Abstract
Canine visceral leishmaniasis (CVL) manifests itself as a broad clinical spectrum ranging from asymptomatic infection to patent severe disease. Despite relevant findings suggesting changes on lymphocytes subsets regarding the CVL clinical forms, it still remains to be elucidated whether a distinct phenotypic profile would be correlated with degree of tissue parasite density. Herein, we have assessed the correlation between the clinical status as well as the impact of bone marrow parasite density on the phenotypic profile of peripheral blood leucocytes in 40 Brazilian dogs naturally infected by Leishmania chagasi. Our major findings describe the lower frequency of B cells and monocytes as the most important markers of severe CVL. Our main statistically significant findings reveal that the CD8+ T cell subset reflects most accurately both the clinical status and the overall bone marrow parasite density, as increased levels of CD8+ lymphocytes appeared as the major phenotypic feature of asymptomatic disease and dogs bearing a low parasite load. Moreover, enhanced major histocompatibility complex (MHC)-II density as well as a higher CD45RB/CD45RA expression index seems to represent a key element to control disease morbidity. The association between clinical status, bone marrow parasitism and CD8+ T cells re-emphasizes the role of the T cell-mediated immune response in the resistance mechanisms during ongoing CVL. Higher levels of circulating T lymphocytes (both CD4+ and CD8+ T cells) and lower MHC-II expression by peripheral blood lymphocytes seem to be the key for the effective immunological response, a hallmark of asymptomatic CVL.
Keywords: bone marrow parasitism, canine visceral leishmaniasis, clinical status, T cell subsets
Introduction
Leishmaniasis is a parasitic infection of animals and humans caused by protozoan parasites from the Leishmania genus, Trypanosomatidae family, Kinetoplastida order [1]. Disease is transmitted by diptera of the Phlebotomidae family, distributed widely in nature, living in sylvatic and/or domestic environments. Visceral leishmaniasis is currently expanding markedly worldwide, mainly in Brazil, where the typically rural outline has shifted recently to a progressively urbanized profile. In this context, canine visceral leishmaniasis (CVL) is highly relevant epidemiologically, considering its increased incidence in the last decade, in addition to the intense cutaneous parasite density reported in infected dogs which may add to the spread of disease [2,3]. Besides being a reservoir of Leishmania parasites in endemic areas [4,5], dogs are also used as experimental models of human diseases [6], especially in the study of parasite–host interaction, immune response and in the development of new vaccines and therapeutic agents [7].
According to Mancianti et al. [8], CVL can be categorized into three distinct clinical forms, based on the major features observed in infected animals, which can be classified as asymptomatic (AD), oligosymptomatic (OD) and symptomatic (SD).
Several reports have focused attention on the relationship between distinct clinical forms of CVL and disease progression, with the aim of identifying laboratory markers to be used in follow-up studies of Leishmania chagasi infection. We have demonstrated previously that impaired biochemical/haematological status is associated with severe clinical aspects of CVL, suggesting that follow-up of these parameters could be a relevant approach during therapeutic and vaccine evaluation [9]. Moreover, we have also described that immunoglobulin isotype patterns are important hallmarks for clinical status and tissue parasite density in Brazilian dogs naturally infected by L. chagasi[10]. It has been well accepted in experimental infection in dogs that although hypergammaglobulaemia is observed, increased levels of immunoglobulins, mainly IgG, do not provide protection [11]. It has been reported that effective immunity appears to be more dependent on specific cellular immunity. Dogs developing progressive disease revealed clear suppression of cell-mediated immunity [11]. According to Keenan et al.[12], experimental CVL is accompanied by lower activity of lymph node T cell-dependent regions, infiltration and proliferation of macrophages as well as hyperplasia of B cell-dependent areas.
In vitro studies have also confirmed the impaired cellular immune response during CVL. Brandonisio et al. [13] highlighted the reduced phagocytic ability of monocytes in infected dogs compared to healthy controls. In addition, Panaro et al.[14] demonstrated that peripheral blood monocytes from L. infantum-infected dogs lack a nitric oxide-mediated leishmanicidal effect.
A reduced lymphoproliferative response to Leishmania-specific antigen has also been verified in experimentally infected dogs [11,15–17]. Despite the results of in vitro lymphocyte proliferation, suggesting an impaired T cell response to antigenic stimulation, Pinelli et al. [11] pointed out that asymptomatic dogs display a well-preserved response to L. infantum antigens both in vitro and in vivo. Moreover, Pinelli et al. [17] demonstrated that asymptomatic dogs presented higher levels of tumour necrosis factor (TNF)-α compared to symptomatic dogs.
The well-defined in vitro cellular immune profile described for CVL and its relationship to clinical status have also motivated several investigators to search further for a possible association between ex vivo immunological features in the context of the dichotomic clinical status of CVL, aiming to identify laboratory markers to be used promptly as complementary tools. Flow cytometry analysis of canine peripheral blood cells has demonstrated that L. infantum infection is accompanied by lowered levels of both CD4+ T cells and CD21+ B cells, suggesting its relationship with immunosuppression in symptomatic dogs [18].
Despite these findings regarding major lymphocytes subsets in L. infantum-infected dogs, categorized as asymptomatic and symptomatic, it still remains to be elucidated whether a similar pattern of phenotypic features could be observed when L. chagasi naturally infected dogs are classified into three major clinical groups (AD, OD and SD), according to Mancianti et al.[8]. Moreover, in this study we have also assessed the impact of bone marrow parasite density on the phenotypic profile of peripheral blood leucocytes in Brazilian dogs naturally infected by L. chagasi. Bone marrow parasite density has been shown to be the most reliable parasitological marker to decode the clinical status of canine visceral leishmaniasis [10]. Our major findings describe bone marrow parasite density as being related closely to major phenotypic changes reported in peripheral blood leucocytes during canine visceral leishmaniasis. The association between clinical status, bone marrow parasitism and CD8+ T cells re-emphasizes the role of T cell-mediated immune response in resistance mechanisms during ongoing CVL.
