Abstract
Dendritic cells play a crucial role in initiating tumour immunity as well as in the immune response for invading foreign pathogens such as bacteria and viruses. For bacterial and viral infections, the immature dendritic cells (iDCs) residing in peripheral tissues are efficiently activated and matured by pathogen signals for performing the immune response. In contrast, for self-antigens, the naive T cells are not activated by iDCs but proceed to anergy/deletion, and the generation of regulatory T cells for immune tolerance. The induction of immune response and tolerance is regulated strictly by iDCs as the sensor for homeostasis of immune response in the host. Despite the identification of some tumour antigens, tumour immunity is not provoked successfully. Even though there are some critical obstacles to inhibit effective tumour immunity, tumour cells are able to exploit the functional roles of iDCs for tumour progression, which are induced by tumour-derived soluble factors such as vascular endothelial growth factor (VEGF) and functionally modulated in the microenvironment. The iDCs still remain as the critical target for provoking tumour immunity. In this review, the functional roles of tumour-associated iDCs and the strategy for targeting iDCs in effective tumour immunity for the cancer patient are discussed.
Keywords: dendritic cell, immature dendritic cell, immunosuppression, solid tumour, tumour immunity
Introduction
Dendritic cells (DCs) play a crucial role in initiating the immune response as the immunological sensor not only for invading foreign pathogens, but also in provoking tumour immunity [1,2]. For bacterial and viral infections, the immature dendritic cells (iDCs) derived from the bone marrow and residing in peripheral tissues are recruited to the inflamed site by pathogen signals. They capture foreign antigens and undergo maturation and migration to secondary lymphoid organs such as lymph nodes (LNs) [3]. The migrated mature DCs present foreign antigens such as major histocompatibility complex (MHC) peptides to naive T cells, which are clonally expanded to effector T cells for the primary immune response in which some are differentiated to memory T cells for the second immune response [4]. In contrast, apoptotic cells that result from normal cell turnover in peripheral tissues are an important source of self-antigens [5]. Self-antigens derived from apoptotic cells during tissue homeostasis are generally engulfed by iDCs, which are recruited to LNs, and then presented as the MHC-peptide to naive T cells. However, without the amplification of co-stimulatory molecules the migrated DCs produce T cell anergy/deletion [6] and generation of regulatory T cells (Tregs) for immune tolerance [5,7]. Thus, the systemic immune response upon invading foreign pathogens as well as the induction of tolerance to self-antigens is controlled strictly by iDCs under steady state conditions [8].
In tumour cells, the tumour-derived soluble factors (TDSFs) such as vascular endothelial growth factor (VEGF) act as a strong stimulator of iDCs from bone marrow, which can be constitutively stimulated as long as the tumours persist [9,10]. The induced iDCs, including myeloid and plasmacytoid DCs from the bone marrow, are recruited to the primary tumour site through chemokines and their receptors [11], and are functionally modulated as tumour-associated iDCs (TiDCs) that contribute to tumour immune privilege [12]. The TiDCs are able to capture apoptotic tumour cells, which were derived from the high growth fraction of tumour cell kinetics. Apoptotic cells are phagocytosed via a complex composed of the scavenger receptor CD36 and αvβ3/5 integrins [13]. Many receptors have been implicated in the uptake of dying cells such as CD14 [14], LOX1 [15] and the phosphatidylserine receptor in macrophages [16]. The TiDCs-captured apoptotic cells (TiDCs-Cp) become more stable and resistant to apoptosis than iDCs, in which they contribute to induction of immune tolerance [17]. Importantly, despite the fact that the TiDCs-Cp can be matured by inflammatory signals, the MHC-tumour antigen cannot be presented efficiently to naive T cells, resulting in the continued induction of immune tolerance [18,19]. The emerging existence of constitutively increased iDCs triggered by VEGF and subsequent production of TiDCs-Cp plays a critical role in exerting immune privilege for tumour progression in the cancer patient. In this review, we discuss the mechanism by which iDCs induce immune privilege and how they are targeted for provoking tumour immunity, compared to the regulation of immune response to microbial invasion and self-antigens in the host.
