Abstract
The human neuromuscular disease myasthenia gravis (MG) is characterized by the generation of autoantibodies reactive with nicotinic acetylcholine receptors (AChR) that cause loss of AChR from the neuromuscular end-plate with resultant failure of neuromuscular transmission. A role for complement (C) in AChR loss has been suggested based upon morphological identification of C at the end-plate in MG and from the effects of C inhibition in murine models. Here we provide further evidence implicating C, and specifically the membrane attack complex (MAC), in a mouse model of MG. Mice deficient in the C regulators Daf1 and/or Cd59a were tested in the model. Wild-type mice were resistant to disease while mice deficient in Daf1 had mild disease symptoms with evidence of C activation and AChR loss at end-plates. Cd59a-deficient mice had very mild disease with some muscle inflammation and essentially undamaged end-plates. In contrast, mice deficient in both C regulators developed a severe paralytic disease with marked muscle inflammation and loss of end-plates. Inhibition of MAC assembly abrogated clinical disease in these double-deficient mice, demonstrating conclusively that MAC formation was driving pathology in the model. These findings provoke us to suggest that current anti-C therapeutics targeting MAC assembly will be beneficial in MG patients resistant to conventional therapies.
Keywords: autoimmunity, complement, neuroimmunology, rodent, transgenic/knock-out mice
Introduction
Activation of complement (C) generates strong pro-inflammatory and cytotoxic activities that collaborate to destroy pathogens [1,2]. The small anaphylactic fragments C3a and C5a recruit and activate phagocytes while the large opsonic fragments C4b and C3b bind targets, labelling them for phagocyte uptake. The membrane attack complex (MAC) causes cytolysis by disrupting cell membrane integrity. While C activation is critical for host defence, aberrant activation occurs in numerous inflammatory and autoimmune diseases [3]. C activation in tissues is controlled by a battery of membrane C regulatory protein (CReg) that include decay accelerating factor (DAF; CD55) which accelerates decay of the enzymes of the activation pathways, and CD59 which inhibits assembly of the MAC [4]. When local C activation is severe and sustained, Creg-mediated defence may be overwhelmed and damage ensues. In these circumstances, therapeutic inhibition of C may be beneficial [5].
Myasthenia gravis (MG) is a debilitating and potentially life-threatening condition characterized by episodes of profound muscle weakness [6]. Therapeutic options are currently limited; some patients are resistant to available therapy, and acute myasthenic crises may require urgent respiratory support. MG is one of the best understood of the ‘organ-specific’ autoimmune diseases. Autoantibodies against the acetylcholine receptor (AChR) are generated that bind AChR at the neuromuscular junction (end-plate) and block neuromuscular transmission, causing the severe muscle weakness that typifies the condition [6–8]. The anti-AChR antibodies are not directly blocking, but instead recruit other effectors to cause end-plate damage. C was implicated over 40 years ago with the demonstration that serum C levels were decreased in MG patients, suggesting activation of C [9]. When dense localized deposits of C activation products were identified at the end-plate in electron micrographs of muscle from MG patients, a direct role for C activation in the destruction of the end-plate was proposed [10,11]. The abundance of MAC at this site provoked thesuggestion that ‘microlysis’ caused by MAC deposition and lytic pore formation was the cause of the observed destruction [12]. Given the dearth of current therapeutic options for MG, strategies targeting C activation are attractive. However, it is first essential to understand precisely how C causes damage at the end-plate.
Rodent models of MG have provided a number of important insights into the disease, including a growing body of evidence supporting a key role of C activation (summarized in the discussion). Here we have used a well-established mouse model of MG, passively induced experimental autoimmune MG (EAMG) [13,14], to dissect the contributions of the different CReg in defence of the end-plate. The data show that absence of two of the major murine CRegs, CD55 and CD59a, profoundly exacerbates disease. Further, we show that the observed acute pathology is mediated by the MAC and that inhibition of MAC formation protects from disease even in this ‘deregulated’ severe disease model. These findings suggest that targeted inhibition of MAC formation will be of benefit in human MG.
Materials and methods
Antibodies and other reagents
The rat monoclonal antibody (mAb) McAb-3 against AChR was the kind gift of Professor Vanda Lennon (Mayo Clinic, Rochester, MN, USA). The mouse mAb BB5·1 against mouse C5 was the kind gift of Professor Brigitta Stockinger (NIMR, London, UK). Polyclonal goat anti-serum against rat C3c (cross-reactive with mouse C3c) was purchased from Nordic Laboratories (Tilburg, the Netherlands) and diluted 1:400 for staining sections. Polyclonal rabbit anti-serum against rat C9 (cross-reactive with mouse C9/MAC) was generated and characterized in house and diluted 1:300 for staining. Rabbit anti-human C3 was generated in-house, IgG was purified from anti-serum by passage over protein A Sepharose and the purified IgG labelled with biotin using standard methods (Perbio Science, Cramlington, UK). Streptavidin peroxidase was from Bio-Rad (Hemel Hempstead, UK). α-Bungarotoxin–rhodamine conjugate (T-1175) was from Molecular Probes (Invitrogen, Paisley, UK). Anti-rabbit Ig fluorescein isothiocyanate (FITC) was from Bio-Rad.