Materials and methods
Animals
Sixty mixed-breed adult dogs of both genders, 2–6 years old, were selected. They were maintained in the kennels of the Institute of Biological Sciences of Federal University of Minas Gerais or provided by the Control Zoonosis Center in Belo Horizonte City Council, Minas Gerais state, Brazil. Clinical preselection was carried out in the latter location. The animals were kept in quarantine with drinking water and a balanced feed given ad libitum. The dogs used in this study were stray or domiciled mongrel dogs, selected based on their serological results on indirect immunofluorescence assay test (IFAT), used as a ‘gold standard’ immunological test for diagnosis of CVL. Animals presenting IFAT titres = > 1:40 were considered positive and included into the infected groups. Animals with IFAT negative at 1:40 were considered non-infected and included as a control group. Leishmania-infected dogs did not receive any treatment for CVL. As chemotherapeutic practice for CVL is not allowed officially in Brazil, all infected dogs must be killed. Infection with L. chagasi was confirmed serologically in all IFAT-positive dogs, including enzyme-linked immunosorbent assay (ELISA) extract and ELISA r-K39, as described previously [19] and/or at least one parasitological examination performed at different tissue sites including bone marrow, spleen, skin, poplyteous lymph node and liver, as described below.
Ethics
All procedures in this study were according to the guidelines set by the Brazilian Animal Experimental College (COBEA). This study was approved by the Ethical Committee for the use of Experimental Animals of the Universidade Federal de Minas Gerais, Brazil (CETEA).
Clinical and parasitological evaluation
The dogs were classified clinically according to the presence/absence of infection signs: asymptomatic (AD, n = 12), with no signs suggestive of disease; oligosymptomatic (OD, n = 12), with a maximum of three clinical signs including opaque bristles and/or localized alopecia and/or moderate loss of weight; symptomatic (SD, n = 16), with characteristic clinical signs of visceral leishmaniasis, such as opaque bristles, severe loss of weight, onychogriphosis, cutaneous lesions, apathy and keratoconjunctivitis; and non-infected dogs (CD, n = 20), animals for negative serological and parasitological examination for Leishmania, considered here as control animals.
Assessment of parasitological parameters was performed by diagnoses in tissue smears (bone marrow, spleen, skin, poplyteous lymph node and liver) carried out after necropsy of the animals. The smears were stained by Giemsa and examined under optical microscopy for the identification of amastigote forms of Leishmania. Parasite density evaluation was performed in bone marrow and the results expressed as Leishman Donovan units (LDU index), according to Reis et al. [10], which correspond to the number of Leishmania amastigotes by 1000 nucleated cells. We evaluated the bone marrow parasitism from L. chagasi in naturally infected dogs. Tissue parasitism for bone marrow was classified initially as low (LP), median (MP) and high (HP) parasitism based on bone marrow-specific LDU values categorized statistically into tertiles as follow: LP (0–0); MP (2–33) and HP (46–1104) [10]. This approach further strengthens statistical analysis on well-balanced numbers of dogs into each subgroup. The number of animals included on each subgroup was approximately 12–14 animals.
Blood sample collection
Five millilitres of peripheral blood from the brachiocephalic vein was collected into tubes containing ethylenediamine tetraacetic acid (EDTA) (final concentration of 1 mg/ml). A haemogram was performed in each sample (Coulter MD18; Luton, UK). All samples were maintained at room temperature up to 12 h prior to processing.
Immunophenotyping by flow cytometry
Immunophenotyping analyses of peripheral blood through flow cytometry were undertaken as described by Reis et al. [19]. Briefly, 1 ml of EDTA whole blood was submitted to prefixation and erythrocyte lysis by the slow addition of 13 ml of lysis solution [fluorescence activated cell sorter (FACS) lysing solution; Becton Dickinson San Diego, CA, USA] followed by incubation for 10 min at room temperature (RT). After centrifugation (450 g for 10 min at RT), the pellet was resuspended in 500 µl phosphate-buffered saline (PBS) supplemented with 10% of fetal bovine serum (FBS-10%).
In 96-well U-bottom plates (Limbro Biomedicals, Inc., Aurora, OH, USA), 30 µl of prefixed leucocyte suspension were incubated at RT for 30 min in the dark in the presence of 30 µl of anti-canine cell surface marker monoclonal antibodies (mAbs) diluted previously in PBS-10% in indirect immunofluorescence procedures. A range of cell surface markers that define major canine leucocyte subpopulations were used, including diluted purified anti-dog CD5 1:800 (rat IgG2a, clone YKIX322·3), anti-dog CD4 1:12 500 (rat IgG2a, clone YKIX302·9), anti-dog CD8 1:800 (rat IgG1, clone YCATE55·9), anti-MHCII 1:200 (rat IgG2b, clone YKIX334·2), anti-CD45RA 1:200 (rat IgG2b, clone YKIX753·22·2) and anti-CD45RB 1:800 (rat IgG2b, clone YKIX716·13), all purchased from Serotec (Oxford, UK). Cells were additionally incubated in the same conditions, in the presence of 60 µl of previous diluted fluorescein isothiocyanate (FITC)-conjugated sheep anti-rat IgG antibody.
