Abstract
Background
In order to understand the consequences of persistent enteral feeding in patients with carbohydrate malabsorption, we fed piglets lactulose in sufficient dosage to produce osmotic diarrhea or inulin, using a conventional dose, to determine if this prebiotic can modulate the effects of lactulose. Feeding lactulose increases cecal luminal synthesis of butyrate, with inulin having an intermediate effect. Because clostridia may be a major source of colonic butyrate production, we hypothesized that feeding piglets lactulose or inulin would increase cecal densities of clostridia.
Methods
Piglets were assigned to 3 formula study groups for 6 days: (1) control, fed only sow milk replacer (n = 12); (2) inulin, inulin supplement (3 g/L; n = 11); and (3) lactulose, lactulose supplement (66.7 g/L; n = 6). Cecal fluid for bacteriological studies was sampled intraoperatively.
Results
The wet/dry ratio of the cecal contents (mean ± SEM) was 8.2 ± 0.5, 6.2 ± 0.5, and 18.8 ± 5.5, respectively, in the control, inulin, and lactulose groups (p = .049, Kruskal-Wallis). There were no differences among the diet groups for cecal densities (106 colony-forming units [CFU]/g dry wt cecal contents) of total anaerobes, total aerobes, bifidobacteria, or lactobacilli. Densities of clostridia were markedly reduced in the lactulose group (1.14 ± 0.41) vs the control (18.39 ± 4.44; p = .001) or inulin groups (8.87 ± 2.20; p = .04).
Conclusions
In piglets, feeding lactulose at a dose known to cause diarrhea reduces cecal densities of clostridia.
Because of our clinical interest in how to approach enteral feeding of patients with osmotic diarrhea secondary to carbohydrate malabsorption (and incomplete fermentation), we developed a piglet model of carbohydrate malabsorption: persistent feeding of lactulose, an indigestible disaccharide of galactose and fructose.1–3 Our research suggests that osmotic diarrhea caused by lactulose malabsorption has minimal, if any, clinical effects, but lactulose feeding consistently causes decreased cecal cell proliferation.1–3 Because, in vivo, butyrate produced by bacteria and well absorbed from the colonic lumen4 causes increased cell proliferation,5–7 we hypothesized that osmotic diarrhea might cause a form of in vivo butyrate deficiency, in part characterized by decreased cell proliferation in the colon.1
We then sought ways to enhance colonic fermentation so that when challenged with severe carbohydrate malabsorption, fermentation (and butyrate production) might be enhanced. Previously, others8 had shown that giving adult volunteers a prolonged, non-diarrheogenic dose of lactulose attenuated the subsequent diarrhea caused by a “laxative” dose of lactulose, and we theorized that augmenting the fermentation capacity with a fermentable carbohydrate other than lactulose might have a similar effect.1 Inulin is an indigestible and highly fermentable fructooligosaccharide (FOS), with an average of 35 fructosyl units. FOSs are found naturally in foods such as wheat, onion, and garlic, in an increasing number of processed foods used as low-energy, low-glycemic-index, noncariogenic sweeteners (functional foods), and in various formulas, cereals, and other foods for infants.4,9 In healthy adults, the threshold for diarrhea with FOS is about 55 g/d (0.8 g/kg/d).9 Inulin (3 g/L of formula, or about 0.4 g/kg/d) has been used by others to increase colonic cell proliferation in piglets.10 There is controversy about the extent to which feeding inulin enhances butyrate formation.3,10,11 In an initial study, we showed a trend in lactulose-treated piglets toward enhanced cecal cell proliferation and reduced diarrhea when inulin was prefed for 7 days and then discontinued before a challenge with lactulose.2 These results seemed consistent with our original hypothesis that fermentation and butyrate production were inhibited by high purging rates, but enhancing bacterial activity in the colon by prefeeding inulin could reverse these effects.