Mice
In mice there are two genes for both CD55 and CD59, and in each case one gene (Daf1; CD59a) is widely expressed while expression of the other (Daf2; CD59b) is essentially restricted to testis [15–17]. Mice deficient in the broadly expressed forms of CD55 (Daf1–/–) and CD59 (Cd59a–/–) have been described and shown to lack expression of the respective proteins [16,18–20]. Each of these strains had been back-crossed onto C57Bl6 for at least 10 generations. Daf1–/–Cd59a–/– double knock-out mice were created in-house by intercrossing the two strains. Wild-type (WT) mice were littermates from these intercrosses confirmed to express each protein. All mice were maintained in standard animal house conditions and diet.
Testing C activating capacity of McAb-3
Plates (96-well, Nunclon Maxisorb, Nunc, Roskilde, Denmark) were coated with a Fab fragment of a polyclonal anti-rat IgG (made in-house) at 10 µg/ml in carbonate buffer pH 8·0, blocked in phosphate-buffered saline (PBS) containing 0·1% Tween-20 (PBS-T) and 5% non-fat dried milk and incubated with various dilutions of McAb-3 ascites in PBS-T [13,21]. Unbound antibody was removed by washing in PBS-T, wells blotted dry and dilutions of mouse serum in veronal-buffered saline (CFD; Oxoid, Basingstoke, UK) added to the wells. Plates were incubated for 60 min at 37°C, washed in PBS-T and incubated with biotinylated anti-C3 IgG (10 µg/ml in PBS-T) for 30 min at 37°C. After washing, plates were incubated with avidin–peroxidase (Bio-Rad; 1:1000 in PBS-T), washed and developed using orthophenylene diamine (OPD) substrate.
Induction and assessment of disease
All animal studies were reviewed and approved by the UK Home Office. Mice (groups of five) were injected intraperitoneally (i.p.) with McAb3 ascites (40 µl diluted to 400 µl in PBS) at time zero. This dose of ascites was chosen based upon information from the donor that it was the lowest dose required to generate disease in C57Bl6 mice. In some experiments, mice were administered simultaneously anti-C5 mAb BB5·1 (1 mg in 100 µl PBS). Mice were weighed prior to disease induction and at frequent intervals thereafter. Disease severity was assessed clinically, essentially as described [22], and scored on a scale from 0 (no disease) to 4 (severe paralytic disease necessitating sacrifice). A quantitative measure of grip strength was obtained using a home-built apparatus in which mice were allowed to grip with forepaws a metal bar attached to an electronic balance and gentle traction applied until the grip was broken. The grip strength (in grams) was assessed in three repetitions for each measurement and the mean of the three recorded. Mice were killed at 48 or 72 h and the calf muscles dissected. From each mouse, one calf muscle set was snap-frozen in isopentane on dry ice, the other fixed in 10% paraformaldehyde. Fixed tissue was resin-embedded, sectioned on a microtome and sections mounted on slides. Snap-frozen muscles were sectioned (10 µm) on a cryostat and transferred to Superfrost slides (Surgipath, Peterborough, UK). Sections were fixed in acetone for 5 min, and stored at −20°C until use.
Histology and immunohistology
Fixed muscle tissue was sectioned and stained with haemotoxylin and eosin (H&E). At least 10 sections from similar regions of each muscle were assessed in a blinded manner for the presence of inflammatory infiltrates. A semiquantitative scoring system was employed as follows: 0, no inflammatory infiltrates; 1, infiltrates < 1 per low-power field; 2, infiltrates 1–5 per field; 3, infiltrates 6–10 per field; 4, infiltrates > 10 per field.
Frozen tissue was sectioned and stained with α-bungarotoxin–rhodamine diluted to 1:200 in block buffer [PBS, 1% bovine serum albumin (BSA)] for 40 min at room temperature in a humid chamber. Following three washes in PBS, sections were mounted using VectorShield and analysed under a fluorescent microscope. The density slicing function of the Openlab software (Improvision, Coventry, UK) was used to calculate the area occupied by α-bungarotoxin-reactive AChR in each section. Twenty fields were captured from comparable areas of the soleus muscle. For analysis of deposition of C3 fragments and C9/MAC, frozen sections were incubated for 1 h at room temperature with the primary anti-serum diluted 1:100 in block buffer in a humid chamber, washed three times in PBS and incubated with anti-rabbit Ig-FITC (Bio-Rad; 1:200 in block buffer) for 40 min at room temperature in a humid chamber. To confirm end-plate staining, α-bungarotoxin–rhodamine was included in this second incubation. After washing, sections were mounted in VectorShield and analysed on a fluorescent microscope. As a negative control, sections from a naive mouse were stained as described. Sections were scored blind for degree of staining for C3 fragments and C9/MAC on an arbitrary scale from 0 (no specific staining) to 4 (strong staining at most or all end-plates). For confirmation of inflammatory cell infiltration, frozen sections were stained with rat anti-mouse CD11b-FITC conjugate.
Statistical analysis
For analysis of differences between two groups, Student's t-test was used. Where more than two groups were compared, the analysis of variance (anova) test was applied with post-hoc analysis using the t-test with Bonferroni correction. Statistical analysis utilized the InStat package (GraphPad, San Diego, CA, USA). Results were considered significantly different when P < 0·05.
