Abstract
Apoptosis of blood monocytes was studied in experimental sepsis by multi-drug-resistant Pseudomonas aeruginosa. Thirty-six rabbits were used, divided into the following groups: A (n = 6), sham; B (n = 6), administered anaesthetics; and C (n = 24), acute pyelonephritis induced after inoculation of the test isolate in the renal pelvis. Blood was sampled at standard time intervals for estimation of tumour necrosis factor (TNF)-α and isolation of monocytes. Half the monocytes were incubated and the other half was lysed for estimation of the cytoplasmic activity of caspase-3 by a kinetic chromogenic assay. No animal in groups A and B died; those in group C were divided into two subgroups, CI (n = 8) with present activity of caspase-3 of blood monocytes at 3·5 h and CII (n = 16) with absent activity. Their median survival was 2·0 and 3·5 days, respectively (P = 0·0089). Ex vivo secretion of TNF-α from monocytes was higher by monocytes of subgroup CII than subgroup CI at 3·5 h (P = 0·039) and of group A than CII at 48 h (P = 0·010). Median change of caspase-3 activity between 3·5 and 24 h of sampling was 56·1 and −5·8 pmol/min per 104 cells for subgroups CI and CII (P = 0·040), respectively. Respective changes between 3·5 and 48 h were 28 981·0 and 0 pmol/min per 104 cells (P = 0·036). Early induction of apoptosis in blood monocytes is of prime importance for the survival of the septic host and might be connected to changes of monocyte potential for the secretion of TNF-α.
Keywords: apoptosis, Pseudomonas, sepsis
Introduction
Apoptosis is a central process involved in the evolution of sepsis. Triggering of the apoptotic cascade has been described in lymphocytes, in tissue macrophages and in intestinal epithelia and is connected to organ dysfunction [1]. Although data for the apoptosis of cells of the adaptive immune system are available, little evidence exists for the implication of the innate immune system, i.e. of blood monocytes. The need for knowledge in that field is aggravated further by the central role of monocytes in the pathogenesis of sepsis [2]. The existing theory for the pathogenesis of sepsis is based on the overproduction of proinflammatory cytokines by blood monocytes when triggered by the cell wall constituents of bacterial pathogens [3]. Apoptosis of blood monocytes has been described in a limited number of patients with severe sepsis [4], whereas more data have been derived by a prospective study of our group in 90 patients with nosocomial sepsis by ventilator-associated pneumonia [5].
In clinical studies it is not possible to follow-up cellular apoptosis after bacterial challenge, but only after diagnosis based on full-blown symptoms is conducted. The time of presentation of symptoms may differ from the time of bacterial challenge in each individual host. In a former study by our group [5], monocyte apoptosis greater than 50% on the first day of presentation of signs of sepsis was connected to prolonged 28-day survival compared to patients with monocyte apoptosis of less than 50%. However, no data were possible to present for apoptosis of monocytes in the time-points preceding clinical diagnosis of sepsis.
The present study was designed in an attempt to explain the effect of monocyte apoptosis taking place following bacterial challenge on the overall survival of the septic host. Apoptosis of monocytes was studied over time in an experimental model of sepsis and pyelonephritis by multi-drug-resistant Pseudomonas aeruginosa. The latter isolate was selected in order to simulate a setting of nosocomial sepsis.
Methods and materials
Bacterial isolate
One blood isolate of P. aeruginosa derived from a female patient with acute pyelonephritis and severe sepsis was applied. Minimal inhibitory concentrations (MICs) of ticarcillin/clavulanate, piperacillin, ceftazidime, imipenem, meropenem, ciprofloxacin and amikacin determined by a microdilution technique were equal to > 256/2, > 512, 16, 64, 32, > 512 and 256 μg/ml, respectively; the isolate was considered multi-drug-resistant [6].