In a subsequent study, we measured the rate of colonic luminal synthesis of butyric acid in growing, chronically catheterized piglets orally fed sow milk replacement formula, with or without supplements with lactulose, inulin, or inulin plus lactulose.3 We observed a doubling of the rate of butyrate synthesis in the group fed lactulose (15.2 g/kg/d)3; a combination of both prefeeding and then cofeeding inulin (0.4 g/kg/d) with lactulose (15.7 g/kg/d) prevented the decrease in cecal cell proliferation observed with lactulose feeding and was associated with normalization of butyrate synthesis.3 When compared with the control group, piglets fed inulin without lactulose (0.4 g/kg/d) manifested lower cecal cell proliferation, more diarrhea, and an intermediate rate of butyrate synthesis that was not statistically different from controls or lactulose-fed piglets.3 One hypothetical explanation for these data is that fructose-containing sugars, lactulose or inulin, exert a “prebiotic” effect,12 namely, suppression of growth of specific bacterial species, which via an independent, unknown mechanism normally stimulate cecal cell proliferation. However, when fed together, they may competitively inhibit the use of either sugar as a substrate for fermentation by this/these bacterial species, resulting in no change in cecal cell proliferation from normal.2,3 Butyrate synthesis, then, could be a function of the amount of fermentable sugar reaching the colon (much greater for lactulose in our model), rather than related to the prebiotic effect of the sugars. So, in order to gain more insight into the putative effects of lactulose and inulin on the cecal bacterial flora of piglets, we conducted the following piglet study. Because clostridia species have been thought to be major sources for colonic butyrate production,13,14 we nominally hypothesized that feeding piglets with supplementary lactulose or inulin would increase cecal densities of clostridia.
MATERIALS AND METHODS
Animals, Feedings, and Design
Twenty-nine standard Yorkshire/Hampshire piglets were studied at the University of Vermont, where the Institutional Animal Care and Use Committee approved the research protocol. On approximately day 12 of life, the piglets were transported from the pig farm to the laboratory, where the piglets were housed individually and fed orally a sow milk substitute formula (Control formula, C; SPF Lac; sterile milk replacer; PetAG Inc, Hampshire, IL). According to analysis (Covance Laboratories, Madison, WI), the macronutrient composition was as follows: energy, 3.7 MJ/L; protein, 48.2 g/L; fat, 60.5 g/L; total carbohydrate, 33.9 g/L; and lactose, 28.7 g/L. The formula was further supplemented with lactose to achieve a final concentration of 60 g/L.
The piglets were assigned to 3 treatment groups and fed milk replacer for 6 days. The control group (C; n = 12) received only sow milk replacer. The first experimental group (I; n = 11) was fed milk replacer with inulin (3 g/L), and the second experimental group (L; n = 6) was fed milk replacer with lactulose (66.7 g/L). Piglets assigned to the C and I groups were randomly assigned to diets as part of a longer study involving cecal infusions of butyrate or phosphate-buffered saline, commencing after the conclusion of the present study. Piglets assigned to the L group were not part of the cecal infusion study. Thus, the number of piglets in the L group was half that of the other 2 groups. During the study, body weight, formula intake, and stool characteristics were monitored. Diarrhea was quantified by computing the fraction of the observation period when diarrhea was observed, as previously described.1,2
After 6 days of feeding, the piglets underwent a surgical procedure for collection of cecal luminal fluid for bacteriology studies. Details of the surgical procedures have been described previously.3,15–18 Anesthesia was induced with a combination of Telazol (Fort Dodge Animal Health, Wyeth, Madison, NJ)/xylazine administered intramuscularly (Telazol, 6 mg/kg; xylazine, 4 mg/kg). General anesthesia was then maintained with isoflurane via an endotracheal tube. A laparotomy and cecal incision were performed as previously described.15 After collection of cecal fluid, piglets in the C and I groups then underwent insertion of a cecal infusion cannula and additional studies, but the piglets in the L group were euthanized with pentobarbital.