Results
The anti-AChR mAb McAb-3 efficiently activates mouse C
To confirm that the rat IgG2b mAb McAb-3 activated mouse C, the mAb was first immobilized through specific capture onto a 96-well plastic plate, and dilutions of mouse serum incubated in the coated wells. C activation was assessed by measuring C3 fragment deposition in the wells. C was activated in a dose-dependent manner in wells coated with the mAb, while BSA-blocked wells in which no mAb had been immobilized showed trace activation at the highest concentration of mouse serum, confirming that the mAb McAb-3 activated C (Fig. 1a).
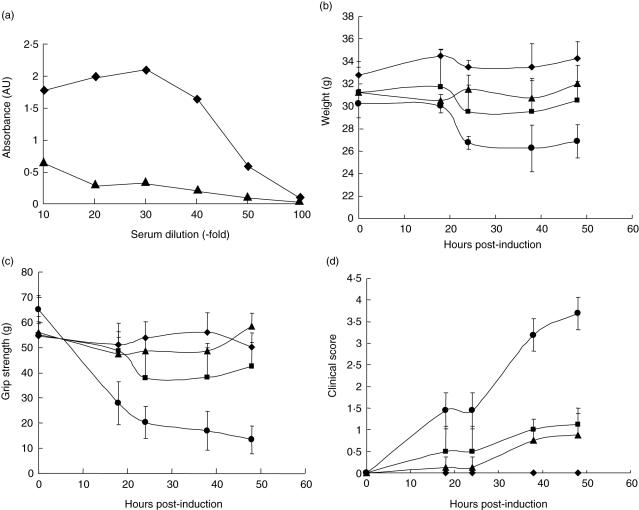
Fig. 1.
Experimental autoimmune myasthenia gravis (EAMG) is markedly exacerbated in Daf1–/–CD59a–/– mice. (a) To confirm that the monoclonal antibody (mAb) McAb-3 activated complement (C), a plate assay was established in which the mAb was coated onto enzyme-linked immunosorbent assay (ELISA) plate wells and capacity to activate C from serum assessed by measuring C3b deposited on the plastic. Antibody-coated wells are shown as diamonds and wells without mAb as triangles. Results are means of quadruplicate determinations at each point. Standard errors are plotted but are not visible in this highly reproducible assay. (b–d) EAMG was induced in age- and sex-matched groups of five wild-type (diamonds), Daf1–/–(squares), Cd59a–/– (triangles) and Daf1–/–CD59a–/– (circles) mice. Weight (b), grip strength (c) and clinical score (d) were measured at intervals. Data are means ± standard deviation (s.d.) (n = 5) for each point. Weight was reduced significantly compared to controls (P < 0·05) only in Daf1–/–Cd59a–/– mice from 24 h onwards. Grip strength was reduced significantly compared to controls in Cd59a–/– mice at 24, 38 and 48 h (P < 0·05) and Daf1–/–Cd59a–/– mice at all time-points (P < 0·01). Clinical score was increased significantly compared to controls in Daf1–/– and Cd59a–/– mice at 38 and 48 h (P < 0·05) and in Daf1–/–Cd59a–/– mice (P < 0·05 at 18 and 24 h, P < 0·01 at 38 and 48 h).
Absence of membrane CReg exacerbates weakness in EAMG
EAMG was induced in groups of five mice deficient in CD55 (Daf1–/–), CD59 (Cd59a–/–) or both (Daf1–/–CD59a–/–) and disease compared with that in WT controls. WT mice did not show significant clinical disease or reduced grip strength when compared to that measured prior to injection of mAb (Fig. 1c,d), nor did they lose weight over the course of the experiment (Fig. 1b). Both Daf1–/– and Cd59a–/– mice developed mild clinical disease and a small but consistent and statistically significant (P < 0·05) increase in clinical score at 38 and 48 h when compared to pre-induction measurements or to WT controls (Fig. 1c,d); weight loss was not significantly different between the groups (Fig. 1b). In marked contrast, Daf1–/–Cd59a–/– mice developed severe clinical disease with profound weakness within 24 h of administration of mAb (for grip strength, P < 0·01 at all time-points; for clinical score, P < 0·05 at 18 and 24 h, P < 0·01 at 38 and 48 h; Fig. 1c,d). At 48 h, the degree of weakness and the marked weight loss (significant at P < 0·05 at all time-points from 24 h; Fig. 1b) in this experimental group necessitated the mice to be killed.
