI-1640 (Dutch Modification; Gibco) containing 0·5 μCi [3H]-thymidine per well was added, and incubation was continued for 16 h. Cultures were then harvested on glass-fibre filters, and [3H]-thymidine incorporation was measured by liquid scintillation counting. The results are expressed as stimulation indexes (SI); that is, the median of triplicates in the presence of stimulus divided by the median of triplicates with medium alone.
Stimuli
The stimuli used were as follows: recombinant IL-2, insulin (Sigma), glutamic acid decarboxylase (GAD)65 (Diamyd Medicals, Stockholm, Sweden), recombinant human pro-insulin and IA-2602–979, human islet homogenate (kindly provided by Dr Ezio Bonifacio, San Rafaele Institute, Milan, Italy), tetanus toxoid (1·5 Limes flocculationes/ml or 12·0 international units/ml) (National Institute of Public Health and Environmental Protection, the Netherlands), purified protein derivative (PPD) (Staten Serum Institute, Copenhagen, Denmark), Haemophilus influenzae matrix protein M1 and p53 [29]. Recombinant human IA-2 cytoplasmic domain (residues 602–979) with N-terminal affinity tag was produced in Escherichia coli BL21/DE3 using isopropyl β-D-1-thiogalactopyranoside (IPTG) induction and purified as described, including preparative electrophoresis and electroelution [30]. Endotoxin levels were 0·8 EU/mg protein as determined by the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD, USA).
Statistical significance was determined using the Mann–Whitney U-test. P-values were corrected for the number of comparisons; P < 0·05 was considered significant.
Results
Immune responses in patients not requiring second antibody therapy
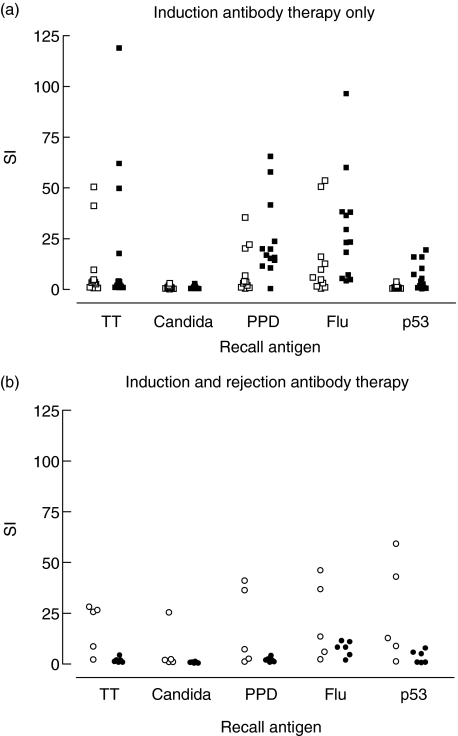
Proliferative responses upon in vitro stimulation with recall antigen were significantly different. In particular, the anti-bacterial (PPD) and the anti-viral (H. influenzae virus matrix protein M1) T cell response was decreased in patients with ATG induction versus daclizumab induction. Stimulation indices for ATG and daclizumab induction were 3·7 versus 16·9 for PPD, respectively (P = 0·02), and 5·9 versus 23·4 for H. influenzae virus matrixprotein M1 (P = 0·03; Fig. 1).
Fig. 1.
Recall responses after anti-thymocyte globulin (ATG) Fresenius (□) daclizumab (▪) induction therapy only (a) and after second antibody therapy with ATG Merieux in ATG Fresenius (○) and daclizumab (•) treated patients (b); the anti-bacterial [purified protein derivative (PPD)] and the anti-viral (Haemophilus influenzae virus matrix protein M1) T cell response was decreased in patients with ATG induction versus daclizumab induction. (a) Significant differences in recall immunity were found for the daclizumab-treated patients with versus without second antibody treatment. Stimulation indices in these treatment groups were, respectively, 2·0 versus 16·9 for PPD (P = 0·002) and 8·3 versus 23·4 for H. influenzae virus matrix protein M1 (P = 0·03) (a and b).