Bacteriology Studies
Cecal contents collected via the incision were placed in a sterile vial such that the vial was completely filled. The vial was then capped tightly and wrapped with parafilm. The vial was immediately refrigerated until it was mailed on ice the same day to the laboratory of Carol Williams at Mississippi State University (overnight express). Following preparation of dilutions in prereduced, sterile, phosphate-buffered (0.06 M, pH 7.0) gelatin (1% wt/vol) diluent, aliquots were immediately plated to both selective and nonselective agar media for quantification of total anaerobes, total aerobes, Bifidobacterium species, Clostridium species, and Lactobacillus species.1,19–21 General plate media included Wilkins-Chalgreen blood agar (for both total anaerobes and total aerobes), BIM-25 agar (for Bifidobacterium species), MRS agar (for Lactobacillus), and MacConkey agar for total enteric count. Selective recovery of Clostridium species was accomplished using the ethanol shock treatment method, followed by plating onto anaerobic blood agar. Identification of bacteria was performed to the genus level using standard microbiologic methods. All manipulations for the recovery and identification of anaerobic bacteria were performed in an anaerobic chamber. Bacterial counts were expressed as colony forming units/g dry weight cecal content according to the wet/dry weight ratio of the cecal luminal fluid sample.
Data Analysis and Statistics
During bacteriological examination, the investigators were masked to the identity of the treatment group for each piglet. Because of lack of homogeneity of variance, we generally used the Kruskal-Wallis test initially to determine if there was a significant diet-group effect. Significant results were supplemented with an analysis of variance (ANOVA) performed on the ranks,22 followed by Scheffe’s test to allow comparisons between specific groups (SPSS Base 10.0; SPSS Inc, Chicago, IL). All results are expressed as mean ± SEM.
RESULTS
There was no significant difference among the groups in weight gain and milk replacer intake. There was a significantly higher fraction of days with diarrhea in the lactulose-fed piglets (0.61 ± 0.16) compared with the controls (0.08 ± 0.04; p = .008) or to the inulin-fed piglets (0.17 ± 0.08; p = .029). The difference between the inulin and control groups would have been statistically significant (p < .05) with a power of 0.81, with 43 in each group. The wet/dry ratio of the cecal contents was 8.2 ± 0.5, 6.2 ± 0.5, and 18.8 ± 5.5, respectively, in the C, I, and L groups (p = .049, Kruskal-Wallis).
Table I shows a lack of differences among the groups for cecal densities of total anerobes, total aerobes, bifidobacteria, or lactobacilli. Figure 1 shows that densities of clostridia (106 colony-forming units [CFU]/g dry wt cecal contents) were markedly reduced in the L group (1.14 ± 0.41) compared with the C group (18.39 ± 4.44; p = .001) or the I group (8.87 ± 2.20; p = .04). The 52% reduction in the I group compared with the C group was not statistically significant.
TABLE I.
Densities (106 colony-forming units/g dry weight of cecal contents) of bacteria in the cecum of piglets fed milk replacer alone or supplement with inulin or lactulose
| Feeding group | Total anaerobes | Total aerobes | Bifidobacteria | Lactobacillus |
|---|---|---|---|---|
| Control | 20,850 ± 6127 | 4641 ± 1692 | 2670 ± 684 | 17,477 ± 5002 |
| Inulin | 23,936 ± 5992 | 6525 ± 2184 | 1925 ± 623 | 15,856 ± 4504 |
| Lactulose | 100,152 ± 56,442 | 23,623 ± 13,958 | 6481 ± 4229 | 33,605 ± 10,667 |
Figure 1.
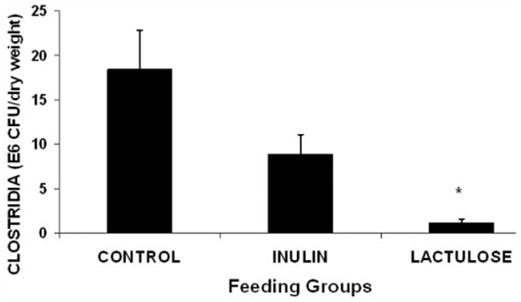
Cecal clostridial colonization in piglets (106 colony-forming units per g dry weight). * Lactulose vs control, p = .001; lactulose vs inulin, p = .04 (rank transformation; analysis of variance followed by Scheffe’s test).