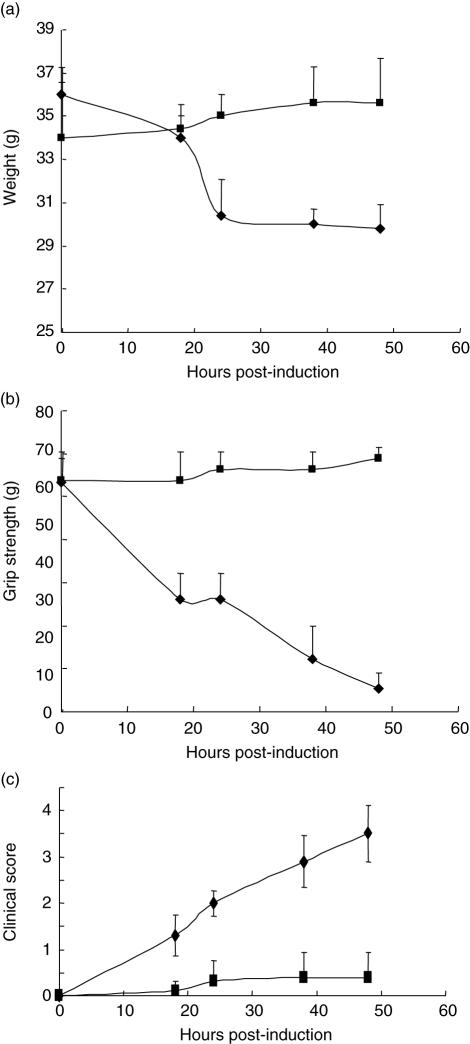
In a separate experiment, disease was induced in two groups of five Daf1–/–Cd59a–/– mice; one group was treated with anti-C5 mAb BB5·1 at 1 mg/animal given i.p. with the disease-inducing mAb (Fig. 2). In preliminary experiments, this dose of BB5·1 caused complete inhibition of haemolytic C activity in C57Bl6 mice through 48 h (data not shown). Administration of anti-C5 mAb with the disease-triggering mAb completely abrogated disease in the Daf1–/–Cd59a–/– mice. While untreated mice developed severe paralysis and disease, as in the first experiment, the anti-C5 mAb-treated group showed minimal clinical disease and no reduction in grip strength when compared to pre-induction measurements, and exhibited no other clinical signs of disease throughout the 48-h experimental time-course (Fig. 2b,c). Anti-C5-treated Daf1–/–Cd59a–/– mice also continued to gain weight following induction of EAMG, while those not treated with anti-C5 showed marked weight loss (Fig. 2a). Differences between treated and untreated groups for each of the three measured parameters were significant (P < 0·05 to P < 0·01) at all time-points post-induction.
Fig. 2.
Inhibition of C5 cleavage protects from disease in Daf1–/–CD59a–/– mice. In a separate experiment, experimental autoimmune myasthenia gravis (EAMG) was induced in two age- and sex-matched groups of five Daf1–/–CD59a–/– mice. One group was given anti-C5 monoclonal antibody (mAb) with the disease-initiating antibody (squares), while the other group was given initiating antibody alone (diamonds). Weight (a), grip strength (b) and clinical score (c) were measured at intervals. Data are means ± standard deviation (s.d.) (n = 5) for each point. Differences between treated and untreated groups for each of the three measured parameters were significant (P < 0·05 to P < 0·01) at all time-points post-induction.
Absence of membrane CReg exacerbates end-plate AChR loss in EAMG
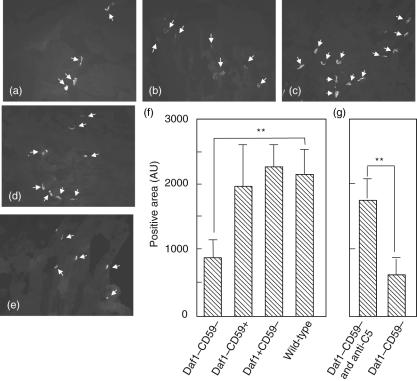
Number and integrity of end-plates was assessed in muscle by staining for AChR in muscle sections with α-rhodamine-labelled bungarotoxin, followed by imaging and quantification. In untreated WT mice, well-circumscribed brightly stained end-plates were evident in most sections; this appearance was essentially identical in WT mice treated with the anti-AChR mAb (Fig. 3a). In contrast, α-bungarotoxin staining in Daf1–/–CD59a–/– mice revealed a marked reduction in staining with a few residual weakly stained and poorly circumscribed end-plates (Fig. 3b). Muscle from Cd59a–/– mice showed well-preserved end-plate staining while that from Daf1–/– mice showed intermediate loss of staining and structure (Fig. 3c,d). The pooled data from each experimental group is summarized in Fig. 3f. The total number of α-bungarotoxin-positive end-plates was reduced significantly in Daf1–/–Cd59a–/– mice compared with each of the other groups (P < 0·01). Differences between other groups failed to reach significance. End-plate loss was significantly reduced (P < 0·01) in Daf1–/–Cd59a–/– mice treated with anti-C5 mAb, as demonstrated by the preservation of α-bungarotoxin-positive end-plates (Fig. 3e,g).
Fig. 3.
Acetylcholine receptor (AChR) loss is exacerbated in Daf1–/–CD59a–/– mice. Muscle sections were stained with α-bungarotoxin–rhodamine and imaged. Twenty fields were captured for each muscle analysed. Representative fields for wild-type (a), Daf1–/–CD59a–/– (b), Cd59a–/– (c) and Daf1–/– (d) mice are shown. Plate (e) is a representative section for Daf1–/–Cd59a–/– mice treated with anti-C5 monoclonal antibody (mAb). Arrows indicate positive areas. Magnification × 200. The area occupied by α-bungarotoxin-positive end-plates was measured automatically using the density-slicing feature of the Openlab software and the mean value from 20 fields for each mouse calculated. (f) Mean of these means ± standard deviation (s.d.) (n = 5) for the first experiment comparing each of the four mouse strains used; end-plate area was reduced significantly in Daf1–/–Cd59a–/– mice compared to all other groups (only comparisons with wild-type are shown for clarity). (g) Means of means for the second experiment, testing effects of C5 inhibition in Daf1–/–Cd59a–/– mice; end-plate area was preserved by anti-C5 treatment. *P < 0·05; **P < 0·01.