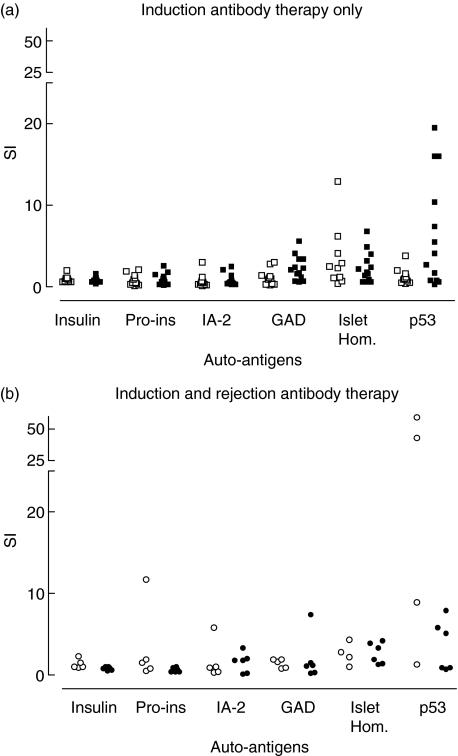
In both the ATG-treated group and the daclizumab-treated group, low autoreactivity was found. No statistical differences were found between both groups for all autoantigens, except for GAD65. Stimulation indices for GAD65 were 1·0 versus 2·1 for ATG and daclizumab induction, respectively (P = 0·02; Fig. 2).
Fig. 2.
Recall responses after anti-thymocyte globulin (ATG) Fresenius (□) daclizumab (▪) induction therapy only (a) and after second antibody therapy with ATG Merieux in ATG Fresenius (○) and daclizumab (•) treated patients (b). In both the ATG-treated group and the daclizumab-treated group, low autoreactivity was found. No statistical differences were found between both groups for all auto-antigens except for glutamic acid decarboxylase (GAD)65. Stimulation indices for GAD65 were 1·0 versus 2·1 for ATG Fresenius and daclizumab induction, respectively (P = 0·02) (a). No significant differences were found in proliferative responses upon stimulation with either auto or recall antigens in ATG-treated patients with versus without second antibody therapy, except for p53 stimulation. Stimulation index was 0·9 versus 12·8 (P = 0·02) (a and b).
Proliferative responses to p53 protein for ATG- versus daclizumab-treated patients were significantly higher in daclizumab-treated patients (SI 0·9 versus 4·1; P = 0·02).
Immune responses in patients treated with second antibody therapy due to rejection episode
Kidney rejection episodes (diagnosed by kidney graft biopsies) were treated with steroids. In the case of steroid resistant rejection episodes, a second antibody therapy with ATG of a different source (Merieux) was used. Five patients in the ATG Fresenius group received rejection therapy with ATG Merieux, whereas six patients in the daclizumab group were exposed to ATG Merieux as rejection therapy. Second antibody therapy with ATG Merieux effectively reduced proliferative immune reactivity to recall antigens in daclizumab-treated patients, but appeared less effective in patients who had received ATG (Fresenius) as transplant induction therapy.
No significant differences were found in proliferative responses upon stimulation with either auto- or recall antigens in ATG-treated patients with versus without second antibody therapy with ATG Merieux, except for p53 stimulation. Mean stimulation index was 0·9 versus 12·8 (P = 0·02; Fig. 2).
In the daclizumab-treated patients, no difference in proliferative responses upon stimulation with autoantigens was found between patients with versus without second antibody therapy with ATG Merieux.