Expressing the bacteriology data per gram dry weight accounts for differences in water content of feces, which may be independently affected by diet group, as noted above. However, changes in total bacterial mass, not quantified in this study, will certainly affect the ratio of bacterial numbers to dry weight.23 Therefore, because we are interested in how lactulose and inulin alter the relative proportions of the different bacterial groups, we also examined ratios between bacterial classes. The ratio of clostridia/lactobacilli (per dry weight) in the L group (0.0001 ± 0.00002) was significantly lower compared with the C group (0.0042 ± 0.00210; p < .001) or the I group (0.0010 ± 0.00032; p = .004) (rank transformation, Scheffe’s test), but there was no significant difference between the C and I groups. There was no effect of treatment group on the ratio of clostridia/bifidobacteria.
DISCUSSION
This study shows that lactulose lowers the cecal density of clostridial species when fed at a dose that produces watery feces (diarrhea) but does not cause nutrition problems or, consistently, cecal inflammation.1–3 We have been using lactulose as a surrogate for mal-absorbed disaccharides such as lactose. Clostridial species ferment lactose24 and are cultured from the feces of both bottle-fed and breast-fed infants.25 However, a literature review did not reveal studies of the effects of dietary lactose on the clostridial colonization of the colon of infant mammals. Kleesen et al11 showed that lactose supplementation caused a decrease in the density (per dry weight) of clostridia in the feces of elderly subjects. The biologic (“clinical”) significance of lowering the normal cecal densities of clostridia is unclear, and we were unable to differentiate among the various species of cecal clostridia, which vary in potential pathogenic effects on the intestine.14,24 Bacteria of the Clostridium genus have been recently implicated in causing necrotizing enterocolitis (NEC)24,26–28 and enteritis necroticans (pigbel).29 Moreover, NEC has been linked to feeding and, specifically, the feeding of carbohydrates partially fermented in the colon.4,30 Cecal, NEC-like lesions were produced frequently in response to lactose feeding and oral inoculation of clostridial strains in alactasic, previously gnotobiotic quails; this pathology was associated with high cecal concentrations of butyric acid.24 The particular lactulose feeding regimen used in our study has been shown to cause a higher rate of cecal luminal synthesis of butyrate and lower cecal luminal concentration of butyrate.3 Further studies would be required to determine, for example, if feeding lactulose would lower the risk of NEC or cecitis in a suitable model.24,28,31 However, apart from relevance to NEC, lactulose-induced changes in the assemblages of various bacteria species in the colon could alter fermentation pathways (eg, the concentrations and relative proportion of organic acids that are produced during fermentation of a given substrate).32 This, in turn, would affect energy lost as heat during fermentation (eg, lactate vs acetate production)33 or the production of butyrate and other short-chain fatty acids with specific effects on endogenous enzymatic pathways.34,35
In the present study, although lactulose feeding (~66 g/L formula) caused diarrhea, it still decreased the cecal density of clostridia expressed either per dry weight cecal contents or as a ratio to another common bacterial species (lactobacilli). Thus, lactulose did not simply purge the cecum of all bacteria but selectively diminished the density of clostridia. Whether this “pre-biotic” effect on clostridia was related to changes in osmolality of the cecal contents cannot be determined from our study, but we have not found evidence in the literature for selective effects of osmolality on the growth of this bacterium. Compared with controls, the inulin group, fed a much lower amount of indigestible carbohydrate (~3 g/L) than the lactulose group, did not exhibit a statistically significant change in either diarrhea frequency (despite a mean 112% increase) or cecal density of clostridia (52% lower). In a previous study, we did find that feeding inulin without lactulose caused more than a doubling of the fraction of days when diarrhea was observed (152% increase), although lactulose had a greater effect (274%).3 Thus, inulin, at the dose used, may have an intermediate effect on both diarrhea and the cecal density of clostridia, compared with lactulose, given at a much larger daily dose of indigestible carbohydrate.