General roles of DCs in immune response to foreign pathogens and self-antigens
The general immune response for invading foreign pathogens such as bacteria and viruses is initiated by iDCs residing in peripheral tissues in response to a pathogen signal. The signal can recruit iDCs transiently through the CD34+ haematopoietic progenitor cells (HPCs) from the bone marrow to the inflamed site, where the iDCs capture foreign antigens, leading to maturation and migration through lymphatic vessels to T cell areas of lymphoid organs in response to the chemotactic gradients of the CCR7 ligands CCL21 and CCL19 [20,21]. The migrated mature myeloid DCs are able to present MHC peptides to naive T cells leading to clonal expansion of antigen-specific effector T cells in the primary immune response, in which some are differentiated to memory T cells [22]. In addition, DCs represent a critical source of interleukin (IL)-12, a cytokine that is involved in innate responses and drives T helper 1 (Th1) polarization [23]. IL-12 production by DCs is tightly controlled, requiring first a priming signal provided by microbial products or interferon (IFN)-γ and then an amplifying signal provided by T cells through the CD40 ligand (CD40L) [24]. Thus, DCs are capable of integrating signals from pathogens, cytokines and T cells, leading to the generation of an adaptive immune response of the appropriate class.
Foreign antigens such as bacteria and viruses are engulfed by iDCs, which are recognized by pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) that sense microbial products and trigger DC maturation and cytokine production, effectively bridging innate and adaptive immunity [25]. For example, TLR2 is triggered by peptidoglycan [26]; TLR3, by double-stranded RNA [27]; TLR4, by lipopolysaccharide [26]; TLR7, by single-stranded RNA [28]; and TLR9, by unmethylated oligonucleotide (CpG) [29]. TLRs are localized in different components. Although most TLRs are present on the cell surface, TLR7, TLR8 and TLR9 are in the endosomal compartments [30] and TLR3 is intracellular. In addition, TLRs are expressed differentially by distinct DC subsets. For example, TLR9 is expressed only by plasmacytoid DCs (pDCs), whereas myeloid DCs (mDCs) preferentially express TLR2 and TLR4 [31]. Foreign antigens can be captured by iDCs either by phagocytosis or receptor-mediated endocytosis that proceed to present MHC peptides to naive T cells as non-self antigens [32]. After eliminating foreign antigens by antigen-specific effector T cells, most of these T cells are committed to die by activation-induced cell death (AICD) [33]. Despite the commitment of T cell death after completion of the immune response, some effector T cells are differentiated to effector memory T cells [34].
DCs are also important in immune tolerance to self-antigens [35]. Two mechanisms were created to avoid the immune system attack on the components of self, central and peripheral tolerance, both of which are controlled and maintained by DCs. Central tolerance occurs in the thymus and is dependent on mature thymic DCs, which are essential for the deletion of newly generated T cells with a receptor that recognizes self-components. However, as many self-antigens may not access the thymus, the need for peripheral tolerance is fulfilled in lymphoid organs and is mediated by iDCs. Peripheral tolerance involves induction of T cell anergy, i.e. unresponsiveness, or under certain circumstances, deletion [5,36]. Immature DCs within tissues capture the remains of cells that die in the process of physiological tissue turnover. As there is no inflammation accompanying this process, the DCs remain immature. These iDCs, which lack co-stimulatory molecules, migrate to draining lymph nodes where they present the tissue antigens to T cells. T cells presented with antigens in the absence of co-stimulation enter either into a state of anergy or are deleted. Further, in extrinsic tolerance, iDCs are able to expand CD4+ CD25+ Tregs[37], and are also able to induce the Tr1-type Tregs[38]. Thus, DCs play a critical role in the control of cellular immunity.