Five microlitres of undiluted FITC-labelled mouse anti-human CD21 (mouse IgG1, clone IOBla; Immunotech Co., Marseille, France) and 50 µl of previously diluted PE/Cy-5-conjugated mouse anti-human CD14 1:200 (mouse IgG2a, clone TÜK4; Serotec Oxford, UK) were used in direct immunofluorescence procedures.
Before flow cytometric data collection and analysis, labelled cells were fixed for 30 min with 200 µl of FACS FIX solution (10·0 g/l paraformaldehyde; 10·2 g/l sodium cacodylate and 6·65 g/l sodium chloride, pH 7·2).
Flow cytometric acquisition and data analysis
Flow cytometric measurements were performed on a FACScan instrument (Becton Dickinson, Mountain View, CA, USA). The CellQuest software package was used in both data acquisition and analysis. A total of 10 000 events was acquired for each preparation. Canine whole blood leucocytes were identified on the basis of their specific forward (FSC) and side (SSC) light-scatter properties. Following FSC and SSC gain adjustments, the lymphocytes were selected based on their characteristic FSC versus SSC gain distribution. Fluorescence was evaluated based on the spectra of FITC and Cy5-PE on FL1 or FL3 single-histogram representation. The monocytes were analysed by fluorescence intensity detection on single histograms directly on ungated leucocytes. For data analysis, a marker was set on the internal control in order to confine over 98% of the unlabelled cells.
The results were expressed in absolute counts (cell number/mm3) that allow better characterization of cell subpopulation frequencies, considering that overall leucocyte counts may differ between groups. The absolute counts for lymphocyte subsets were calculated as the product of the percentage of positive cells (CD5+, CD4+, CD8+, CD21+) within gated lymphocytes by the absolute lymphocyte counts derived from the haemogram. The absolute counts for monocytes were obtained as the product of CD14+ cells obtained within ungated leucocytes by the total white blood cell counts derived from the haemogram.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 3·1 software package (San Diego, CA, USA). Considering the nonparametric nature of all data sets, the Kruskal–Wallis test was used for between-group comparative study, followed by Dunns' test for multiple comparisons. Spearman's rank correlation was also computed to investigate associations between phenotypic features with clinical status and bone marrow parasite density. In all cases, the differences were considered significant when the probabilities of equality, P-values, were < 0·05.
Results
Marked anaemia and leucopenia are hallmarks of haematological dysfunction associated with high bone marrow parasite density during canine visceral leishmaniasis
The assessment of haematological parameters was evaluated according to the tissue parasitism for bone marrow was initially classified as low (LP), median (MP) and high (HP) parasitism based on bone marrow-specific LDU values categorized statistically into tertiles as follows: LP (0–0); MP (2–33) and HP (46–1104) [10]. Our results demonstrated severe anaemia in HP animals, with significant decreases in haemoglobin, erythrocytes and haematocrit, in relation to LP (P < 0·01), MP (P < 0·05) and CD (P < 0·001) (Table 1). In addition, white blood cell counts demonstrated significant decreases of eosinophil (P < 0·001) and monocyte (P < 0·05) absolute values in HP dogs compared to CD/MP and CD/LP, respectively. Additionally, lower lymphocyte counts were observed in HP compared to LP (P < 0·05) and MP (P < 0·01).
Table 1.
Evaluation of haematological parameters of naturally infected and non-infected dogs.
| Parasite density | ||||
|---|---|---|---|---|
| Haematological parameters | CD | LP | MP | HP |
| Erythrocytes, million/mm3 | 6·8 ± 0·8 | 5·6 ± 0·7a | 5·8 ± 0·6 | 3·9 ± 1·2a,b,c |
| Haemoglobin, g/dl | 15·7 ± 1·9 | 13·6 ± 2·2 | 14·1 ± 2·0 | 9·1 ± 3·1a,b,c |
| Haematocryt, % | 46·4 ± 5·4 | 39·4 ± 5·2 | 40·1 ± 4·9 | 27·0 ± 9·7a,b,c |
| Leucocytes × 103/mm3 | 12·8 ± 2·7 | 13·9 ± 4·6 | 12·8 ± 3·9 | 10·7 ± 4·3 |
| Neutrophils | 7·3 ± 3·1 | 7·3 ± 3·4 | 7·0 ± 3·0 | 7·8 ± 2·9 |
| Eosinophils | 1·8 ± 0·8 | 1·3 ± 1·2 | 0·8 ± 0·5a | 0·5 ± 0·5a |
| Lymphocytes | 2·7 ± 1·5 | 4·1 ± 3·5 | 4·1 ± 3·0 | 1·8 ± 1·7b,c |
| Monocytes | 1·1 ± 0·5 | 1·0 ± 0·5 | 0·7 ± 0·3 | 0·5 ± 0·4a,b |
The results are shown as the average values ± standard deviation. The superscript letters a, b and c represent statistically significant differences for the control group (CG) and bone marrow parasite density (low: LP; median: MP; and high: HP parasitism, respectively), when p < 0.05.