This study was not intended as a direct comparison of the effects of inulin and lactulose on cecal density of bacteria but rather as a follow-up to our previous studies examining the effects of a very large dose of lactulose on cell proliferation.1–3 As explained in the introduction, we have been studying a “laxative dose” of lactulose in order to simulate severe sugar malabsorption in infants. Inulin was purposefully not given at such a high dose that it would induce a similarly high purging rate as lactulose, with the realized expectation that, when fed before or with lactulose, it would alter fermentation pathways and prevent the inhibition of cecal cell proliferation.2,3,8 We had hypothesized that lactulose, more than inulin, would stimulate colonization with clostridia, which ostensibly are important in producing butyrate.13,14 Because we now have evidence that lactulose (and perhaps inulin as well) inhibits clostridia colonization, it certainly would be reasonable in future studies to compare the effects of these 2 sugars at equal doses of total sugar and at equal doses of fructose. Previous studies have not shown a consistent effect of FOS on clostridia in the colon either in humans36,37 or nonhuman mammals.10,38,39 However, in the quail model, FOS supplementation lowered clostridial colonization.13
In conclusion, cecal densities of clostridia were markedly decreased by the inclusion of lactulose in a milk replacer fed to 6-day-old piglets at a dose known to cause diarrhea, to lower cecal proliferation, and to cause higher colonic luminal butyrate synthesis. It is not clear whether this prebiotic effect of lactulose would be seen at lower doses or if it could be used to lower abnormally high densities of clostridia under other conditions. These results are consistent with the hypothesis that indigestible carbohydrates including fructose alter the assemblages of bacteria in the cecum.
Acknowledgments
The study was supported in part by National Institutes of Health Grant DK61775. We appreciate the technical assistance of Rhonda Maple, Karen Everingham, and the personnel of the Animal Resources Center, University of Vermont and by Cristen L. Williams, Cary L. Williams, and Candace L. Williams, Mississippi State University.
References
- 1.Kien CL, Murray RD, Qualman SJ, Marcon M. Lactulose feeding in piglets: a model for persistent diarrhea and colitis induced by severe sugar malabsorption. Dig Dis Sci. 1999;44:1476–1484. doi: 10.1023/a:1026672306929. [DOI] [PubMed] [Google Scholar]
- 2.Kien CL, Chang JC, Cooper JR, Frankel WL. Effects of prefeeding a prebiotic on diarrhea and colonic cell proliferation in piglets fed lactulose. JPEN J Parenter Enteral Nutr. 2004;28:22–26. doi: 10.1177/014860710402800122. [DOI] [PubMed] [Google Scholar]
- 3.Kien CL, Schmitz-Brown M, Solley T, Sun D, Frankel WL. Increased colonic luminal synthesis of butyric acid is associated with lowered colonic cell proliferation in piglets. J Nutr. 2006;136:64–69. doi: 10.1093/jn/136.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21:351–366. doi: 10.1177/0115426506021004351. [DOI] [PubMed] [Google Scholar]
- 5.Sakata T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: a possible explanation for trophic effects of fermentable fibre, gut microbes and luminal tropic factors. Br J Nutr. 1987;58:95–103. doi: 10.1079/bjn19870073. [DOI] [PubMed] [Google Scholar]
- 6.Frankel WL, Zhang W, Singh A, et al. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology. 1994;106:375–380. doi: 10.1016/0016-5085(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 7.Sakata T, Tamate H. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J Dairy Sci. 1978;61:1109–1113. doi: 10.3168/jds.S0022-0302(78)83694-7. [DOI] [PubMed] [Google Scholar]