The isolate was stored as multiple aliquots in skimmed milk (Oxoid Ltd, London, UK) at −70°C. Before each experiment, one aliquot was thawed and cultured onto McConkey agar plates (Becton Dickinson, Cockeysville, MD, USA). Single colonies were suspended in Mueller–Hinton broth (Oxoid) and incubated for 12 h at 37°C in a shaking water-bath. The resulting inoculum was washed three times with NaCl 0·9% to remove any free endotoxins.
Animals
A total of 36 white New Zealand male rabbits were used, mean ± standard deviation (SD), weight 3·02 ± 0·34 kg. The study received permission from the Veterinary Directorate of the Perfecture of Athens according to the Greek legislation in accordance with the 160/1991 Council Directive of the EU. Animals were housed in single metal cages and had access to tap water and standard balanced rabbit chow ad libitum. Room temperature ranged between 18 and 22°C; relative humidity was between 55 and 65% and the light/dark cycle was 6 a.m.−6 p.m.
Study design
Animals were divided into three groups, as follows:
Group A (n = 6): sham-operated rabbits.
Group B (n = 6): rabbits that were sedated initially by intramuscular injection of 25 mg/kg ketamine and 5 mg/kg xylazine. Anaesthesia was maintained by the intramuscular administration of 15 mg/kg of xylazine at 30-min time intervals for a total of 1·5 h.
Group C (n = 24): rabbits subject to the induction of acute pyelonephritis, as described previously [7–9]. Anaesthesia was administered as in animals in group B. Through an upper midline abdominal incision, the peritoneal cavity was entered and the intestines were displaced to the left. The right ureter was recognized and ligated with a 3·0 suture just below the pelvis. A total of 1 × 107 colony-forming units (CFU) of the pathogen, at a volume of 0·1 ml, was injected by a 26-gauge needle into the renal pelvis, proximal to the suture. The peritoneal cavity and the abdominal wall were closed in layers.
Resuscitation of each animal was achieved by the intravenous administration of normal saline (N/S) 0·9% at a rate of 30 ml/h via an ear vein for 2 h. Blood was sampled from the left ear vein of each animal before the operation and at 3·5, 24 and 48 h; 0·5 ml was applied for quantitative culture and the remainder was added into pyrogen-free tubes (Vacutainer; Becton Dickinson). Another 1·5 ml of blood drawn at 3·5, 24 and 48 h was collected into heparin-coated syringes (Becton Dickinson) for isolation of monocytes and neutrophils. Isolation of neutrophils was performed in all animals in groups A and B and in six animals in group C. After centrifugation, serum was kept refrigerated at −70°C until assayed for endotoxins [lipopolysaccharide (LPS)], tumour necrosis factor (TNF)-α and malondialdehyde (MDA).
Animals were transported to their cages and survival was recorded each 12 h for a total period of follow-up of 7 days.
Assays for LPS, TNF-α and MDA
For the estimation of LPS, serum samples were diluted 1 : 10 in sterile and pyrogen-free water (BioWhitaker, Walkersville, MD, USA) and incubated for 5 min at 70°C. The concentration of LPS was then measured by the QCL-1000 limulus amoebocyte lysate kinetic assay (BioWhitaker; lower limit of detection 0·05 EU/ml) using a standard curve created by known concentrations of LPS by Escherichia coli serotype O111:B4. All determinations were performed in duplicate and the mean of two observations was applied.