Lower frequency of circulating B cells and monocytes are important markers of severe CVL, whereas increased levels of CD8+ lymphocytes appear to be the major phenotypic feature of asymptomatic disease
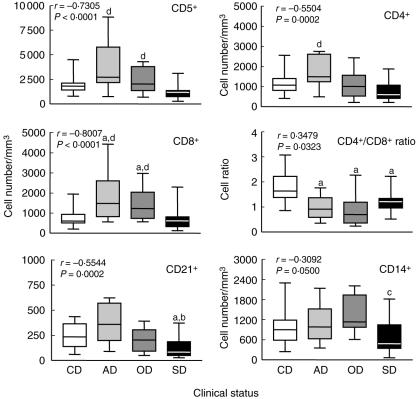
In order to evaluate the immunological profile of dogs naturally infected with L. chagasi we enumerated the frequency of T lymphocytes (CD5+) and the major T cell subpopulations (CD4+ and CD8+). Animals were classified according to their clinical status as AD, OD and SD dogs. Our data demonstrated that AD (P < 0·001) and OD (P < 0·05) presented significant increases in absolute counts of T lymphocytes CD5+ in comparison to SD. Analysis of T cell subpopulations showed a distinct profile for CD4+ and CD8+ lymphocytes. Higher CD4+ cell counts were observed in the AD compared to the SD groups (P < 0·001). Conversely, increased values of CD8+ cells were documented in AD (P < 0·01) and OD (P < 0·05) in comparison to both CD and SD (Fig. 1). In order to explore more effectively the balance between this major T cell subpopulation, we have also reported a decreased CD4+/CD8+ cell ratio in all infected dogs (AD, P < 0·01; OD, P < 0·001 and SD, P < 0·05) compared to CD (Fig. 1).
Fig. 1.
Immunophenotypic profile of peripheral blood leucocytes in Leishmania chagasi naturally infected dogs categorized according to their clinical status as asymptomatic (AD =  ), oligosymptomatic (OD =
), oligosymptomatic (OD =  ) and symptomatic (SD = ▪) dogs. Uninfected dogs were used as a control group (CD = □). The results are expressed as absolute cell counts and cell ratio in box-plot format highlighting the gap of 50% of data set measurement, the median as well as the maximum and minimum values. Significant differences at P < 0·05 are indicated by the letters ‘a’, ‘b’, ‘c’ and ‘d’ in comparison to CD, AD, OD and SD, respectively. Spearma's correlation indexes (r and P-values) are shown on the graphs.
) and symptomatic (SD = ▪) dogs. Uninfected dogs were used as a control group (CD = □). The results are expressed as absolute cell counts and cell ratio in box-plot format highlighting the gap of 50% of data set measurement, the median as well as the maximum and minimum values. Significant differences at P < 0·05 are indicated by the letters ‘a’, ‘b’, ‘c’ and ‘d’ in comparison to CD, AD, OD and SD, respectively. Spearma's correlation indexes (r and P-values) are shown on the graphs.
In addition, analysis of circulating B lymphocytes (CD21+) revealed lower absolute numbers in SD compared to the CD (P < 0·05) and AD (P < 0·01) groups. No significant differences were observed for the CD5+/CD21+ cell ratio (data not shown).
To confirm and extend our initial findings, we performed a detailed correlation analysis of major lymphocyte subset counts in the peripheral blood within all infected groups (statistics shown in Fig. 1). Results from this investigation show that as disease progresses to a higher morbidity score (r = −0·7305, P < 0·0001) there is a sustained restoration of CD5+ lymphocytes to normal values observed in the peripheral blood of non-infected dogs. A statistically significant restoration in the absolute number of CD4+ T lymphocytes was also observed (r = −0·5504, P = 0·0002). Increased levels of CD8+ lymphocyte counts in Leishmania-infected dogs also correlated with low disease morbidity (r = −0·8007, P < 0·0001). These findings corroborate earlier studies indicating that asymptomatic L. chagasi infection of dogs results in an increased CD8+ subset in the peripheral T cells compartment (Fig. 1).
Enhanced major histocompatibility complex (MHC)-II density on circulating lymphocytes as well as a higher CD45RB/CD45RA expression index may represent a key element to control disease morbidity in asymptomatic dogs
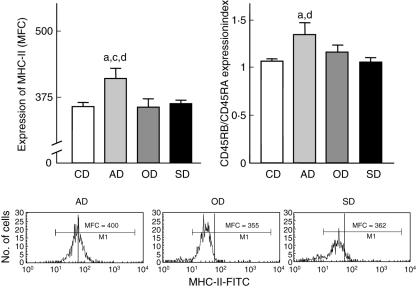
Expression of MHC II, CD45RA and CD45RB by peripheral blood lymphocytes was evaluated through semiquantitative analyses in order to verify whether disease morbidity may be associated with an altered pattern of these constitutive cell surface markers involved directly in lymphocyte activation, as well as effector functions (Fig. 2). Our data demonstrated higher MHC-II expression by circulating lymphocytes from AD in comparison to the OD, SD and CD groups (P < 0·05). Although no significant differences were observed for CD45RA and CD45RB expression considered as isolated parameters, analysis of the CD45RB/CD45RA expression index revealed higher values in AD in comparison to the SD and CD groups (P < 0·05). Figure 2 shows a typical profile of MHC-II expression on single-colour histograms of fluorescence intensity (FL-1). Representative overlay flow cytometric diagrams highlighting the higher CD45RB/CD45RA expression index are also shown in Fig. 2.
Fig. 2.
Activation status of peripheral blood leucocytes in Leishmania chagasi naturally infected dogs categorized according to their clinical status as asymptomatic (AD =  ), oligosymptomatic (OD =
), oligosymptomatic (OD =  ) and symptomatic (SD = ▪) dogs. Uninfected dogs were used as a control group (CD = □). The results are expressed as average of major histocompatibility complex (MHC)-II expression reported as mean fluorescence channel (MFC) and CD45RB/CD45RA expression index plus standard deviation. Significant differences at P < 0·05 are indicated by the letters ‘a’, ‘b’, ‘c’ and ‘d’ in comparison to CD, AD, OD and SD, respectively. Representative histograms illustrating the higher MHC-II expression on circulating lymphocytes from AD are also presented.