- 8.Flourie B, Briet F, Florent C, Pellier P, Maurel M, Rambaud JC. Can diarrhea induced by lactulose be reduced by prolonged ingestion of lactulose? Am J Clin Nutr. 1993;58:369–375. doi: 10.1093/ajcn/58.3.369. [DOI] [PubMed] [Google Scholar]
- 9.Briet F, Achour L, Flourie B, et al. Symptomatic response to varying levels of fructooligosaccharides consumed occasionally or regularly. Eur J Clin Nutr. 1995;49:501–507. [PubMed] [Google Scholar]
- 10.Howard MD, Gordon DT, Pace LW, Garleg KA, Kerley MS. Effects of dietary supplementation with fructooligosaccharides on colonic microbiota populations and epithelial cell proliferation in neonatal pigs. J Pediatr Gastroenterol Nutr. 1995;21:297–303. doi: 10.1097/00005176-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kleessen B, Sykura B, Zunft H-J, Blaut M. Effects of inulin and lactose on fecal microflora, microbial activity, and bowel habit in elderly constipated persons. Am J Clin Nutr. 1997;65:1397–1402. doi: 10.1093/ajcn/65.5.1397. [DOI] [PubMed] [Google Scholar]
- 12.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 13.Butel MJ, Waligora-Dupriet AJ, Szylit O. Oligofructose and experimental model of neonatal necrotising enterocolitis. Br J Nutr. 2002;87(suppl 2):S213–S219. doi: 10.1079/BJNBJN/2002540. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Moriwaki H, Muto Y, Kato N, Watanabe K, Ueno K. Effect of lactulose on short-chain fatty acids and lactate production and on the growth of faecal flora, with special reference to Clostridium difficile. J Med Microbiol. 1997;46:80–84. doi: 10.1099/00222615-46-1-80. [DOI] [PubMed] [Google Scholar]
- 15.Kien CL, Ailabouni AH, Murray RD, Powers PA, McClead RE, Kepner J. Technical note: pig model for studying nutrient assimilation by the intestine and colon. J Anim Sci. 1997;75:2161–2164. doi: 10.2527/1997.7582161x. [DOI] [PubMed] [Google Scholar]
- 16.Kien CL, Chang JC, Cooper JR. Quantitation of colonic luminal synthesis of butyric acid in piglets. J Pediatr Gastroenterol Nutr. 2002;35:324–328. doi: 10.1097/00005176-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Ebner S, Schoknecht P, Reeds P, Burrin D. Growth and metabolism of gastrointestinal and skeletal muscle tissues in protein-malnourished neonatal pigs. Am J Physiol. 1994;266:R1736–R1743. doi: 10.1152/ajpregu.1994.266.6.R1736. [DOI] [PubMed] [Google Scholar]
- 18.Reeds PJ, Burrin DG, Jahoor F, Wykes L, Henry J, Frazer EM. Enteral glutamate is almost completely metabolized in first pass by the gastrointestinal tract of infant pigs. Am J Physiol. 1996;270:E413–E418. doi: 10.1152/ajpendo.1996.270.3.E413. [DOI] [PubMed] [Google Scholar]
- 19.Onderdonk AB, Allen SD. Clostridium. In: Balows A, Hausler WJ, Herrmann KL, Isenberg HD, Shadomy HJ, editors. Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology; 1995. pp. 574–586. [Google Scholar]
- 20.Summanen P, Baron EJ, Citron DM, Strong CA, Wexler HA, Finegold SM. Wadsworth Anaerobic Bacteriology Manual. Belmont, CA: Star Publishing Co; 1993. [Google Scholar]
- 21.Biavati B, Sgorbati B, Scardovi V. The genus Bifidobacterium. In: Balows A, Triiper HG, Dworkin M, Harder W, Schleifer K-H, editors. The Prokaryotes: A Handbook on the Biology of Bacteria. New York, NY: Springer-Verlag; 1992. pp. 816–833. [Google Scholar]
- 22.Conover WJ, Iman RI. Rank transformations as a bridge between parametric and nonparametric statistics. Am Stat. 1981;35:124–128. [Google Scholar]
- 23.Stephen AM, Cummings JH. The microbial contribution to human faecal mass. J Med Microbiol. 1980;13:45–56. doi: 10.1099/00222615-13-1-45. [DOI] [PubMed] [Google Scholar]