Functional roles of immature DCs in the impaired tumour immunity
Several tumour and tumour-associated antigens have been identified and cancer immunotherapy using peptide vaccine and DCs loaded with peptide have been tried, but the clinical response has been poor [39]. Several factors that inhibit tumour immunity have been reported, including loss of tumour antigen and human leucocyte antigen (HLA) [40], immunosuppressive factors [41] and Tregs[21]. The impairment of an effective anti-tumour immune response is due to the lack of an efficient immune synapse between DCs and T cells [42]. Nevertheless, in the initial phase of carcinogenesis abnormal cells can be eliminated by tumour immunity, following which the tumour growth is in equilibrium with the capacity for elimination by tumour immunity [43]. If it exceeds the threshold, tumour cells escape from tumour immunity. The functional role of tumour immunity in the initial phase can be disrupted gradually according to tumour progression. Tumour cells can grow in an autocrine and paracrine fashion supported by angiogenesis. TDSFs such as VEGF increase according to tumour growth, and play an important role in promoting immune escape from tumour immunity [44]. The tumour-secreted VEGF is a strong stimulator of iDCs from the bone marrow, which are recruited to the primary tumour site via chemokines and their receptors [11]. These iDCs can be modulated functionally in the tumour site, then referred to as TiDCs, which are not only resistant to apoptosis but also express the immunosuppressive enzyme, indoleamine 2,3-dioxygenase (IDO) [45]. IDO-high expression by tumour cells enables certain cancer subsets to initially avoid immune attack and defeats the invasion of T cells via local tryptophan depletion and the production of pro-apoptotic tryptophan catabolites [46]. The accumulated TiDCs tend to expand immune privilege by exploiting the functional roles of iDCs for immune tolerance: TiDCs may capture tumour antigens derived from apoptotic cells and migrate to tumour-draining lymph nodes, where they cannot present these antigens to naive T cells without amplification of co-stimulatory signals, resulting in immune tolerance [36]. Further, TiDCs induce CD4+ CD25+ Tregs from naive T cells, and suppress T cell proliferation of both CD4+ and CD8+ T cells [47,48]. In addition, CD4+ CD25+ Tregs down-regulated CD80/CD86 co-stimulatory molecules on bone marrow-derived DCs, suggesting that their antigen-presenting function might be affected [49]. Thus, TiDCs extensively reconstitute immunological tolerance to tumour antigens for immune escape in cancer.
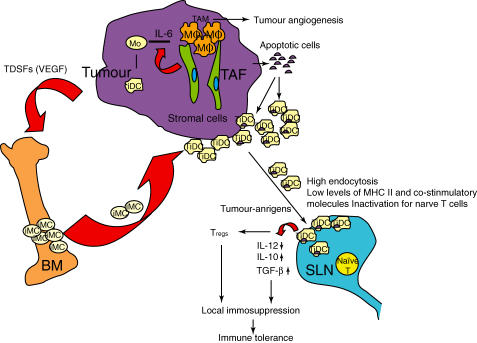
The other important causative factor to immune tolerance is the anti-inflammatory property of iDCs, which is caused by apoptotic tumour cells during tumour progression. The high rate of tumour growth produces a number of apoptotic cells for the homeostasis of cell kinetics during tumour progression. The released apoptotic cells are phagocytized by macrophages and iDCs through interaction of apoptotic cells and surface receptors on the APCs, such as phosphatydilserine and its receptor [50]. The apoptotic cells are captured very efficiently by macrophages and iDCs [51,52], resulting in an increase in anti-inflammatory cytokines such as IL-10 and transforming-growth factor-β (TGF-β) [53], and a decrease in proinflammatory cytokines such as IL-1, IL-6 and tumour necrosis factor (TNF)-α[54]. Apoptotic cells prevent the release of intracellular proteases that activate antigen-presenting cells (APCs), and in the DC microenvironment where the endocytosis takes place. The anti-inflammatory response produced by apoptotic tumour cells abrogates the initiation of an anti-tumour immune response, which is due to inhibition of the maturation of iDCs [55]. The iDCs have failed to express co-stimulatory molecules and the MHC peptide complex on the surface even after exposure to DC-activation, resulting in impairment of effective presentation of the antigen to naive T cells [18,19]. The reason for the impairment of exposure of the MHC antigen complex on the surface in TiDCs is unclear. Although the captured apoptotic tumour cells by iDCs might be processed to an appropriate peptide for antigen presentation, the complex may not be transported effectively to the cell surface. Thus, it is unlikely that most of TiDCs are appropriate for the presentation of tumour-associated antigens in the cancer patient, even though these TiDCs had been stimulated (Fig. 1).