TNF-α was measured by a bioassay on L929 fibrosarcoma cell line, as described previously [7–9]. Briefly, confluent cells were washed thoroughly with Hank's solution and harvested with 0·25% thrypsin/0·02% ethylenediamine tetraacetic acid (EDTA) (Biochrom AG, Berlin, Germany). Cells were centrifuged, resuspended in RMPI-1640 supplemented with 10% fetal bovine serum (FBS) and 2 mM of glutamine (Biochrom AG) and distributed into a 96-well cell culture plate at a density of 1 × 105 cells/well, with a final volume of fluid into each well of 0·05 ml. After incubation for 2–3 h at 37°C at 5% CO2, 0·06 ml of serum or of standard dilutions of known concentrations of human TNF-α (Sigma, St. Louis, MO, USA; range 5·75–375·00 pg/ml) were added to each well followed by 0·05 ml of a 0·3-mg/ml dilution of cycloheximide (Sigma). Incubation continued overnight; the supernatant of each well was then discarded by aspiration and 0·1 ml of a 0·5-mg/ml methylene blue solution in methanol 99% was added. After 10 min, the dye was removed and wells were washed thoroughly three times with 0·9% sodium chloride. Wells were left to dry and remnants of the dye in each well became soluble by the addition of 0·1 ml of 50% glacial acetic acid (Merck, Darmstadt, Germany). Optical density in each well was read at 495 nm (Hitachi Spectrophotometer, Tokyo, Japan) against blank wells and control wells without added serum. Concentrations of TNF-α were estimated by reduction of the optical density of control wells by unknown samples applying a standard curve generated by standard concentrations. All determinations were performed in quadruplicate. The interday variation of the assay was 13·75%.
Lipid peroxidation was estimated by the concentration of MDA, as described previously [10]. Briefly, a 0·1-ml aliquot of each sample was mixed with 0·9 ml of trichloroacetic acid 20% (Merck) and centrifuged at 12 000 g and 4°C for 10 min. The supernatant was removed and incubated with 2 ml of thiobarbituric acid 0·2% (Merck) for 60 min at 90°C. After centrifugation, a volume of 10 μl of the supernatant was injected into a high-performance liquid chromatography (HPLC) system (Agilent 1100 Series; Waldbronn, Germany) with the following elution characteristics: Zorbax Eclipse XDB-C18 (4·6 × 150 mm, 5 μm) column under 37°C; mobile phase consisting of a 50-mM K3PO4 (pH: 6·8) buffer and methanol 99% at a 60 : 40 ratio with a flow rate of 1 ml/min; fluorometric detection with signals of excitation at 515 nm and emission at 535 nm. The retention time of MDA was 3·5 min and was estimated as μmol/ml by a standard curve created with 1, 1, 3, 3-tetramethoxy-propane (Merck). All determinations were performed in duplicate.
Blood cultures
Volumes of 0·5 ml blood were added into glass tubes with 2 ml thioglycolate medium (Becton Dickinson). A 0·1-ml aliquot was then diluted 1 : 10 into sterile sodium chloride four consecutive times. Another aliquot of 0·1 ml of each dilution was plated onto McConkey agar and incubated at 35°C for a total period of 3 days. Plates were incubated at 35°C and the number of viable colonies were counted into each dilution and multiplied by the appropriate dilution factor. The lower detection limit was 50 CFU/ml. The number of bacterial cells in blood was expressed by their log10 value.
Blood monocytes assay
For the isolation of blood monocytes, heparinized venous blood was layered over Ficoll Hypaque (Biochrom, Berlin, Germany) and centrifuged. The separated mononuclear cells were washed three times with phosphate-buffered saline (PBS; pH 7·2) and resuspended in RPMI-1640 supplemented with 10% FBS and 2 mM of glutamine in the presence of 100 U/ml of penicillin G and 0·1 mg/ml of streptomycin (Sigma). After incubation for 1 h at 37°C in 5% CO2, non-adherent cells were removed while adherent monocytes were washed three times with Hank's solution. Cells were then harvested by 0·25% trypsin/0·02% EDTA and counted. Half were treated with an ice-cold cell lysis buffer [50 mM HEPES buffer, 0·1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulphonate (CHAPS), 5 mM dithiothreitol (DTT), 0·1 mM EDTA, pH 7·4]. After centrifugation for 10 min at 10 000 g under 4°C, activity of caspase-3 was estimated in the cytosolic extract by an enzymatic chromogenic assay (Biomol Research Laboratories, Plymouth, PA, USA). It was based on the rate of hydrolysis at 37°C of a substrate releasing p-nitroaniline over time, as assessed by sequential photometry at 410 nm. The assay was also performed in the presence of a caspase-3 inhibitor. The activity of caspase-3 in cell extracts was expressed as pmol/min per 104 cells.