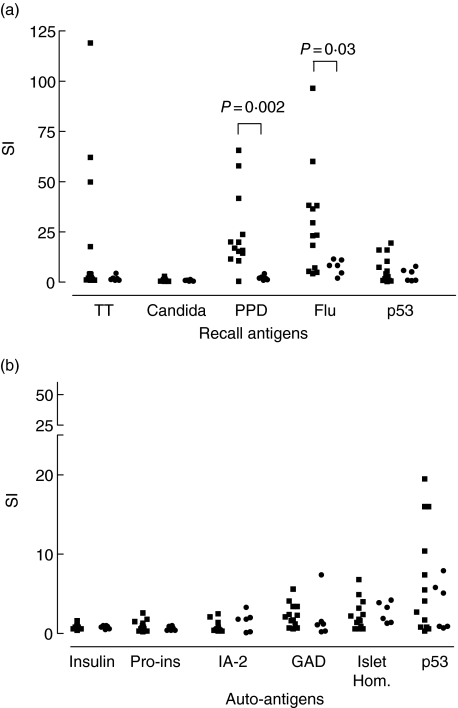
Significant differences in recall immunity were found for the daclizumab-treated patients with versus without second antibody therapy with ATG Merieux. Stimulation indices in these treatment groups were, respectively, 2·0 versus 16·9 for PPD (P = 0·002) and 8·3 versus 23·4 for H. influenzae virus matrixprotein M1 (P = 0·03; Fig. 3).
Fig. 3.
Recall responses after daclizumab without second antibody therapy with anti-thymocyte globulin (ATG) Merieux rejection episode treatment (▪) and with second antibody therapy with ATG Merieux rejection episode treatment (•) (a) and auto responses after daclizumab without second antibody treatment (▪) and with second antibody treatment (•) (b); Significant differences in recall immunity were found for the daclizumab-treated patients with versus without second antibody treatment. Stimulation indices in these treatment groups were, respectively, 2·0 versus 16·9 for purified protein derivative (PPD) (P = 0·002) and 8·3 versus 23·4 for Haemophilus influenzae virus matrixprotein M1 (P = 0·03) (a). No significant differences were found in proliferative responses upon stimulation with autoantigens (b). Data from Figs 1 and 2 have been combined for this comparison.
IL-2 stimulation
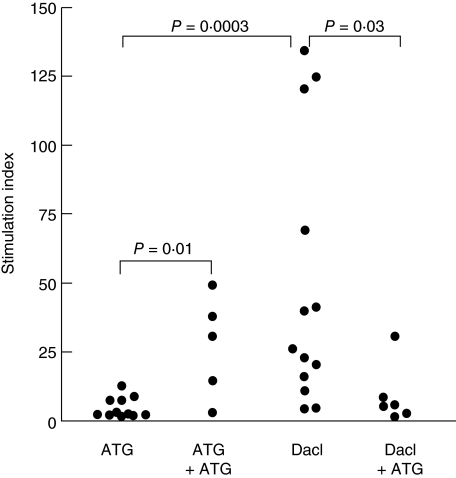
Similar to the effect on recall immunity, the response to recombinant IL-2 was affected differentially by ATG versus daclizumab (Fig. 4). ATG and daclizumab induction therapy was associated with stimulation indices of 2·7 and 26·1, respectively (P = 0·0003). After second antibody therapy with ATG Merieux the stimulation index was significantly higher compared to ATG-treated patients without ATG Merieux therapy (SI 31·0 versus 2·7, respectively; P = 0·01). For daclizumab induction, second antibody therapy with ATG Merieux was associated with lower stimulation indexes of 5·6 compared to 26·1 in daclizumab-treated patients without ATG Merieux (P = 0·03).
Fig. 4.
Interleukin (IL)-2 responses after anti-thymocyte globulin (ATG) Fresenius (□) and daclizumab (▪) induction therapy only and after second antibody therapy with anti-thymocyte globulin (ATG) Merieux in ATG Fresenius (○) and daclizumab (•) treated patients; ATG and daclizumab induction therapy was associated with stimulation indices of: 2·7 and 26·1, respectively (P = 0·0003). After second antibody therapy with ATG Merieux the stimulation index was significantly higher compared to ATG-treated patients without second antibody therapy (SI 31·0 versus 2·7, respectively; P = 0·01). For daclizumab induction, second antibody therapy was associated with lower stimulation indexes of 5·6 compared to 26·1 in daclizumab-treated patients without second antibody therapy (P = 0·03) (a and b).