Absence of membrane CReg permits C activation at the end-plate in EAMG
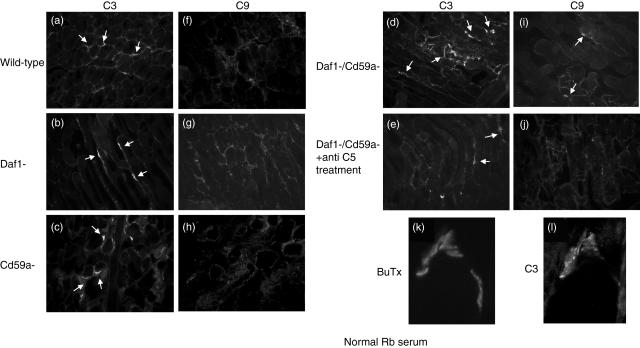
Muscle sections were stained for deposition of C3 fragments and C9 as a surrogate for MAC. Muscle sections from untreated WT or knock-out animals were negative for both these anti-sera (data not shown). C3 fragment deposition was present in all mice given the anti-AChR mAb, present in a linear pattern along the end-plates (Fig. 4a–e). Presence at the end-plates was confirmed by double-staining in all sections (example shown in Fig. 4k,l from Cd59a– mouse). C3 fragment deposition was assessed and scored in sections from all mice in each group. All Daf1–/–Cd59a–/– mice had moderate to heavy C3 staining at end-plates [scores 3·4 ± 0·6; mean ± standard deviation (s.d.)]; the majority of Daf1–/– and Cd59a–/– mice had moderate C3 staining at end-plates (scores 2·8 ± 0·36 and 2·0 ± 0·24, respectively; mean ± s.d.); WT mice had mild to moderate C3 staining (score 1·2 ± 0·2; mean ± s.d.). C3 staining in each of the gene knock-out animals was significantly higher than in WT (P < 0·05), but differences between the other groups were not statistically significant. Specific C9/MAC staining was present only in Daf1–/–Cd59a–/– mice (Fig. 4i). Staining was strong in this group (score 3·2 ± 0·4; mean ± s.d.) and co-localized with residual α-bungarotoxin-positive end-plates. There was no specific staining for C9/MAC in any of the other groups, including the anti-C5-treated Daf1–/–Cd59a–/– group (negative data not shown).
Fig. 4.
Complement (C) activation is present at the end-plate in experimental autoimmune myasthenia gravis (EAMG). Muscle sections were stained for C3b (a–e) or C9/MAC (f–j) and double-stained with rhodamine–bungarotoxin (example in k,l). Representative fields for wild-type (a,f), Daf1–/– (b,g), Cd59a–/– (c,h) and Daf1–/–CD59a–/– (d,i) mice are shown. Plates (e,j) are representative sections for Daf1–/–Cd59a–/– mice treated with anti-C5 monoclonal antibody (mAb). Arrows indicate C3b/C9-positive bungarotoxin-positive end-plates. Magnification × 200; plates k,l × 1000.
Absence of membrane CReg exacerbates muscle inflammation in EAMG
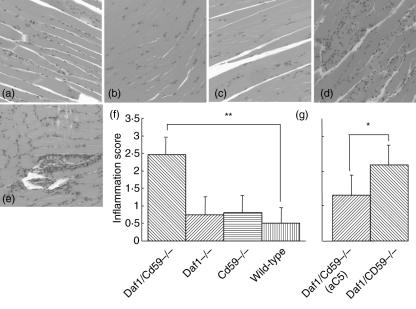
Muscle histology with H&E staining at 48 h revealed the presence of inflammatory foci in the majority of mice given the anti-AChR mAb, absent in untreated controls, confirmed by staining for infiltrating macrophages and/or neutrophils using anti-mouse CD11b (Fig. 5a–c). Inflammatory scores were calculated for each mouse and the results for each group are shown in Fig. 5f. WT, Daf1–/– and CD59a–/– mice all showed mild inflammation with average inflammatory scores < 1 in each group. In contrast, all Daf1–/–Cd59a–/– mice had moderate to severe inflammatory changes in muscle that were significantly increased (P < 0·01) compared with each of the other groups. Treatment with anti-C5 in these mice significantly reduced but did not ablate inflammation in Daf1–/–Cd59a–/– mice (Fig. 5g; P < 0·05).
Fig. 5.