In all animals in groups A and B and in six animals in group C, one-quarter of the isolated monocytes were applied for estimation of the cytoplasmic activity of caspase-3, as described above. In another quarter of monocytes, the rate of apoptosis was also estimated after incubation for 15 min in the dark with the protein annexin-V at the fluorocolour fluorescein isothiocyanate (FITC) (emission 520 nm; Immunotech, Marseille, France) and with propidium iodide (PI) (emission 550 nm; Immunotech). Cells staining positive for annexin-V and negative for PI after running through the Epics XL/MSL flow cytometer (Beckman Coulter) were considered apoptotic.
The remaining half of the monocytes was added into 12-well plates at a volume of 2·4 ml per well with RPMI-1640 supplemented with 10% FBS and 2 mM of glutamine. They were incubated for 18 h at 37°C under 5% CO2. TNF-α of supernatants was estimated as described above and expressed as pg per 104 cells.
The pellet remaining after removal of mononuclear cells derived from the first centrifugation was rich in red blood cells and neutrophils. Red blood cells were lysed with the application of 0·1 M ammonium chloride (Biochrom) for 5 min. After three washings with PBS (pH 7·2), neutrophils were suspended in RPMI-1640 supplemented with 10% FBS and 2 mM glutamine in a 12-well plate; a final volume of 2·4 ml fluid per well was applied. After incubation for 18 h at 37°C under 5% CO2, TNF-α of supernatants was estimated as described above and expressed as pg per 105 cells.
Statistical analysis
Results were expressed by their median and range or interquartile range (IQR). For the purpose of analysis, animals in group C were divided into two groups: those with present activity of caspase-3 of monocytes at 3·5 h of sampling, namely CI, and those with absent activity, i.e. equal to zero activity of caspase-3 at the same time interval, namely CII. Groups A and B were compared with subgroups CI and CII by Mann–Whitney U-test with Bonferroni's correction. Survival was estimated by Kaplan–Meier analysis; comparisons were performed by the log-rank test.
Any value of P below 0·05 was considered significant.
Results
No animals in groups A and B died within the 7-day period of follow-up. Twenty-three animals died in group C.
Cytoplasmic activity of caspase-3 of monocytes isolated at 3·5 h was equal to zero in all animals of groups A and B. At 24 h, median (IQR) caspase-3 activity of monocytes of group A was 76·0 (532·7) pmol/min per 104 cells (P = 0·109 compared to 3·5 h); and of group B 131·3 (290·6) pmol/min per 104 cells (P = 0·109 compared to 3·5 h). At 48 h, median (IQR) caspase-3 activity of monocytes of group A was 0 (378·9) pmol/min per 104 cells (P = 0·180 compared to 3·5 h); and of group B 12·2 (684·7) pmol/min per 104 cells (P = 0·180 compared to 3·5 h).
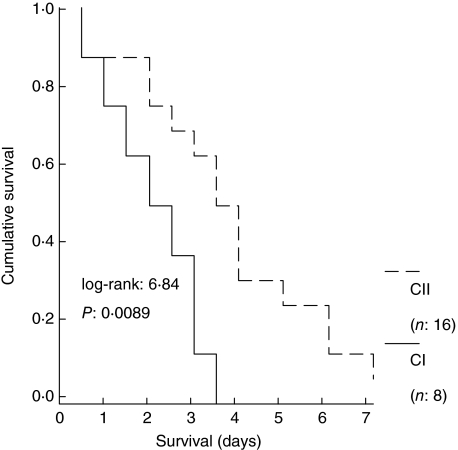
Based on the cytoplasmic activity of caspase-3 at 3·5 h, animals in group C were divided into two subgroups: subgroup CI, comprising eight animals with present activity (n = 8) and subgroup CII, comprising 16 animals with absent activity (n = 16). Median survival of these subgroups was 2 and 3·5 days, respectively (P of comparisons = 0·0089; Fig. 1).