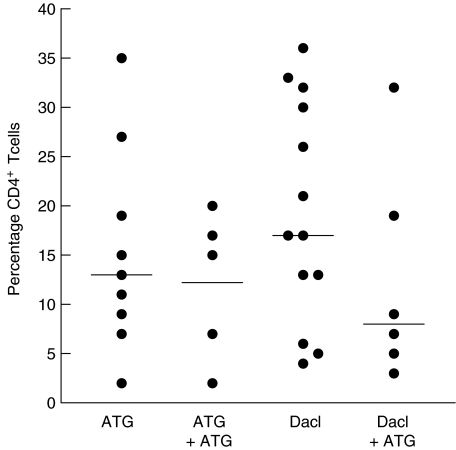
CD4+ T cells
No significant differences were found in the percentage of CD4+ T cells [mean ± standard deviation (s.d.)] for ATG- versus daclizumab-treated patients (Fig. 5). ATG 15% ± 10%, daclizumab 19% ± 11%, ATG with second antibody therapy 12% ± 7%, daclizumab with second antibody therapy 13% ± 11%.
Fig. 5.
Percentages of CD4+ T cells (mean ± standard deviation) for the different treatment groups.
Discussion
The past decade has seen several major breakthroughs in monoclonal antibody therapeutics [31]. The realization that antibodies to tumour necrosis factor-α can ameliorate the symptoms of autoimmune diseases, while monoclonals against CD3 may preserve β cell function in new-onset type 1 diabetes patients illustrate the potential for their application in type 1 diabetes [32].
In this study we demonstrate that daclizumab induction is affecting diabetes-related immune responses more specifically than ATG induction treatment. Although no significant differences were found in the percentage of CD4+ T cells, the autoimmunity appeared to be affected effectively in both ATG- and daclizumab-treated patients, while anti-bacterial, -viral and -tumour immunity was preserved after daclizumab induction treatment in this in vitro test system.
Interestingly, this advantage of preserved memory responses is lost in the case of rejection episode treatment with a second polyclonal antibody therapy with ATG Merieux following the daclizumab induction therapy. Our results support strongly the idea that daclizumab has a more specific activity on islet autoimmunity with less immunological adverse effects than ATG treatment. Indeed, patients treated with daclizumab developed cytomegalovirus viraemia less frequently than those treated with ATG induction therapy [28]. Second antibody therapy after ATG induction was as effective in reversing the alloreactivity/rejection episode clinically as it was after daclizumab induction. Despite this effect, surprisingly, we found higher responses to recall antigens compared to ATG-treated patients without a second antibody therapy. Although repeated therapy with ATG could suffer from the induction of neutralizing anti-rabbit antibodies, we favour as explanation that the time span between the last antibody therapy affected this outcome. Higher responses were found in patients tested later after the second antibody therapy, pointing to a possible recovery, while no association for time span between last antibody therapy and test date and immune responses were found in the other groups.
The same response pattern was obtained after stimulation with recombinant IL-2. IL-2 is a T cell growth factor. Our results on IL-2-related proliferation imply that ATG affects the proliferation capacity of the T cell repertoire even more than 1 year after antibody therapy. This long-lasting ATG effect accords with previous studies [33–35]. In contrast, IL-2-related proliferation was restored several months after daclizumab treatment, while the autoimmunity is still affected. Daclizumab might temporarily affect activated T cells (in this case reactivated autoreactive T cells), but does not influence T cell reactivity after clearance.
Several studies suggest an important role for p53-specific CD4+ T cells in anti-tumour immunity [29, 36]. The immune response towards the tumour protein p53 is more preserved in the daclizumab-treated patients compared to the ATG-treated patients. Although tested in vitro, this might have clinical implications. The proliferative capacity to the tumour protein p53 serves as a marker for tumour surveillance capability. Tumour development is a possible consequence of immune suppressive drug treatment. Preservation of tumour surveillance with daclizumab, therefore, could have favourable consequences for the incidence of tumours.