Fig. 1.
Mechanisms by which immature dendritic cells (iDCs) produce immune privilege in tumour progression. Tumour and stromal cells-secreted inflammatory cytokine, interleukin (IL)-6 enhances the differentiation from monocyte (Mo) to macrophage rather than to iDC, which is functionally modulated as tumour-associated macrophage (TAM) that promotes tumour angiogenesis by secreting inflammatory cytokines such as tumour necrosis factor (TNF)-α and IL-1. Tumour-driven soluble factors (TDSFs) such as vascular endothelial growth factor (VEGF) stimulate recruitment of iDCs from bone marrow (BM) to the tumour site, which are functionally modulated as tumour-associated iDCs (TiDCs) that contribute to immune privilege. The TiDCs are primed with apoptotic tumour cells in the tumour site, and migrate to sentinel lymph node (SLN), resulting in the increased production of anti-inflammatory cytokines such as IL-10 and transforming growth factor (TGF)-β, and decreased capacity for antigen presentation to naive T cells for immune tolerance despite the presence of maturation signal. The TiDCs also induce regulatory T cells that inhibit T cell proliferation.
The iDCs derived from peripheral blood monocytes in the cancer patient are a uniform population of iDCs that cannot be matured efficiently in the presence of IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) (Mo-DCs) because a small population had the morphology (large cells with thin projections), immunophenotype (including CD83+) and function of mature DC. In contrast, haematopoietic stem cells, when cultured with GM-CSF and TNF, yield a heterogenous population of mature DCs including Langerhans cells and interstitial DCs with DC function (CD34-DCs) [56]. Using Mo-DCs which were loaded with the antigen for adoptive immunotherapy, the clinical effect of treatment with peptide vaccine or peptide-loaded DCs (DC/peptide) for malignant tumours was not satisfied, due to many variables remaining to be explored. These included antigen sources, modes of DC antigen-loading, multiple tumour antigens and tumour microenvironment [56]. Even though there were some critical factors to inhibit effective tumour immunity, including immunosuppressive cytokines and Tregs, the distributed TiDCs might have already captured apoptotic cells in the tumour site and peripheral tissues. Because of insufficient antigen presentation, the release of anti-inflammatory cytokines had less cross-priming ability to naive T cells resulting in less anti-tumour immune response in the cancer patient. In fact, tumour-induced DC dysfunction generates an ineffective immune response. In the absence of TDSFs, DCs differentiate and mature. Mature DCs increase their capacity to process antigens and express the co-stimulatory molecules and release the cytokines for initiating anti-tumour immune response. In contrast, under the presence of TDSFs, iDCs and apoptotic DCs are generated and accumulated. Although iDCs can present antigens, they can suppress T cell activation through the production of reactive oxygen species. Immature DCs and apoptotic DCs can present antigens, but in the absence of amplification by co-stimulation and cytokine secretion they promote Th2 response, induce anergy or inhibit T cell proliferation that lead to tumour immune evasion. Nevertheless, despite the fact that treatment with peptide for tumour antigens for malignant melanoma recruited a considerable number of CD8+ cytotoxic T cells (CTLs) to the tumour site [57], it appears that localization of CTLs at the tumour site were required for the antigen-specific immune response. Because less than 20% effector CD8+ T cells expressed perforin, however, this was not sufficient to reduce tumour volume clinically [57,58], even though other factors such as antigen heterogeneity and immunosuppressive reactions were also involved.