) and symptomatic (SD = ▪) dogs. Uninfected dogs were used as a control group (CD = □). The results are expressed as average of major histocompatibility complex (MHC)-II expression reported as mean fluorescence channel (MFC) and CD45RB/CD45RA expression index plus standard deviation. Significant differences at P < 0·05 are indicated by the letters ‘a’, ‘b’, ‘c’ and ‘d’ in comparison to CD, AD, OD and SD, respectively. Representative histograms illustrating the higher MHC-II expression on circulating lymphocytes from AD are also presented.
Bone marrow parasite density is related closely to major phenotypic changes reported for peripheral blood leucocytes pointed as hallmarks of the clinical status of canine visceral leishmaniasis
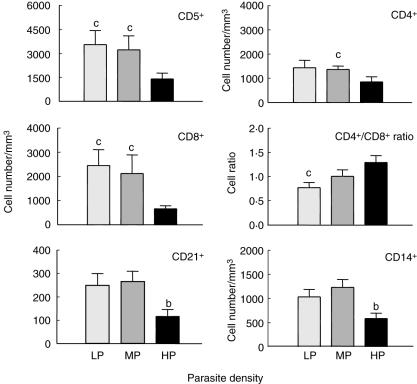
In order to evaluate whether the peripheral blood phenotypic profile reflects the parasite load during CVL, we conducted an analysis of the phenotypic features of circulating leucocytes of L. chagasi-infected dogs, classified according to the degree of bone marrow parasite density (Fig. 3). Our results showed that absolute counts of the T lymphocytes CD5+ cells were higher in LP and MP compared to HP (P < 0·05). Moreover, the CD4+ T cell subpopulation was higher in MP in comparison to HP, whereas CD8+ T cells was higher in LP and MP in comparison to HP. Further analysis of T cell subsets revealed a lower CD4+/CD8+ cell ratio in LP in comparison to HP (P < 0·05) (Fig. 3). No significant differences were observed for the CD5+/CD21+ cell ratio (data not shown). A difference was also observed in B cell (P < 0·05) and monocyte (CD14+) counts (P < 0·01), revealing a lower frequency in HP in comparison to MP.
Fig. 3.
Immunophenotypic of peripheral blood leucocytes in Leishmania chagasi naturally infected dogs categorized according to parasite density (LDU) in bone marrow as low parasitism (LP =  ), medium parasitism (MP =
), medium parasitism (MP =  and high parasitism (HP = ▪) dogs. The results are expressed as mean absolute cell counts and cell ratio plus standard deviation. Significant differences at P < 0·05 are indicated by the letters ‘a’, ‘b’ and ‘c’ in comparison to LP, MP and HP, respectively.
and high parasitism (HP = ▪) dogs. The results are expressed as mean absolute cell counts and cell ratio plus standard deviation. Significant differences at P < 0·05 are indicated by the letters ‘a’, ‘b’ and ‘c’ in comparison to LP, MP and HP, respectively.
We have also investigated whether dogs displaying higher bone marrow parasite density would be more likely to develop severe CVL. Our data further support this hypothesis, demonstrating that HP showed a higher relative risk of belonging to the SD group (odds ratio = 82·5, P < 0·0001).
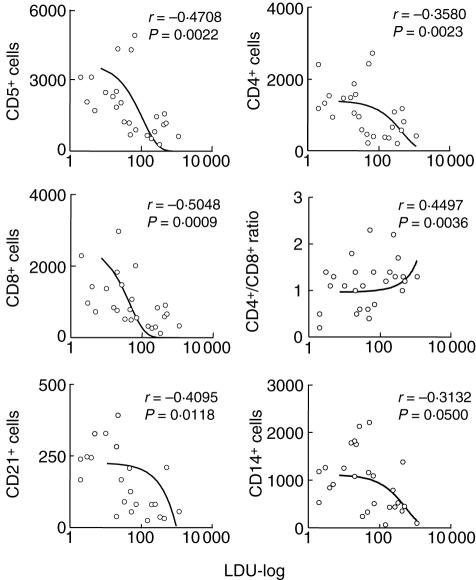
Correlation analysis revealed that the CD8+ T cell counts reflect more effectively the overall bone marrow parasite density
Correlation analysis of phenotypic features and bone marrow parasite density further confirms the association between high cell counts and low bone parasite density during CVL (Fig. 4). A negative correlation between T cell subsets (CD5+, r = −0·4708; P = 0·0022; CD4+, r = −0·3580; P = 0·0023 and CD8+ cells, r = −0·5048; P = 0·0009) was also observed. These data re-emphasize that high CD8+ T cell counts appear to be better peripheral markers of low bone marrow parasitism. Moreover, our data reveal that impaired CD21+ (r = −0·4095; P = 0·0118) and CD14+ counts (r = −0·3132; P = 0·0500) are correlated with disease morbidity in dogs infected with L. chagasi (Fig. 4).
Fig. 4.
Correlation between parasite density (LDU-log) in bone marrow and absolute numbers of peripheral blood leucocyte subpopulations from Leishmania chagasi naturally infected dogs. The results are expressed as scattering of individual values. Spearman's correlation indexes (r and P-values) are shown on the graphs. Connecting lines illustrate positive and negative correlation indexes.