Muscle inflammation is exacerbated in Daf1–/–CD59a–/– mice. Muscle sections were stained with haematoxylin and eosin to identify inflammatory cell infiltrates. Representative fields for wild-type (a), Daf1–/– (b), Cd59a–/– (c) and Daf1–/–CD59a–/– (d) mice are shown. Plate (e) is a representative section from Daf1–/–CD59a–/– mice treated with anti-C5. Multiple sections were scored as described in Methods and the mean inflammatory score for each animal calculated. Plate (f) shows the summed data for each set in experiment 1; columns represent means of means and lines show standard deviations (n = 5 for each group). Plate (g) shows summed data for experiment 2 represented on the same scale as (f). For experiment 2, only four animals were analysed per group because of technical difficulties that rendered tissue from one animal unusable in the study. Here, too, columns represent means and lines show standard deviations. Magnification × 100. *P < 0·05; **P < 0·01.
Discussion
There is an abundance of data implicating C in pathogenesis in rodent models of MG induced either actively by immunizing with AChR purified from the torpedo ray electric organ, or passively by administering MG patient sera, EAMG serum or mAb generated against the AChR [23]. The first evidence was provided by the demonstration of C deposition at the end-plate in rats with actively induced EAMG [11]. A causative role was suggested by Lennon and coworkers who showed that removal of C activity by treatment with the C-consuming agent cobra venom factor (CVF) markedly inhibited both actively induced and passively induced (by serum transfer) EAMG in Lewis rats [14]. These findings were confirmed in rats in which EAMG was passively induced using the anti-AChR mAb35; treatment with soluble C receptor 1 (sCR1), a specific inhibitor of C activation, markedly inhibited disease [22]. A role for MAC in passively induced EAMG was suggested by the demonstration that neutralization of C6 inhibited disease [24]. In these studies, Fab fragments of a polyclonal anti-C6 antibody were administered to rats prior to injection of anti-AChR mAb35. Treated rats were protected as assessed by measurement of weight loss, evoked muscle potential and clinical disease. In mice, C5-deficient strains were shown to be resistant to active induction of EAMG, despite having levels of anti-AChR antibodies similar to C sufficient controls [25]. More recently, these workers showed that knock-out mice lacking either C3 (required for all C activation pathways) or C4 (specific for the classical pathway) are resistant to active induction of EAMG despite the generation of strong anti-AChR titres, implying an essential requirement for classical pathway activation [26].
We and others have used mice deficient in the membrane CReg to define roles of C in mouse models of disease. CD59a-deficient (CD59a–/–) mice showed increased disease when compared to WT mice in experimental demyelination, arthritis models and models of nephritis [27–29]. CD55-deficient (Daf1–/–) mice were more susceptible to disease in nephritis models [20], and mice deficient in both these regulators showed the most severe disease in nephritis models [30]. The Daf1–/– mice have been tested in the passive EAMG model and reported to show a marked increase in disease susceptibility compared to WT littermates [31]. To clarify further the contributions of the various C-derived effectors and specifically the role of the MAC in end-plate damage, we have utilized mice deficient in one or two membrane CReg and pathway-specific murine anti-C reagents in passively induced EAMG in the mouse. As noted by others [31], WT mice on the C57Bl6 background were resistant to clinical disease in this model, despite evidence of muscle inflammation and C3 deposition at the end-plate, probably reflecting the known high safety factor for neuromuscular transmission in this species. In our hands, Daf1–/– mice developed a mild and acute disease characterized by mild muscle weakness and pathologically by muscle inflammation, and end-plate damage as shown by increased C3 deposition and reduced α-bungarotoxin staining.
These findings differ qualitatively from the report of Lin et al. using the same passive induction EAMG model and the same dose of inducing mAb, where Daf1–/– mice displayed a marked increase in disease susceptibility and severity, reduced AChR numbers and increased C3 staining at the end-plate compared to WT [31]. The reasons why these two independently derived strains of Daf1–/– mice behave so differently in their response to passive induction of EAMG using the same initiating mAb are unclear, but may hint at subtle differences between the strains. Cd59a–/– mice also developed only very mild disease in this model with minimal muscle inflammation and end-plate damage. Of note, C3 deposition, present in the Daf1–/– mice, was reduced in Cd59a–/–, indicating that control in the activation pathways was adequate to prevent significant C activation in the model. In marked contrast, mice deficient in both of these regulators developed a severe disease with profound weakness, associated with abundant inflammation in muscle and severe damage to end-plates with deposition of C3 and C9 and loss of α-bungarotoxin staining. Inflammation was not restricted to the vicinity of the end-plates, as has been shown in some previous studies [13,14], due probably to the severity of disease in these animals. These findings provide further evidence of the key role of C in end-plate damage in this model of MG and show that CReg control in the activation and terminal pathways collaborate to protect the tissues. Further, they imply that the observed destruction is mediated by the MAC and regulation of MAC formation can restrict or prevent the destruction of the end-plate and development of paralysis despite the presence of muscle inflammation and C3 deposition.
To confirm the importance of C activation and MAC formation, the effects of inhibiting C5 activation were tested in the Daf1–/–Cd59a–/– mice. The mAb BB5·1 acts by binding C5 and preventing its cleavage, thus preventing the generation of C5a and MAC. C5 inhibition using this mAb rescued the severe disease phenotype seen in the Daf1–/–Cd59a–/– mice. Minimal clinical signs were detected in the BB5·1-treated Daf1–/–Cd59a–/– mice, while C3 deposition at the end-plate and inflammatory cell infiltration were reduced significantly compared to untreated Daf1–/–Cd59a–/– mice.