Fig. 1.
Survival curves of 24 animals with acute pyelonephritis by multi-drug-resistant Pseudomonas aeruginosa. Animals were divided into two subgroups: subgroup CI comprising eight animals with present activity of cytoplasmic caspase-3 of monocytes at 3·5 h after bacterial challenge; and subgroup CII comprising 16 animals with absent activity at the same time interval.
Median (IQR) caspase-3 activity of subgroup CI at 3·5 h was 188·9 (48 767·3) pmol/min per 104 cells, becoming 0 (387·9) pmol/min per 104 cells at 24 h and 0 (2258·9) pmol/min per 104 cells at 48 h. Median change of caspase-3 activity of subgroup CI between 3·5 and 24 h was 56·1 pmol/min per 104 cells and between 3·5 and 48 h was 28 981·0 pmol/min per 104 cells.
Median (IQR) caspase-3 activity of subgroup CII at 3·5 h was 0 (0) pmol/min per 104 cell, becoming 5·8 (34 782) pmol/min per 104 cells at 24 h and 0 (19 726) pmol/min per 104 cells at 48 h. Median change of caspase-3 activity of subgroup CII between 3·5 and 24 h was −5·8 pmol/min per 104 cells (P = 0·040 compared to subgroup CI) and between 3·5 and 48 h was 0 pmol/min per 104 (P = 0·036 compared to subgroup CI).
At 3·5 h, median (IQR) annexin-V(+)/PI(–) monocytes of groups A, B and C were 24·3 (26·3)%; 17·8 (25·4)%; and 11·6 (11·6)%, respectively [P not significant (n.s.) between groups]. At 24 h, median (IQR) annexin-V(+)/PI(–) monocytes of groups A, B and C were 4·2 (13·5)%; 6·6 (16·2)% and 58·9 (76·3)%, respectively (P of comparisons group A versus C 0·016; group B versus C 0·020). At 48 h, median (IQR) annexin-V(+)/PI(–) monocytes of groups A, B and C were 18·4 (26·4)%, 17·3 (26·8)% and 27·3 (38·4)%, respectively (P n.s. between groups).
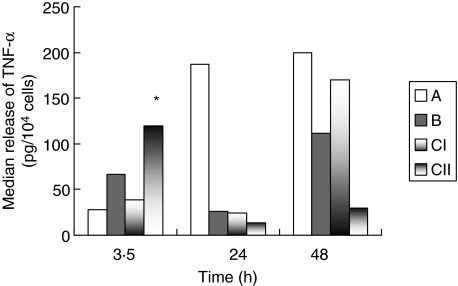
The ex vivo secretion of TNF-α from monocytes isolated on consecutive time intervals in relation to the presence or absence of activity of caspase-3 of monocytes at 3·5 h is shown in Fig. 2. It was higher in subgroup CII than subgroup CI at 3·5 h (P = 0·039). It was also higher in group A than subgroup CII at 48 h (P = 0·010). Furthermore, TNF-α released by monocytes of subgroup CII isolated at 24 h was lower compared to concentrations released at 3·5 h (P = 0·005).
Fig. 2.
Comparative release of tumour necrosis factor (TNF)-α from blood monocytes isolated from 36 rabbits divided into three groups. Group A: sham-operated; group B: animals administered anaesthetics; and group C: animals with acute pyelonephritis by multi-drug-resistant Pseudomonas aeruginosa. The latter group is further divided into two subgroups: subgroup CI with present and subgroup CII with absent activity of cytoplasmic caspase-3 of their monocytes at 3·5 h after bacterial challenge. P of comparisons between CI and CII: *0·039.