Discussion
Flow cytometric methods have been used largely to access the immunological status of domestic animals, such as cats and dogs, becoming a powerful tool in veterinary medicine [7,19,20]. In this context, the analysis of leucocyte absolute counts performed routinely in the veterinary clinical laboratory represents complementary tools to support veterinarian physicians to evaluate clinical cases. We have previously reported significant alterations of haematological parameters associated with the clinical status of CVL [9]. In this study we have addressed this issue further, demonstrating that severe disease is associated with altered erythroid and leucocytic compartments, with significant decrease of red blood cell counts, haemoglobin and haematocrit values as well as the number of circulating leucocyte subsets (eosinophils, lymphocytes and monocytes) in dogs presenting high bone marrow parasite density (Table 1). Moreover, we have also observed that phenotypic features of circulating leucocytes, expressed as absolute values, appear to be important immunological markers associated with clinical status as well as bone marrow parasite density in dogs naturally infected by L. chagasi.
It is believed that Leishmania virulence results from the interaction of different parasite determinants with the host immune system. In this model of parasite–host interaction, L. chagasi antigens interact sequentially with different components of the host immune system to cause leishmaniasis. The virulence molecules of parasites help them to overcome the host immune and non-immune barriers to establish intracellular infection of macrophages. Infection alone does not cause severe disease, but it is a prerequisite for this stage of high morbidity. In hosts bearing asymptomatic or mild disease, immunity becomes highly regulated with an increased number of T cells, creating a microenvironment efficient to remove the parasites, as shown herein for AD and OD as well as LD and MD. High activity of the host immune response seems to be efficient at removing the parasite in the asymptomatic phase, but cannot promote parasite clearance in the final stages of disease or the start of the symptomatic phase (SD or HP). In this severe stage of the disease the host immune response is down-regulated, the number of immune cells returns to normal and the parasite spread through many host tissues. Therefore, asymptomatic canine visceral leishmaniasis is characterized by sustained T cell-mediated immune responses to parasite antigens [11]. Studies in L. major murine models have also shown that the development of protective events during leishmaniasis is dependent on an effective type 1 T cell-mediated immune response [21,22]. Our data are in agreement with these statements demonstrating that dogs with asymptomatic or mild disease, both AD and OD groups, presented increased absolute values of the circulating T cells (CD5+) within their peripheral blood lymphocytes compared to SD and HP (Figs 1 and 3).
Although all infected dogs displayed lower CD4+/CD8+ cell ratios, compared to CD, we have observed that dichotomic phenotypic alterations led to these findings in dogs presenting distinct clinical status. Increased CD8+ T cell values represent the major phenotypic feature associated with the inversion of the CD4+/CD8+ cell ratio in AD/LP and OD/MP (Figs 1 and 3). Indeed, these findings re-emphasize the protective role of T CD8+ cells during CVL. Much attention has been given to the role of T CD4+ lymphocytes on leishmaniasis; however, several studies have shown that the T CD8+ lymphocytes act in protective events during canine visceral leishmaniasis [11,17]. Pinelli et al. [23] suggested a special role for T CD8+ lymphocytes in protective immunity during L. infantum infection, observing that these cells are capable of mounting an efficient type 1 immune response, with interferon (IFN)-γ production and ability to lysis infected L. infantum macrophages. Further analyses have been conducted by our group to elucidate if the impaired T cell compartment observed in this study for SD/HP is associated with a type 2 immune response.
It has been well documented that an important characteristic found in Leishmania-infected dogs is a typical follicular hyperplasia owing to the increase of plasmocytes into lymph node, tonsils, Peyer's patches and spleen follicles [16].Analysis of lymphoid organs from dogs naturally infected with Leishmania revealed enhanced areas of B cells, mainly plasmocytes, which are associated with an increased production of anti-Leishmania antibodies, determined by serological tests [16]. These findings suggest that migration of B cells from peripheral sites into lymphoid tissues might be occurring during active CVL, with activation and differentiation of these cells in plasmocytes and consequent polyclonal activity leading to high production of anti-Leishmania antibody. Consistent with this hypothesis we have observed a marked reduction in absolute values of B lymphocytes (CD21+) in SD and HP, which might be related to the selective migration of this cellular population into lymphoid organs (Figs 1 and 3). Previous analyses have highlighted this proposal by showing a positive correlation between clinical and parasitological features with higher immunoglobulin titres [10].
A decrease of absolute values of circulating monocytes was also observed as a hallmark of HP compared to CD (Table 1). Moreover, analysis of CD14+ cells also revealed lower cell counts in SD (Fig. 1) and HP (Fig. 3) compared to OD and MP, respectively. These data may suggest, during active CVL, the recruitment of monocytes to lymphoid tissue, where they might play an important role in immunological connections throughout antigen presentation and parasite clearance. The increase in macrophage proportions in the spleen of dogs with CVL has already been documented [24]. This issue is currently under investigation in our laboratory.
The sustained T cell compartment in the peripheral blood of AD and LP was further confirmed by our findings that asymptomatic disease was associated with higher activation status of circulating lymphocytes, as demonstrated by higher expression of MCH-II and increased CD45RB/CD45RA expression index in comparison to SD and CD (Fig. 2). Cobbold and Metcalfe [25] developed pioneering investigations about MHC-II molecules in dogs. These authors observed that, in contrast with other species, the MHC-II of dogs is expressed constitutively in all circulating lymphocytes. It has been proposed that increased expression of MHC-II may reflect an antigenic priming-related immunological event [25]. Based on this statement, our findings support the hypothesis that AD is more prone to be primed by Leishmania antigens and mounts a vigorous immune response. The increased MHC-II expression in AD might reflect an increased ability of antigen presentation leading to effective activity of the immune system. On the other hand, SD displaying lower MHC-II expression is more vulnerable to disease severity due to a down-regulated immune response, allowing parasite distribution throughout an effective antigen presentation and the number of T cells coming back to normal range. Lower levels of circulating antigen-presenting cells (including B lymphocytes and monocytes) are consistent with this proposal. Moreover, additional correlation analysis further confirmed the association between these phenotypic features (Fig. 4). Supplementary studies will be necessary to better elucidate this hypothesis.