Strategies for targeting immature DCs for provoking tumour immunity
Dendritic cells play a central role in the initiation and control of T cell-mediated immunity. Immature DCs residing in tissues endocytose soluble antigens, microbes or apoptotic cells, and receive microbial or inflammatory maturation cues depending on the type of pathogen and the nature and extent of tissue damage [59]. Given that activation and maturation of TiDCs in the cancer patient is important for initiating an anti-tumour immune response, the problem remains of understanding how TiDCs can be manipulated to initiate an immune response to tumour cells. It seems that even though TiDCs are activated, effective presentation of tumour antigens by TiDCs cannot be expected. One possible way for targeting iDCs is the recruitment of newly produced CD34+ HPCs from the bone marrow. This can be induced by GM-CSF that preferentially expands the myeloid DC (mDC) subset in vivo[60]. Because the newly induced iDCs do not capture apoptotic cells rapidly in steady state conditions, iDCs recruited from the bone marrow may undergo differential maturation and activation compared to TiDCs. The only recruitment of iDCs by GM-CSF is not enough for initiating an anti-tumour immune response, because the release of a number of tumour antigens is required for the phagocytosis by iDCs. The increased release of dead cells is provided by neoadjuvant chemotherapy, after which treatment with GM-CSF can recruit new iDCs from the bone marrow. When massive cell death occurs following neoadjuvant chemotherapy, a number of damaged cells and subsequent dead tumour cells produce not only proinflammatory stimuli such as TNF-α, IL-1, regulated upon activation normal T cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1 and monocyte chemoattractant protein (MCP)-1 [61], but also secondary necrosis [62]. This is captured by newly induced iDCs that proceed to maturation and migrate to LNs, and then present MHC-peptide to naive T cells, resulting in clonal expansion of effector T cells in the secretion of IL-12 for Th1 polarization [63]. Given that apoptotic and necrotic cells are a strong source of tumour antigens as well as self-antigens [64], the chemotherapy-induced massive cell damage and resulting cell death are able to activate iDCs as in a proinflammatory situation. In fact, iDCs can capture various killed tumour cells, including Jurkat T cell lymphoma, malignant melanoma and prostate carcinoma. DCs loaded with killed tumour cells induce MHC class I- and class II-restricted proliferation of autologous CD8+ and CD4+ T cells, demonstrating cross-presentation of tumour cell-derived antigens [65]. Of note, IL-12 production is potentially boosted by activated T cells through CD40L [24]. However, IL-12 is not induced by some maturation stimuli, such as TNF-α and IL-1. IL-12 production can be modulated by cytokines and mediators present during induction of maturation [66].
A second method of generating newly produced iDC from bone marrow is the induction of pDCs from the bone marrow by G-CSF [67]. This is activated by CpG oligonucleotides (ODNs) through TLR9 in the accumulated tumour-derived components contained with hypomethylated CpG ODNs following neoadjuvant chemotherapy. The activated pDCs are matured with the expression of co-stimulatory molecules and the production of IL-12 for Th1 polarization, and migrate to LNs where they present MHC II-peptide to naive T cells for induction of effector T cells through CD4+ T cells [68]. The production of IFN-γ from effector T cells activates mDCs, which co-operate with pDCs for expansion of antigen-specific effector T cells in provoking tumour immunity. Although there is little evidence that pDCs are important for antigen presentation in the induction phase of immunity, their key property seems to be the production of IFN-α/β[69]. This ability is clearly important for blocking viral replication, but it has also been reported to activate other DCs, including those involved in cross-priming [70]. Further, in another study, using G-CSF-mobilized stem cells obtained before initiation of chemotherapy, lymphodepleting chemotherapy followed by the transfer of highly avid anti-tumour lymphocytes can mediate significant tumour regression in heavily pretreated patients with IL-2 refractory metastatic melanoma [71].