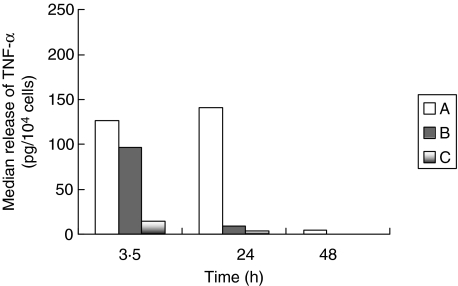
The ex vivo release of TNF-α from neutrophils isolated at consecutive time intervals is shown in Fig. 3. The observed differences in median values were not proved to be significant after non-parametric comparisons at any time of sampling.
Fig. 3.
Comparative release of tumour necrosis factor (TNF)-α from blood neutrophils isolated from 18 rabbits divided into three groups. Group A: sham-operated; group B: animals administered anaesthetics; and group C: animals with acute pyelonephritis by multi-drug-resistant Pseudomonas aeruginosa.
The number of bacteria in blood and serum levels of LPS, MDA and TNF-α of the studied animals is shown in Table 1. The only difference between subgroups CI and CII was shown in serum levels of TNF-α at 48 h. Both subgroups had higher numbers of bacteria in blood and greater serum levels of LPS at 24 and 48 h compared to groups A and B.
Table 1.
Comparisons of the number of bacteria in blood and of serum levels of endotoxins (LPS), malondialdehyde (MDA) and tumour necrosis factor (TNF)-α of 36 rabbits divided into three groups. Group A: sham-operated; group B: animals administered anaesthetics; and group C: animals with acute pyelonephritis by multi-drug-resistant Pseudomonas aeruginosa. The latter group is divided further into two subgroups: subgroup CI with present and subgroup CII with absent activity of cytoplasmic caspase-3 of their monocytes at 3·5 h after bacterial challenge.
| Group C | |||||
|---|---|---|---|---|---|
| Time (h) after challenge | Group A | Group B | Subgroup CI | Subgroup CII | P between subgroups |
| Number of bacteria in blood (log10 CFU/ml, median–range) | |||||
| 0 | ≤ 1·69 | ≤ 1·69 | ≤ 1·69 | ≤ 1·69 | n.s.a |
| 3·5 | ≤ 1·69 | ≤ 1·69 | ≤ 1·69 (1·69–6·40) | ≤ 1·69 (1·69–5·30) | n.s. |
| 24 | ≤ 1·69 | ≤ 1·69 | 7·00 (1·69–7·00)b | 7·00 (1·69–7·00)b | n.s. |
| 48 | ≤ 1·69 | ≤ 1·69 | 5·00 (4·50–7·00)b | 2·28 (1·69–7·00)b | n.s. |
| Serum LPS (EU/ml, median–range) | |||||
| 0 | ≤ 0·05 | ≤ 0·05 | ≤ 0·05 | ≤ 0·05 | n.s. |
| 3·5 | ≤ 0·05 | ≤ 0·05 | 1·03 (0·25–3·64) | 0·32 (≤ 0·05–4·87) | n.s. |
| 24 | ≤ 0·05 | ≤ 0·05 | 4·65 (1·00–13·16)b | 5·25 (≤ 0·05–19·80)b | n.s. |
| 48 | ≤ 0·05 | ≤ 0·05 | 3·83 (1·00–6·66)b | 2·41 (1·00–70·90)b | n.s. |
| Serum MDA (μmol/ml, median–range) | |||||
| 0 | 0·76 (0·13–1·19) | 1·46 (0·09–2·93) | 1·11 (0·31–3·32) | 1·52 (0·30–8·68) | n.s. |
| 3·5 | 0·79 (0·44–1·82) | 0·50 (0·29–2·66) | 1·23 (0·16–19·80) | 0·91 (0·20–12·99) | n.s. |
| 24 | 1·47 (0·11–4·54) | 0·60 (0·44–2·05) | 2·80 (0·85–7·78)c | 1·14 (0·31–11·70)c | n.s. |
| 48 | 2·66 (0·16–2·84) | 0·85 (0·21–1·55) | 1·57 (0·59–2·26) | 1·41 (0·53–9·43) | n.s. |
| Serum TNF-α (pg/ml, median–range) | |||||
| 0 | 19·3 (≤ 5·8–116·7) | ≤ 5·8 (≤ 5·8–167·8) | 13·7 (≤ 5·8–58·8) | 11·5 (≤ 5·8–50·2) | n.s. |
| 3·5 | ≤ 5·8 (≤ 5·8–117·5) | ≤ 5·8 (≤ 5·8–86·3) | 25·6 (≤ 5·8–232·1) | 15·5 (≤ 5·8–6126·7) | n.s. |
| 24 | ≤ 5·8 (≤ 5·8–124·8) | ≤ 5·8 (≤ 5·8) | ≤ 5·8 (≤ 5·8–11·5) | 11·5 (≤ 5·8–1216·0) | n.s. |