It has been reported that naive canine T cells, helper T cells secreting IFN-γ and a wide range of B cells express high molecular weight (220–240 kDa) CD45RA antigen [25,26,27], while the CD45RB antigen has been associated with CD4+ T cells that had been activated by protozoan infection [28]. A pioneering investigation has been conducted recently in L. chagasi-infected dogs showing higher proportions of CD45RB+ cells and lower CD45RA+ cells in the spleen and in peripheral blood compared to healthy dogs [24]. In this study, we have performed a more detailed analysis of CD45 expression by evaluating the density of CD45 expression throughout the mean fluorescence intensity index of CD45RB/CD45RA. Considering the higher CD45RB/CD45RA expression rate observed in AD groups suggested increased proportions of CD45RB and the reduced proportions of CD45RA T cells in AD dogs, in relation to healthy controls and symptomatic dogs, which could reflect an effective activation of T cells [29] during the asymptomatic infection. Whether these changes in CD45 isoforms expression occur simultaneously or sequentially in different tissues, and how they may affect the development of CVL, should be the aim of further studies. Moreover, we cannot discard the possibility that increased expression of CD45RA in SD, due mainly to activated B cells, may also be relevant to the lower CD45RB/CD45RA ratio observed in SD dogs. The increase in the proportion of CD45RB/CD45RA lymphocytes, due mainly to increased proportions of CD45RB, associated with a high frequency of CD8+ T lymphocytes in dogs with asymptomatic disease, could be valuable parameters for the monitoring of CVL development.
In conclusion, our findings highlight the complexity of cellular immunological events related to natural infection from dogs by L. chagasi, further correlating major peripheral blood phenotypic markers with clinical status and bone marrow parasite density. Together, our data suggest that the sustained T cell compartment observed in AD and LP may be resultant from the high activity of the host immune system to perform antigen presentation and remove parasites from affected sites. The higher levels of circulating T lymphocytes (both CD4+ and CD8+ T cells) consistent with higher MHC-II expression by peripheral blood lymphocytes seems to be key for the effective immunological response, a hallmark of asymptomatic CVL.
Acknowledgments
This work was supported by the CNPq/BR/grant 521124/98–0 and FAPEMIG/BR/grant CBS 2222\97. We also thank the people from ‘Fundação Nacional da Saúde’, ‘Ministério da Saúde’ and from the regional district of Belo Horizonte, Minas Gerais, for their special dedication to this work.
References
- 1.Laison R, Shaw JJ. Evolution, classification and geographical distribution. In: Peters W, Killick-Kendrick R, editors. The leishmaniasis in biology and medicine. London: Academic Press; 1987. pp. 1–20. [Google Scholar]
- 2.Tesh RB. Control of zoonotic visceral leishmaniasis: is it time to changes strategies? Am J Trop Med Hyg. 1995;52:287–92. doi: 10.4269/ajtmh.1995.52.287. [DOI] [PubMed] [Google Scholar]
- 3.Molina R, Amela C, Nieto J, et al. Infectivity of dogs naturally infected with Leishmania infantum to colonized Phlebotomus perniciosus. Trans R Soc Trop Med Hyg. 1994;88:491–3. doi: 10.1016/0035-9203(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 4.Molina R, Lohse JM, Pulido F, et al. Infection of sand flies by humans coinfected with Leishmania infantum and human immunodeficiency virus. Am J Trop Med Hyg. 1999;60:51–3. doi: 10.4269/ajtmh.1999.60.51. [DOI] [PubMed] [Google Scholar]
- 5.Guarga JL, Moreno J, Lucientes J, et al. Canine leishmaniasis transmission: higher infectivity amongst naturally infected dogs to sand flies is associated with lower proportions of T helper cells. Res Vet Sci. 2000;69:249–53. doi: 10.1053/rvsc.2000.0419. [DOI] [PubMed] [Google Scholar]
- 6.Faldyna M, Sinkora J, Knotigova P, et al. Lymphatic organ development in dogs: major lymphocyte subsets and activity. Vet Immunol Immunopathol. 2005;104:239–47. doi: 10.1016/j.vetimm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Williams DL. Studies of canine leucocyte antigens: a significant advance in canine immunology. The Vet J. 1997;153:31–9. doi: 10.1016/s1090-0233(97)80006-8. [DOI] [PubMed] [Google Scholar]
- 8.Mancianti F, Meciani N. Studies on canine leishmaniasis control. 1. Evolution of infection of different clinical forms of canine leishmaniasis following antimonial treatment. Trans R Soc Trop Med Hyg. 1988;82:566–7. doi: 10.1016/0035-9203(88)90510-x. [DOI] [PubMed] [Google Scholar]