While combined kidney–pancreas transplantation is currently a standard therapy offered to type 1 diabetes patients with end-stage renal failure, there is a great demand for β cell substitution in other groups of patients. With more specific and less severe immunosuppressive regimes, pancreas or islet transplantation alone in non- or pre-uraemic type I diabetes patients will become a good alternative for simultaneous pancreas–kidney transplantation. In this way, organs can be used more efficiently in this era of organ shortage, while a larger group of type 1 diabetes patients will benefit from β cell replacement.
This concept of affecting activated type I diabetes-associated T cells could also be useful for specific immunotherapy strategies with less immunological side effects in recent-onset diabetes. While targeting the diabetes-related T cells, the benign recall immunity can be spared. As a consequence, β cell neogenesis without the destructive influence of autoreactive T cells is conceivable.
In our study, we applied T cell bioassays that measure T cell reactivity against autoantigens and infectious agents to determine the efficacy of antibody therapy to preserve β cells transplanted into type 1 diabetes patients. Although islet autoantibodies can be useful to determine loss of islet graft function in some patients, their value is the subject of debate [37–39]. In our experience in simultaneous pancreas–kidney transplantation, changes in antibody titres or seroconversions did not correlate with loss of graft function or occurrence of rejection episodes, as all patients retained insulin-independent at 1 year after implantation. In the past, we have applied T cell assays to demonstrate a correlation between T cell autoreactivity and the presence of insulitis and recurrent chronic progressive destruction of β cells after islet transplantation [3, 4]. Here we demonstrate the applicability of T cell technology to measure the efficacy of antibody therapy in type 1 diabetes.
Acknowledgments
This study was supported by the Dutch Diabetes Research Foundation (project no. DFN 2001·06·001) and the Juvenile Diabetes Research Foundation (JDRF 4/2001/434). We thank Ezio Bonifacio, Kees Franken and Sjoerd van de Burg for providing islet homogenate and recombinant autoantigens and professors René R.P. de Vries and Onno T. Terpstra for critical discussions.
References
- 1.Sibley RK, Sutherland DE, Goetz F, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. A light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest. 1985;53:132–44. [PubMed] [Google Scholar]
- 2.Roep BO, Arden SD, de Vries RR, Hutton JC. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature. 1990;345:632–4. doi: 10.1038/345632a0. [DOI] [PubMed] [Google Scholar]
- 3.Roep BO, Kallan AA, Duinkerken G, et al. T-cell reactivity to beta-cell membrane antigens associated with beta-cell destruction in IDDM. Diabetes. 1995;44:278–83. doi: 10.2337/diab.44.3.278. [DOI] [PubMed] [Google Scholar]
- 4.Roep BO, Stobbe I, Duinkerken G, et al. Auto- and alloimmune reactivity to human islet allografts transplanted into type 1 diabetic patients. Diabetes. 1999;48:484–90. doi: 10.2337/diabetes.48.3.484. [DOI] [PubMed] [Google Scholar]
- 5.Roep BO. The role of T-cells in the pathogenesis of Type 1 diabetes: from cause to cure. Diabetologia. 2003;46:305–21. doi: 10.1007/s00125-003-1089-5. [DOI] [PubMed] [Google Scholar]
- 6.Lampeter EF, Homberg M, Quabeck K, et al. Transfer of insulin-dependent diabetes between HLA-identical siblings by bone marrow transplantation. Lancet. 1993;341:1243–4. doi: 10.1016/0140-6736(93)91148-f. [DOI] [PubMed] [Google Scholar]