A third possibility for the effective presentation of tumour antigens is to disrupt the compartmentalization within the tumour site, in which iDCs are capsulized with tumour and non-tumorous tissues. This would increase the release of iDCs from the tumour site to LNs. In the previous report, iDCs were compartmentalized within the component of breast cancer cells and non-cancerous tissues, which differed from mature DCs being located in a peritumour site [72]. The compartmentalization of iDCs in a tumour site can be destroyed and loosened by neoadjuvant chemotherapy, producing massive cell death and proinflammatory cytokines depending on the tissue damage. As the compartmentalized iDCs in the tumour site may have differential functional roles from TiDCs in the peritumour site and peripheral tissues, they may still retain the capacity for activation and maturation by capturing dead cells for tumour immunity under the condition that the iDCs do not capture apoptotic cells. In this regard, although malignant transformation of melanocytes is associated with the recruitment of various subsets of DCs to initiate an immune response, in spontaneous other solid tumours DCs may be unable to capture tumour peptides, as their accumulation is limited to the connective tissue surrounding the tumour [73]. Thus, it is likely that the TiDCs, which capture apoptotic cells, produce anti-inflammatory cytokines and decrease the capacity to present tumour antigens to naive T cells and are not suitable for provoking tumour immunity. The newly induced iDCs from bone marrow by GM-CSF and G-CSF or the released iDCs from compartmentalization may be more beneficial for the activation of anti-tumour immune responses than TiDCs residing in peripheral tissues (Fig. 2).
Fig. 2.
Role of immature dendritic cells (iDCs) in immune response to foreign pathogens and tumour immunity. (a) General roles of iDCs in immune response to foreign pathogens. The iDCs reside in peripheral tissues, peripheral blood DCs (PBDCs) engulf foreign antigens, which are rapidly activated and matured by pathogen signals, and migrate to lymph node in which mature DCs present foreign antigens to naive T cells that are differentiated to effector T cells, resulting in elimination of the pathogens. (b) Differential functional roles of tumour immature dendritic cells (TiDCs) and newly produced iDCs from bone marrow, which are not primed with apoptotic cells. The newly induced iDCs by granulocyte–macrophage colony-stimulating factor (GM-CSF) and G-CSF from CD34+ progenitor cells in bone marrow (BM), bone marrow derived DCs (BMDCs), and the potential iDCs released from compartmentalization within the tumour site can be primed with massive dead cells with inflammatory cytokines following neoadjuvant chemotherapy. These iDCs are activated and matured and migrate to sentinel lymph node (SLN) where they present tumour antigens (TAs) to naive T cells, resulting in proliferation of antigen-specific effector T cells that contribute to tumour response.
The above idea, that the newly produced iDCs may be more effective for priming T cells compared to TiDCs, which have been primed with apoptotic cells, can be supported by several recent studies. In one of these studies the administration of CD34+ progenitor-derived DCs loaded with melanoma-associated epitopes appeared to induce an in vivo expansion of melanoma-specific CTLs that correlated with clinical outcome [74]. Another promising strategy appeared to be effective in the context of breast cancer, in which the iDCs derived from peripheral blood monocytes in healthy volunteers captured radiation-induced apoptotic breast cancer cells, which produced CTLs and killed allogenic breast cancer cells [75]. Another study, using newly produced iDCs from G-CSF-mobilized stem cells before initiation of chemotherapy for metastatic melanoma, showed the induction of melanoma-specific CTL and significant tumour progression [71]. These findings suggest strongly that use of the iDCs which were not primed with apoptotic cells is more effective for producing antigen-specific CTLs for tumour immunity. In addition, some studies have been performed in mouse models showing direct targeting of DCs in vivo. A recent study showed that irradiated tumour cells injected intravenously were captured by efficiently splenic DCs. Furthermore, co-administration of the natural killer T (NK T) cells mobilizing glycolipid α-galactosyl ceramide led to DC maturation and the induction of protective immunity [76]. Differences in functional roles between tumour-associated iDC and peripheral iDC, and newly produced iDC from bone marrow, are summarized in Table 1.