- 24.Waligora-Dupriet AJ, Dugay A, Auzeil N, Huerre M, Butel MJ. Evidence for clostridial implication in necrotizing enterocolitis through bacterial fermentation in a gnotobiotic quail model. Pediatr Res. 2005;58:629–635. doi: 10.1203/01.PDR.0000180538.13142.84. [DOI] [PubMed] [Google Scholar]
- 25.Mevissen-Verhage EAE, Marcelis JH, De Vos MN, Harmsen-van Amerongen WCM, Verhoef J. Bifidobacterium, Bacteroides, and Clostridium spp. in fecal samples from breast-fed and bottle-fed infants with or without iron supplement. J Clin Microbiol. 1987;25:285–289. doi: 10.1128/jcm.25.2.285-289.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosloske AM, Ball WS, Jr, Umland E, Skipper B. Clostridial necrotizing enterocolitis. J Pediatr Surg. 1985;20:155–159. doi: 10.1016/s0022-3468(85)80290-6. [DOI] [PubMed] [Google Scholar]
- 27.Alfa MJ, Robson D, Davi M, Bernard K, Van Caeseele P, Harding GK. An outbreak of necrotizing enterocolitis associated with a novel clostridium species in a neonatal intensive care unit. Clin Infect Dis. 2002;35(Suppl 1):S101–S105. doi: 10.1086/341929. [DOI] [PubMed] [Google Scholar]
- 28.Sangild PT, Siggers RH, Schmidt M, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology. 2006;130:1776–1792. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Petrillo TM, Beck-Sague CM, Songer JG, et al. Enteritis necroticans (pigbel) in a diabetic child. N Engl J Med. 2000;342:1250–1253. doi: 10.1056/NEJM200004273421704. [DOI] [PubMed] [Google Scholar]
- 30.Kien CL. Colonic fermentation of carbohydrate in the premature infant: possible relevance to necrotizing enterocolitis. J Pediatr. 1990;117:S52–S58. doi: 10.1016/s0022-3476(05)81131-x. [DOI] [PubMed] [Google Scholar]
- 31.Butel MJ, Roland N, Hibert A, et al. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J Med Microbiol. 1998;47:391–399. doi: 10.1099/00222615-47-5-391. [DOI] [PubMed] [Google Scholar]
- 32.Florent C, Flourie B, Leblond A, Rautureau M, Bernier J-J, Rambaud J-C. Influence of chronic lactulose ingestion on the colonic metabolism of lactulose in man (an in vivo study) J Clin Invest. 1985;75:608–613. doi: 10.1172/JCI111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kien CL, Sumners JE, Stetina JS, Heimler R, Grausz JP. A method for assessing carbohydrate energy absorption and its application to premature infants. Am J Clin Nutr. 1982;36:910–916. doi: 10.1093/ajcn/36.5.910. [DOI] [PubMed] [Google Scholar]
- 34.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133(7 Suppl):2485S–2489S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 35.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whelan K, Judd PA, Preedy VR, Simmering R, Jann A, Taylor MA. Fructooligosaccharides and fiber partially prevent the alterations in fecal microbiota and short-chain fatty acid concentrations caused by standard enteral formula in healthy humans. J Nutr. 2005;135:1896–1902. doi: 10.1093/jn/135.8.1896. [DOI] [PubMed] [Google Scholar]
- 37.Langlands SJ, Hopkins MJ, Coleman N, Cummings JH. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut. 2004;53:1610–1616. doi: 10.1136/gut.2003.037580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loh G, Eberhard M, Brunner RM, et al. Inulin alters the intestinal microbiota and short-chain fatty acid concentrations in growing pigs regardless of their basal diet. J Nutr. 2006;136:1198–1202. doi: 10.1093/jn/136.5.1198. [DOI] [PubMed] [Google Scholar]
- 39.Flickinger EA, Van Loo J, Fahey GC., Jr Nutritional responses to the presence of inulin and oligofructose in the diets of domesticated animals: a review. Crit Rev Food Sci Nutr. 2003;43:19–60. doi: 10.1080/10408690390826446. [DOI] [PubMed] [Google Scholar]