- 7.Brooks-Worrell BM, Peterson KP, Peterson CM, Palmer JP, Jovanovic L. Peripheral blood mononuclear cell responses from type 1 diabetic patients and subjects at-risk for type 1 diabetes to human fetal pancreatic tissue proteins. Transplantation. 2000;69:1907–12. doi: 10.1097/00007890-200005150-00028. [DOI] [PubMed] [Google Scholar]
- 8.Bougneres PF, Carel JC, Castano L, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine1. N Engl J Med. 1988;318:663–70. doi: 10.1056/NEJM198803173181103. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland DE, Gruessner RW, Gruessner AC. Pancreas transplantation for treatment of diabetes mellitus. World J Surg. 2001;25:487–96. doi: 10.1007/s002680020342. [DOI] [PubMed] [Google Scholar]
- 11.Smets YF, Westendorp RG, van der Pijl JW, et al. Effect of simultaneous pancreas–kidney transplantation on mortality of patients with type-1 diabetes mellitus and end-stage renal failure. Lancet. 1999;353:1915–9. doi: 10.1016/S0140-6736(98)07513-8. [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association. Pancreas transplantation for patients with type 1 diabetes. Diabetes Care. 2000;23:117. doi: 10.2337/diacare.23.1.117. [DOI] [PubMed] [Google Scholar]
- 13.Hesse UJ, Troisi R, Jacobs B, et al. A single center's clinical experience with quadruple immunosuppression including ATG or IL2 antibodies and mycophenolate mofetil in simultaneous pancreas-kidney transplants. Clin Transplant. 2000;14:340–4. doi: 10.1034/j.1399-0012.2000.140410.x. [DOI] [PubMed] [Google Scholar]
- 14.Cantarovich D, Karam G, Giral-Classe M, et al. Randomized comparison of triple therapy and antithymocyte globulin induction treatment after simultaneous pancreas-kidney transplantation. Kidney Int. 1998;54:1351–6. doi: 10.1046/j.1523-1755.1998.00094.x. [DOI] [PubMed] [Google Scholar]
- 15.Mestre M, Bas J, Alsina J, Grinyo JM, Buendia E. Depleting effect of antithymocyte globulin on T-lymphocyte subsets in kidney transplantation. Transplant Proc. 1999;31:2254–5. doi: 10.1016/s0041-1345(99)00326-7. [DOI] [PubMed] [Google Scholar]
- 16.Bourdage JS, Hamlin DM. Comparative polyclonal antithymocyte globulin and antilymphocyte/antilymphoblast globulin anti-CD antigen analysis by flow cytometry. Transplantation. 1995;59:1194–200. [PubMed] [Google Scholar]
- 17.Wiseman LR, Faulds D. Daclizumab: a review of its use in the prevention of acute rejection in renal transplant recipients. Drugs. 1999;58:1029–42. doi: 10.2165/00003495-199958060-00006. [DOI] [PubMed] [Google Scholar]
- 18.Waldmann TA, O'Shea J. The use of antibodies against the IL-2 receptor in transplantation. Curr Opin Immunol. 1998;10:507–12. doi: 10.1016/s0952-7915(98)80215-x. [DOI] [PubMed] [Google Scholar]
- 19.Vincenti F, Kirkman R, Light S, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. N Engl J Med. 1998;338:161–5. doi: 10.1056/NEJM199801153380304. Daclizumab Triple Therapy Study Group. [DOI] [PubMed] [Google Scholar]
- 20.Nussenblatt RB, Fortin E, Schiffman R, et al. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci USA. 1999;96:7462–6. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo A, Stratta RJ, Alloway RR, et al. Initial clinical experience with interleukin-2 receptor antagonist induction in combination with tacrolimus, mycophenolate mofetil and steroids in simultaneous kidney–pancreas transplantation. Transplant Int. 2001;14:396–404. doi: 10.1007/s001470100005. [DOI] [PubMed] [Google Scholar]
- 22.Lehky TJ, Levin MC, Kubota R, et al. Reduction in HTLV-I proviral load and spontaneous lymphoproliferation in HTLV-I-associated myelopathy/tropical spastic paraparesis patients treated with humanized anti-Tac. Ann Neurol. 1998;44:942–7. doi: 10.1002/ana.410440613. [DOI] [PubMed] [Google Scholar]