Table 1.
Differences in functional roles between tumour-associated immature dendritic cells (TiDCs) and peripheral iDCs, and newly produced iDCs from bone marrow.
| Functional role | TiDCs/peripheral iDCs | Newly produced iDCs |
|---|---|---|
| Susceptibility to apoptosis [17] | Resistant/sensitive, pro-apoptotic | Sensitive |
| Response to maturation signal [18,19] | Maturation/maturation | Maturation |
| Anrigen-presenting ability [18,19] | Insufficient/insufficient | Sufficient |
| Secretion of cytokines [53,55] | Most TiDCs are primed with apoptotic cells Anti-inflammatory cytokines (IL-10, TGF-β) | Proinflammatory cytokines (IL-12) after maturation |
| Immunosuppressive factors [45] | Indoleamine 2,3-dioxygenase Reactive oxygen species | None |
| Inducible factors [9,10,60,67] | VEGF | GM-CSF, G-GSF |
| Immune response [18,19] | Immune tolerance | Tumour immunity |
GM-CSF: granulocyte–macrophage colony-stimulating factor; IL: interleukin; TGF: transforming growth factor; VEGF: vascular endothelial growth factor.
Concluding remarks
Despite the fact that tumour immunity is effective for the initial phase in carcinogenesis, numerous TDSFs specifically inhibit effector cells such as CTLs and NK cells in the impairment of tumour immunity. Eventually, tumour cells gain control over the immunosuppressive network facilitating tumour progression and metastasis [77]. The immunosuppressive condition derived from iDCs cannot be modulated easily due to the balance between immunosuppressive and immunopromoting factors in tumours, because the immunosuppressive cytokines and Tregs are distributed predominantly from the tumour site to secondary lymphoid organs such as LNs and peripheral tissues in advanced cancer. Further, tumour cells exploit the inducing tolerogenic mechanisms to self-antigens for expanding the immunosuppressive network. Given that DCs play a crucial role in initiating tumour immunity, and that DCs are composed of a heterogeneous population of cell types rather than a single cell type, the co-operating linkage with pDCs and mDCs appears to be the important event for effective cross-presentation of tumour antigens. Unlike TiDCs, the recruitment of newly produced iDCs from bone marrow may play a differential role in provoking tumour immunity. Future studies will be needed to clarify these hypotheses for a differential role of the iDCs in exerting tumour immunity in the cancer patient.
References
- 1.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 2.Colonna M, Pulendran B, Iwasaki A. Dendritic cells at the host–pathogen interface. Nat Immunol. 2006;7:117–20. doi: 10.1038/ni0206-117. [DOI] [PubMed] [Google Scholar]
- 3.Bell D, Young JW, Banchereau J. Dendritic cells. Adv Immunol. 1999;72:255–324. doi: 10.1016/s0065-2776(08)60023-1. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–6. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 7.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 9.Young MR, Kolesiak K, Wright MA, Gabrilovich DI. Chemoattraction of femoral CD34+ progenitor cells by tumor-derived vascular endothelial cell growth factor. Clin Exp Metastasis. 1999;17:881–8. doi: 10.1023/a:1006708607666. [DOI] [PubMed] [Google Scholar]
- 10.Almand B, Resser JR, Lindman B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–66. [PubMed] [Google Scholar]
- 11.Zou W, Machelon V, Coulomb-L'Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–46. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 12.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–8. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albert ML, Pearce SF, Francisco LM, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, andcross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–68. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–9. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 15.Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 16.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–16. [PubMed] [Google Scholar]
- 17.Finkelman FD, Lees A, Birnbaum R, Gause WC, Morris SC. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol. 1996;157:1406–14. [PubMed] [Google Scholar]
- 18.Verbovetski I, Bychkov H, Trahtemberg U, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196:1553–61. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci USA. 2001;98:8750–5. doi: 10.1073/pnas.151028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morelli AE, Thomson AW. Dendritic cells under the spell of prostaglandins. Trends Immunol. 2003;24:108–11. doi: 10.1016/s1471-4906(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17:326–32. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–8. doi: 10.1126/science.1062416. [DOI] [PubMed] [Google Scholar]