| 48 | ≤ 5·8 (≤ 5·8–237·1) | 37·8 (≤ 5·8–390·6) | 55·5 (18·9–1790·5) | 21·5 (≤ 5·8–48 751·8) | 0·045 |
Non-significant
P < 0·0001 compared to respective values of group A and group B.
P < 0·05 compared to respective values of group A and group B.
Discussion
Apoptosis is a common sequelae in sepsis well studied in lymphocytes, with tissue macrophages and intestinalepithelium being related to organ failure [3]. Published results of our study group from a prospective clinical study in patients with ventilator-associated pneumonia and sepsis reported a clear-cut connection between apoptosis of blood monocytes on the first day of diagnosis and survival [5]. This clinical observation may render apoptosis of monocytes not only a sequelum of the septic process, but also a central mechanism of pathogenesis. In order to clarify the significance of apoptosis of monocytes in the progression to sepsis and death, an animal model of pyelonephritis was applied. Infection was induced by multi-drug-resistant P. aeruginosa in an attempt to simulate nosocomial practice where similar pathogens emerge to those described in the previous cohort of patients with ventilator-associated pneumonia and sepsis [5].
In the present study, apoptosis of monocytes was estimated by two different methods: the cytoplasmic activity of caspase-3 and the presence of annexin-V on cell membranes by flow cytometry. Active caspase-3 signifies induction of the apoptotic cascade that will lead to death [11]. Results revealed that very early induction of apoptosis of monocytes, i.e. within the first 3·5 h after bacterial challenge, was accompanied by shortened survival compared to animals with no apoptosis of monocytes at the same time interval (Fig. 1).
Various hypotheses could be proposed to describe the importance of very early apoptosis of monocytes as a significant denominator of death. Ex vivo transfer and incubation of monocytes that were left unstimulated allowed them to provide an indirect index of their potential for the secretion of TNF-α in the environment of the host in relation to their intracellular signalling for apoptosis. Based on that assumption, the explanation for the increased survival of septic animals with no activity of caspase-3 of their monocytes at 3·5 h post-bacterial challenge might be given by the following hypothesis: with time lapsing post-bacterial challenge, and more precisely at 24 h, these monocytes became apoptotic with biosynthetic inertia for TNF-α. On the contrary, monocytes apoptotic at 3·5 h were replaced by a non-apoptotic cell population at 48 h with high activity for secretion of TNF-α (Fig. 2), thus rendering the septic host prone to earlier death. In accordance with that hypothesis, serum levels of subgroup I were higher than those of subgroup II at 48 h (Table 1). Differences in serum TNF-α seem to reflect the biosynthetic activity of monocytes and not of neutrophils, as no statistical differences were found between groups in the release of TNF-α by neutrophils (Fig. 3).