- 23.Bruce DS, Sollinger HW, Humar A, et al. Multicenter survey of daclizumab induction in simultaneous kidney–pancreas transplant recipients. Transplantation. 2001;72:1637–43. doi: 10.1097/00007890-200111270-00010. [DOI] [PubMed] [Google Scholar]
- 24.Adu D, Cockwell P, Ives NJ, Shaw J, Wheatley K. Interleukin-2 receptor monoclonal antibodies in renal transplantation: meta-analysis of randomised trials. BMJ. 2003;326:789. doi: 10.1136/bmj.326.7393.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 26.Staeva-Vieira T, Peakman M, von Herrath M. Immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol. 2007;148:17–31. doi: 10.1111/j.1365-2249.2007.03328.x. Translational Mini-Review Series on Type 1 Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaden J, May G, Schonemann C, et al. Effect of ATG prophylaxis in sensitized and non-sensitized kidney graft recipients. Transplant Int. 1992;5(Suppl. 1):S75–8. doi: 10.1007/978-3-642-77423-2_25. [DOI] [PubMed] [Google Scholar]
- 28.Huurman VA, van de Kalpoe JS, van de Kalpoe LP, et al. Choice of antibody immunotherapy influences cytomegalovirus viremia in simultaneous pancreas–kidney transplant recipients. Diabetes Care. 2006;29:842–7. doi: 10.2337/diacare.29.04.06.dc05-1647. [DOI] [PubMed] [Google Scholar]
- 29.van der Burg SH, de Cock K, Menon AG, et al. Long lasting p53-specific T cell memory responses in the absence of anti-p53 antibodies in patients with resected primary colorectal cancer. Eur J Immunol. 2001;31:146–55. doi: 10.1002/1521-4141(200101)31:1<146::aid-immu146>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Bergerot I, Elliott JF, et al. Evidence that a peptide spanning the B–C junction of proinsulin is an early autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol. 2001;167:4926–35. doi: 10.4049/jimmunol.167.9.4926. [DOI] [PubMed] [Google Scholar]
- 31.Nabel GJ. Genetic, cellular and immune approaches to disease therapy. past and future. Nat Med. 2004;10:135–41. doi: 10.1038/nm990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 33.Muller TF, Grebe SO, Neumann MC, et al. Persistent long-term changes in lymphocyte subsets induced by polyclonal antibodies. Transplantation. 1997;64:1432–7. doi: 10.1097/00007890-199711270-00010. [DOI] [PubMed] [Google Scholar]
- 34.Martins L, Ventura A, Dias L, Henriques A, Sarmento A, Guimaraes S. Long-term effects of ATG therapy on lymphocyte subsets. Transplant Proc. 2001;33:2186–7. doi: 10.1016/s0041-1345(01)01936-4. [DOI] [PubMed] [Google Scholar]
- 35.Lange H, Muller TF, Ebel H, et al. Immediate and long-term results of ATG induction therapy for delayed graft function compared to conventional therapy for immediate graft function. Transplant Int. 1999;12:2–9. doi: 10.1007/s001470050178. [DOI] [PubMed] [Google Scholar]
- 36.Zwaveling S, Vierboom MP, Ferreira Mota SC, et al. Antitumor efficacy of wild-type p53-specific CD4(+) T-helper cells. Cancer Res. 2002;62:6187–93. [PubMed] [Google Scholar]
- 37.Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–64. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 38.Jaeger C, Brendel MD, Hering BJ, Eckhard M, Bretzel RG. Progressive islet graft failure occurs significantly earlier in autoantibody-positive than in autoantibody-negative IDDM recipients of intrahepatic islet allografts. Diabetes. 1997;46:1907–10. doi: 10.2337/diab.46.11.1907. [DOI] [PubMed] [Google Scholar]
- 39.Bosi E, Braghi S, Maffi P, et al. Autoantibody response to islet transplantation in type 1 diabetes. Diabetes. 2001;50:2464–71. doi: 10.2337/diabetes.50.11.2464. [DOI] [PubMed] [Google Scholar]