Our clear demonstration that end-plate destruction and clinical disease in the mouse passive EAMG model is mediated by C activation, and that MAC formation is necessary to cause destruction of the end-plate, has important implications for human disease. Although primary deficiencies of CReg are extremely rare in man, more subtle changes in C regulatory capacity have been shown to influence susceptibility to disease, for example in the haemolytic uraemic syndrome [32]. C activation has been well documented in MG and a role for the MAC suggested based upon electron microscopic identification of dense MAC deposits and MAC-positive debris at the end-plate [10,12,33]. These data, taken together with the findings presented here, provide strong evidence that C and MAC are indeed causative in the destruction of the end-plate in mice and men. Therapies that target C activation and specifically MAC formation should therefore be considered for treatment of MG, particularly in those difficult cases where conventional therapies fail. Anti-C5 agents have already reached the clinic and have been used with success in several clinical trials, most notably in therapy of myocardial infarction and the post-cardiopulmonary bypass syndrome [34]. A recombinant membrane targeted form of CD59 has been shown to be effective in the haemolytic disorder paroxysmal nocturnal haemoglobinuria [35]. There is now an urgent need to test these agents in MG; all the evidence indicates that inhibition of MAC formation will ablate disease.
Acknowledgments
The authors acknowledge the support of the staff of Biomedical Services, Cardiff University. This work was supported by The Wellcome Trust (Programme Grant no. 068590 to B. P. M. and University Award no. 068823 to C. L. H.) and an MRC Industrial Collaborative Studentship (G 78/7562).
References
- 1.Gasque P, Neal JW, Singhrao SK, McGreal EP, Van Dean YDBJ, Morgan BP. Roles of the complement system in human neurodegenerative disorders: pro-inflammatory and tissue remodeling activities. Mol Neurobiol. 2002;25:1–17. doi: 10.1385/mn:25:1:001. [DOI] [PubMed] [Google Scholar]
- 2.Morgan BP. The complement system: an overview. Meth Mol Biol. 2000;150:1–13. doi: 10.1385/1-59259-056-X:1. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 4.Harris CL, Morgan BP. Complement regulatory proteins. London: Academic Press; 1999. [Google Scholar]
- 5.Morgan BP, Harris CL. Complement therapeutics; history and current progress. Mol Immunol. 2003;40:159–70. doi: 10.1016/s0161-5890(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 6.Hughes BW, De Casillas MLM, Kaminski HJ. Pathophysiology of myasthenia gravis. Semin Neurol. 2004;24:21–30. doi: 10.1055/s-2004-829585. [DOI] [PubMed] [Google Scholar]
- 7.Newsom-Davis J, Pinching AJ, Vincent A, Wilson SG. Function of circulating antibody to acetylcholine receptor in myasthenia gravis: investigation by plasma exchange. Neurology. 1978;28:266–72. doi: 10.1212/wnl.28.3.266. [DOI] [PubMed] [Google Scholar]
- 8.De Baets M, Stassen MHW. The role of antibodies in myasthenia gravis. J Neurol Sci. 2002;202:5–11. doi: 10.1016/s0022-510x(02)00200-9. [DOI] [PubMed] [Google Scholar]
- 9.Nastuk WL, Plescia OJ, Osserman KE. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960;105:177–84. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- 10.Engel AG, Lambert EH, Howard FM. Immune complexes (IgG and C3) at the motoe end-plate in myasthenia gravis: ultrastructural and light microscopic localisation and electrophysiologic correlations. Mayo Clin Proc. 1977;52:267–80. [PubMed] [Google Scholar]
- 11.Sahashi K, Engel AG, Lindstrom J, Lambert EH, Lennon VA. Ultrastructural localisation of immune complexes (IgG and C3) at the end-plate in experimental autoimmune myasthenia gravis. J Neuropath Exp Neurol. 1978;37:212–23. doi: 10.1097/00005072-197803000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Sahashi K, Engel AG, Lambert EH, Howard FM. Ultrastructural localisation of the terminal and lytic ninth complment component (C9) at the motor end-plate in myasthenia gravis. J Neuropath Exp Neurol. 1980;39:160–72. doi: 10.1097/00005072-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Lennon VA, Lambert EH. Myasthenia gravis induced by monoclonal antibodies to acetylcholine receptors. Nature. 1980;285:238–40. doi: 10.1038/285238a0. [DOI] [PubMed] [Google Scholar]
- 14.Lennon VA, Seybold ME, Lindstrom J, Cochrane C, Ulevitch R. Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J Exp Med. 1978;147:973–83. doi: 10.1084/jem.147.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baalasubramanian S, Harris CL, Donev R, et al. CD59a is the primary regulator of membrane attack complex assembly in the mouse. J Immunol. 2004;173:3684–92. doi: 10.4049/jimmunol.173.6.3684. [DOI] [PubMed] [Google Scholar]