- 9.Reis AB, Martins-Filho OA, Teixeira-Carvalho A, et al. Parasite density and impaired biochemical/hematological status are associated with severe clinical aspects of canine visceral leishmaniasis. Res Vet Sci. 2006;81:68–75. doi: 10.1016/j.rvsc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Reis AB, Teixeira-Carvalho A, Vale AM, et al. Isotype patterns of immunoglobulins: hallmarks for clinical status and tissue parasite density in brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet Immunol Immunopathol. 2006;112:102–16. doi: 10.1016/j.vetimm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Pinelli E, Ellick-Kendrick R, Wagenaar J, et al. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect Immun. 1994;62:229–35. doi: 10.1128/iai.62.1.229-235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keenan CM, Hendricks LD, Lightner L, et al. Visceral leishmaniasis in the German shepherd dog-II. Pathol Vet Pathol. 1984;21:80–6. doi: 10.1177/030098588402100114. [DOI] [PubMed] [Google Scholar]
- 13.Brandonisio O, Ceci L, Cedola MC, et al. Phagocytosis of Leishmania infantum promastigotes by monocytes isolated from Leishmania infected dogs. Microbiológica. 1986;9:173–8. [PubMed] [Google Scholar]
- 14.Panaro MA, Lisi S, Mitolo V, et al. Evaluation of killing, superoxide anion and nitric oxide production by Leishmania infantum-infected dog monocytes. Cytobios. 1998;95:151–60. [PubMed] [Google Scholar]
- 15.Abranches P, Santos-Gomes GM, Rachamin N, et al. An experimental model for canine visceral leishmaniasis. Parasite Immunol. 1991;13:537–50. doi: 10.1111/j.1365-3024.1991.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Moreno A, Martinez-Cruz MS, Blanco A, et al. Immunological and histological study of T- and B- lymphocyte activity in canine visceral leishmaniasis. Vet Immunol Immunopathol. 1993;51:49–59. doi: 10.1016/0304-4017(93)90195-s. [DOI] [PubMed] [Google Scholar]
- 17.Pinelli E, Gonzalo RM, Boog CJP, et al. Leishmania infantum- specific T cell lines derived from asymptomatic dogs that lyse infected macrophages in a major histocompatibility complex-restricted manner. Eur J Immunol. 1995;25:1594–600. doi: 10.1002/eji.1830250619. [DOI] [PubMed] [Google Scholar]
- 18.Bourdoiseau G, Bonnefont C, Hoareau E, et al. Specific IgG1 and IgG2 antibody and lymphocyte subset levels in naturally Leishmania infantum-infected treated and untreated dogs. Vet Immunol Immunopathol. 1997;59:21–30. doi: 10.1016/s0165-2427(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 19.Reis AB, Carneiro CM, Carvalho MG, et al. Establishment of a microplate assay for flow cytometric assessment and it is use for the evaluation of age-related phenotypic changes in canine whole blood leukocytes. Vet Immunol Immunopathol. 2005;103:173–85. doi: 10.1016/j.vetimm.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Byrne KM, Reinhart GA, Hayek MG. Standardized flow cytometry gating in veterinary medicine. Meth Cell Sci. 2000;22:191–8. doi: 10.1023/a:1009896109932. [DOI] [PubMed] [Google Scholar]
- 21.Scott P, Pearce E, Cheever AW, et al. Role of cytokines and CD4+ T cell subsets in the regulation of parasite immunity disease. Immunol Ver. 1989;112:161–82. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 22.Liew FY. Regulation of cell-mediated immunity in leishmaniasis. Curr Top Microbiol Immunol. 1990;155:53–64. doi: 10.1007/978-3-642-74983-4_4. [DOI] [PubMed] [Google Scholar]
- 23.Pinelli E. Cytokines in canine visceral leishmaniasis. In: Schijns VECJ, Horzinek MC, editors. Cytokines in veterinary medicine. Netherlands: Utrecht University; 1997. pp. 217–47. [Google Scholar]
- 24.Barrouin-Melo SM, Laranjeira DF, Santos SO, et al. A standardized cytological and immunochemical method for the analysis of fine-needle spleen aspirates: assessment of leukocyte population changes in canine visceral leishmaniosis. Vet Immunol Immunopathol. 2006;111:251–61. doi: 10.1016/j.vetimm.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Cobbold S, Metcalfe S. Monoclonal antibodies that define canine homologues of human CD antigens. Summary of the First International Canine Leukocyte Antigen Workshop (CLAW) Tissue Antigens. 1994;43:137–54. doi: 10.1111/j.1399-0039.1994.tb02315.x. [DOI] [PubMed] [Google Scholar]
- 26.Holmes MA, Lunn DP. Variations of MHC II expression on canine lymphocytes with age. Tissue Antigens. 1994;43:179–83. doi: 10.1111/j.1399-0039.1994.tb02319.x. [DOI] [PubMed] [Google Scholar]
- 27.Zuckermann FA, Peavey C, Schnitzlein WM, et al. Definition of the specificity of monoclonal antibodies against porcine CD45 and CD45R: report from the CD45/CD45R and CD44 subgroup of the Second International Swine CD Workshop. Vet Immunol Immunopathol. 1998;60:367–87. doi: 10.1016/s0165-2427(97)00112-8. [DOI] [PubMed] [Google Scholar]
- 28.Gomes-Pereira S, Rodrigues OR, Santos-Gomes GM. Dynamics of CD62L/CD45RB CD4+ and CD8+ lymphocyte subsets in hepatic and splenic tissues during murine visceral leishmaniasis. Immunol Lett. 2004;95:63–70. doi: 10.1016/j.imlet.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Tipold A, Moore P, Jungi TW, et al. Lymphocyte subsets and CD45RA positive T-cells in normal canine cerebrospinal fluid. J Neuroimmunol. 1998;82:90–5. doi: 10.1016/S0165-5728(97)00192-6. [DOI] [PubMed] [Google Scholar]