Why the apoptotic cascade is triggered in the monocytes of some hosts earlier than others is not clear. Information linked to serum levels of LPS and MDA of subgroups could not provide further help (Table 1).
The animal model described may provide information about the evolution of the apoptotic process in blood monocytes early after inoculation of the pathogen in the host, and to our knowledge is the first in the literature with such a design. There is no doubt that the present animal model may not simulate a clinical situation where patients are subject to both administration of anti-microbials and organ support and where there is not such great mortality. An increase in the activity of caspase-3 at 3·5 h meant that the apoptotic process was initiated, so that its effects were shown clearly by the increase in expression of annexin-V of septic animals compared to controls at 24 h. This is in accordance with findings of our study in humans. In that study apoptosis of monocytes was estimated by the expression of annexin-V on cell membranes within the first 24 h of presentation of clinical signs of sepsis [5].
The present study reveals that early induction of apoptosis in blood monocytes following bacterial challenge is of prime importance for the survival of the septic host. The explanation might rely on the influence of apoptosis on the biosynthetic activity of monocytes for proinflammatory mediators. Because the exact time sequence of events influencing the secretion of proinflammatory mediators from diverse cell populations remains questionable [12], these findings add new perspectives in the pathophysiology of sepsis.
Acknowledgments
This study was supported by an unrestricted educational grant by Abbott Laboratories, Abbott Park, Chicago, USA.
References
- 1.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scand J Infect Dis. 2003;35:585–93. doi: 10.1080/00365540310015692. [DOI] [PubMed] [Google Scholar]
- 2.Ákira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35:555–62. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- 3.Power C, Fanning N, Redmond HP. Cellular apoptosis and organ injury in sepsis: a review. Shock. 2002;18:197–211. doi: 10.1097/00024382-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Adrie C, Bachelet M, Vayssier-Taussat M, et al. Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am J Resp Crit Care Med. 2001;164:389–95. doi: 10.1164/ajrccm.164.3.2009088. [DOI] [PubMed] [Google Scholar]
- 5.Giamarellos-Bourboulis EJ, Routsi C, Plachouras D, et al. Early apoptosis of blood monocytes in the septic host: is it a mechanism of protection in the event of septic shock? Crit Care. 2006;10:R76. doi: 10.1186/cc4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical and Laboratory Standards. Performance standards for antimicrobial susceptibility testing. 14th Int Suppl. 2004;24:30. [Google Scholar]
- 7.Giamarellos-Bourboulis EJ, Adamis T, Laoutaris G, et al. Immunomodulatory clarithromycin treatment of experimental sepsis and acute pyelonephritis caused by multidrug-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2004;48:93–9. doi: 10.1128/AAC.48.1.93-99.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giamarellos-Bourboulis EJ, Tziortzioti V, Koutoukas P, et al. Clarithromycin is an effective immunomodulator in experimental pyelonephritis caused by pan-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2006;57:937–44. doi: 10.1093/jac/dkl084. [DOI] [PubMed] [Google Scholar]
- 9.Giamarellos-Bourboulis EJ, Antonopoulou A, Raftogiannis M, et al. Clarithromycin is an effective immunomodulator when administered late in experimental pyelonephritis by multidrug-resistant Pseudomonas aeruginosa. BMC Infect Dis. 2006;6:31. doi: 10.1186/1471-2334-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal R, Chase SD. Rapid, fluorometric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr. 2002;775:121–6. doi: 10.1016/s1570-0232(02)00273-8. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death is sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–8. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 12.Netea MF, van der Meer JMW, van Deuren M, Kullberg BJ. Proinflammatory cytokines and sepsis syndrome: not enough or too much of a good thing? Trends Immunol. 2003;24:254–8. doi: 10.1016/s1471-4906(03)00079-6. [DOI] [PubMed] [Google Scholar]