- 16.Miwa T, Sun X, Ohta R, Okada N, Harris CL, Morgan BP, Song WC. Characterization of glycosylphosphatidylinositol-anchored decay accelerating factor (GPI-DAF) and transmembrane DAF gene expression in wild-type and GPI-DAF gene knockout mice using polyclonal and monoclonal antibodies with dual or single specificity. Immunology. 2001;104:207–14. doi: 10.1046/j.0019-2805.2001.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin F, Fukuoka Y, Spicer A, et al. Tissue distribution of products of the mouse decay-accelerating factor (DAF) genes. Exploitation of a Daf1 knock-out mouse and site-specific monoclonal antibodies. Immunology. 2001;104:215–25. doi: 10.1046/j.0019-2805.2001.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt DS, Botto M, Bygrave AE, Hanna SM, Walport MJ, Morgan BP. Targeted deletion of the CD59 gene causes spontaneous intravascular haemolysis and haemoglobinuria. Blood. 2001;98:442–9. doi: 10.1182/blood.v98.2.442. [DOI] [PubMed] [Google Scholar]
- 19.Suny XJ, Funk CD, Deng CJ, Sahu A, Lambris JD, Song WC. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proc Natl Acad Sci USA. 1999;96:628–33. doi: 10.1073/pnas.96.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miwa T, Maldonado MA, Zhou L, et al. Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/Ipr mice. Am J Pathol. 2002;161:1077–86. doi: 10.1016/S0002-9440(10)64268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lennon VA, Lambert EH. Monoclonal autoantibodies to acetylcholine receptors: evidence for a dominant idiotype and requirement of complement for pathogenicity. Ann NY Acad Sci. 1981;377:77–96. doi: 10.1111/j.1749-6632.1981.tb33725.x. [DOI] [PubMed] [Google Scholar]
- 22.Piddlesden SJ, Jiang S, Levin JL, Vincent A, Morgan BP. Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol. 1996;71:173–7. doi: 10.1016/s0165-5728(96)00144-0. [DOI] [PubMed] [Google Scholar]
- 23.Mozrzymas JW, Lorenzon P, Riviera AP, Tedesco F, Ruzzier F. An electrophysiological study of the effects of myasthenia gravis sera and complement on rat isolated muscle fibers. J Neuroimmunol. 1993;45:155–62. doi: 10.1016/0165-5728(93)90176-y. [DOI] [PubMed] [Google Scholar]
- 24.Biesecker G, Gomez CM. Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J Immunol. 1989;142:2654–9. [PubMed] [Google Scholar]
- 25.Christadoss P. C5 gene influences the development of murine myasthenia gravis. J Immunol. 1988;140:2589–92. [PubMed] [Google Scholar]
- 26.Tuzun E, Scott BG, Goluszko E, Higgs S, Christadoss P. Genetic evidence for involvement of classical complement pathway in induction of experimental autoimmune myasthenia gravis. J Immunol. 2003;171:3847–54. doi: 10.4049/jimmunol.171.7.3847. [DOI] [PubMed] [Google Scholar]
- 27.Mead RJ, Neal JW, Griffiths MR, et al. Deficiency of the complement regulator CD59a enhances disease severity, demyelination and axonal injury in murine acute experimental allergic encephalomyelitis. Lab Invest. 2004;84:21–8. doi: 10.1038/labinvest.3700015. [DOI] [PubMed] [Google Scholar]
- 28.Williams AS, Mizuno M, Richards PJ, Holt DS, Morgan BP. Deletion of the gene encoding CD59a in mice increases disease severity in a murine model of rheumatoid arthritis. Arthritis Rheum. 2004;50:3035–44. doi: 10.1002/art.20478. [DOI] [PubMed] [Google Scholar]
- 29.Turnberg D, Botto M, Warren J, Morgan BP, Walport MJ, Cook HT. CD59a deficiency exacerbates acclerated nephrotoxic nephritis in mice. J Am Soc Nephrol. 2003;14:2636–42. doi: 10.1097/01.asn.0000083901.47783.2e. [DOI] [PubMed] [Google Scholar]
- 30.Lin F, Salant D, Meyerson H, Emancipator S, Morgan BP, Medof ME. Respective roles of decay-accelerating factor and CD59 in circumventing glomerular injury in acute nephrotoxic nephritis. J Immunol. 2004;172:2636–42. doi: 10.4049/jimmunol.172.4.2636. [DOI] [PubMed] [Google Scholar]
- 31.Lin F, Kaminski HJ, Conti-Fine BM, Wang W, Richmonds C, Medof ME. Markedly enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J Clin Invest. 2002;110:1269–74. doi: 10.1172/JCI16086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41:355–67. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Nakano S, Engel AG. Myasthenia gravis − quantitative immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology. 1993;43:1167–72. doi: 10.1212/wnl.43.6.1167. [DOI] [PubMed] [Google Scholar]
- 34.Fleisig AJ, Verrier ED. Pexelizumab − a C5 complement inhibitor for use in both acute myocardial infarction and cardiac surgery with cardiopulmonary bypass. Expt Opin Biol Ther. 2005;5:833–9. doi: 10.1517/14712598.5.6.833. [DOI] [PubMed] [Google Scholar]
- 35.Hill A, Ridley SH, Esser D, et al. Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: a new approach to PNH therapy. Blood. 2006;107:2131–7. doi: 10.1182/blood-2005-02-0782. [DOI] [PubMed] [Google Scholar]