- 23.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 24.Schulz O, Edwards AD, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 25.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 28.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 30.Heil F, Ahmad-Nejad P, Hemmi H, et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–97. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 33.Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 35.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 36.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamazaki S, Iyoda T, Tarbell K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–47. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+ CD4+ Tr cells. Blood. 2005;105:1162–9. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 39.Parmiani G, Castelli C, Dalerba P, et al. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst. 2002;94:805–18. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 40.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27:184–90. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang L, Carbone DP. Tumor–host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 42.Wang E, Panelli MC, Marincola FM. Understanding the response to immunotherapy in humans. Springer Semin Immunopathol. 2005;27:105–17. doi: 10.1007/s00281-004-0198-7. [DOI] [PubMed] [Google Scholar]
- 43.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Ohm JE, Gabrilovich DI, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–86. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 45.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 46.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–51. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 47.Bacchetta R, Gregori S, Roncarolo MG. CD4+ regulatory T cells: mechanisms of induction and effector function. Autoimmun Rev. 2005;4:491–6. doi: 10.1016/j.autrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Ghiringhelli F, Puig PE, Roux S, et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+ CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cederbom L, Hall H, Ivars F. CD4+ CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–43. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 50.Fadok VA, Chimini G. The phagocytosis of apoptotic cells. Semin Immunol. 2001;13:365–72. doi: 10.1006/smim.2001.0333. [DOI] [PubMed] [Google Scholar]
- 51.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–9. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 52.Iyoda T, Shimoyama S, Liu K, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morelli AE, Larregina AT, Shufesky WJ, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–20. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 55.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–35. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 56.Paczesny S, Ueno H, Fay J, Banchereau J, Palucka AK. Dendritic cells as vectors for immunotherapy of cancer. Semin Cancer Biol. 2003;13:439–47. doi: 10.1016/j.semcancer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Panelli MC, Riker A, Kammula U, et al. Expansion of tumor-T cell pairs from fine needle aspirates of melanoma metastases. J Immunol. 2000;164:495–504. doi: 10.4049/jimmunol.164.1.495. [DOI] [PubMed] [Google Scholar]
- 58.Kammula US, Lee KH, Riker AI, et al. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol. 1999;163:6867–75. [PubMed] [Google Scholar]
- 59.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 60.Pulendran B, Smith JL, Caspary G, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 62.Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte-derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J Immunol. 2004;173:189–96. doi: 10.4049/jimmunol.173.1.189. [DOI] [PubMed] [Google Scholar]
- 63.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases. implications for the pathogenesis of systemic autoimmune disease. Cell Death Diff. 1999;6:6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- 65.Nouri-Shirazi M, Banchereau J, Bell D, et al. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. 2000;165:3797–803. doi: 10.4049/jimmunol.165.7.3797. [DOI] [PubMed] [Google Scholar]
- 66.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 67.Pulendran B, Banchereau J, Burkeholder S, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566–72. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 68.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–9. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 69.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 70.Le Bon A, Etchart N, Rossmann C, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–15. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 71.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bell D, Chomarat P, Broyles D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–26. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vermi W, Bonecchi R, Facchetti F, et al. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. 2003;200:255–68. doi: 10.1002/path.1344. [DOI] [PubMed] [Google Scholar]
- 74.Paczesny S, Banchereau J, Wittkowski KM, Saracino G, Fay J, Palucka AK. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199:1503–11. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neidhardt-Berard EM, Berard F, Banchereau J, Palucka AK. Dendritic cells loaded with killed breast cancer cells induce differentiation of tumor-specific cytotoxic T lymphocytes. Breast Cancer Res. 2004;6:R322–8. doi: 10.1186/bcr794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu K, Idoyaga J, Charalambous A, et al. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–16. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]