Abstract
The local cytokine environment and presence of stimulatory signals determine whether monocytes acquire dendritic cell (DC) or macrophage characteristics and functions. Because enhanced platelet activation is reported in patients with many allergic disorders, such as atopic dermatitis, platelet-derived factors may influence monocytic differentiation into DC. In this study we examined the effect of serotonin, a prototypic mediator of allergic inflammation released mainly by activated platelets at the inflammatory site, on the granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4-driven differentiation of monocytes into monocyte-derived DC. Monocytes from healthy adult donors were cultured with GM-CSF and IL-4 in the presence or absence of serotonin, and the phenotypes and function of these cells were analysed. In the presence of serotonin, monocytes differentiated into DC with reduced expression of co-stimulatory molecules and CD1a, whereas expression of CD14 was increased. These serotonin-treated DC exhibited significantly reduced stimulatory activity toward allogeneic T cells. However, these cells showed enhanced cytokine-producing capacity, including IL-10 but not IL-12. There was no significant difference between both types of DC in phagocytic activity. Experiments using agonists and antagonists indicated that serotonin 5-hydroxytryptamine (5-HT) induced the alteration of their phenotype and reduction in antigen-presenting capacity were mediated via 5-HTR1/7. It is therefore suggested that serotonin-driven DC may have a regulatory function in the inflammatory process.
Keywords: atopic dermatitis, dendritic cell, differentiation, macrophage, platelet, serotonin
Introduction
The local cytokine environment and presence of stimulatory signals determines whether monocytes will acquire dendritic cell (DC) or macrophage characteristics and functions [1–5]. Macrophages are characterized by their capacity for phagocytosis and production of pro- and anti-inflammatory cytokines that regulate inflammatory reactions [5]. In contrast, DC can initiate primary and secondary T cell responses, and are the most efficient professional antigen-presenting cells (APC) [1,2,6,7]. Moreover, the functional and phenotypical characteristics of DC are modified in association with the environment surrounding them [8–10]. The functional divergences may be of importance for the putative regulatory role of monocyte-derived DC (MoDC) or macrophages in tissues, and consequently for the outcome of the local inflammatory response [11]. Thus far several factors, such as corticosteroids, interleukin (IL)-10, histamine and signalling evoked by the engagement of high affinity receptor for IgE (FcεRI), are known to influence monocyte differentiation into DC [11–13].
In vivo, monocytes are the primary leucocytes that interact with activated platelets by forming aggregates with them [14,15]. Platelets contain many mediators and may play a critical role in immunological and non-immunological inflammation [16]. Indeed, enhanced platelet activation in patients with atopic dermatitis (AD) was reported [17]. In AD, scratching due to severe itch often results in excoriation and subsequent platelet aggregation at the lesion. Serotonin (5-hydroxytryptamine, 5-HT) isone of the mediators released by platelets following their aggregation after haemorrhage or the engagement of the IgE receptor on their surface with antigens [18–20]. 5-HT contributes to initiate inflammation by the direct activation of endothelial cells of the local vascular capillaries, inducing an increase in permeability and resulting in local recruitment into the tissues of effector T cells that mediate cutaneous inflammation [21–23]. In addition, it has been reported that 5-HT affects the phenotype and function of monocytes/macrophages, i.e. the suppression of human leucocyte antigen D receptor (HLA-DR) expression and tumour necrosis factor (TNF)-α synthesis and the induction of the release of chemotactic factors for neutrophils and monocytes [24,25].
In this report, we examined the effect of 5-HT on the granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4-driven differentiation of monocytes into DC. In monocytes from non-atopic healthy individuals we found that 5-HT down-regulates the expression of CD86, HLA-DR and CD1a, whereas the expression of CD14 was increased. These 5-HT-treated DC exhibited significantly reduced stimulatory activity towards allogeneic T cells. These cells showed an enhanced cytokine-producing capacity, including IL-10 but not IL-12. Experiments using agonists and antagonists indicate that the 5-HT-induced alteration of their phenotypic and antigen-presenting capacity are mediated via 5-HTR1/7.
Methods
Reagents
Phycoerythrin (PE)-labelled anti-CD1a monoclonal antibody (mAb) (CTB6) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). PE-, fluorescein isothiocyanate (FITC)-conjugated or unconjugated anti-CD1b, CD11b, CD14, CD40, CD45RO, CD68, CD80, CD83, CD86 and HLA-DR were obtained from BD PharMingen (San Diego, CA, USA). mAb against M-colony stimulating factor receptor (CSFR) were purchased from R&D systems (Minneapolis, MN, USA). Anti-human mannose receptor (HyCult Biotechnology, Uden, the Netherlands) and factor XIIIa (FXIIIa, Laboratory Vision, CA, USA) mAb were also purchased. FITC-conjugated and unconjugated F(ab)2 goat anti-mouse IgG antibodies were acquired from Jackson Immunoresearch (West Grove, PA, USA). The 5-HTR1/6/7 antagonist methiothepin maleate (Tocris Bioscience, Bristol, UK), 5-HTR2A antagonist MCI-9042 (Mitsubishi Pharma Corporation, Osaka, Japan), 5-HTR3 antagonist MDL-72222 (Tocris), 5-HTR1/6/7 agonist 5-carboxamidotryptamine maleate (Sigma Chemical Co., St Louis, MO, USA), 5-HTR2 agonist α-methyl-5-hydroxytryptamine (Sigma) and 5-HTR3 agonist 2-methyl-5-hydroxytryptamine hydrochloride (Sigma) were used. Other reagents were obtained from Sigma unless indicated otherwise.
Cell purification and culture
Whole blood was obtained from informed, non-atopic, healthy volunteers under approval of our Institutional Ethical Committee. Monocytes were isolated using Nycoprep (Nycomed, Oslo, Norway) and a monocyte negative isolation kit (Dinal Biotech, Oslo, Norway) according to the manufacturer's instructions. The monocytes were cultured with 100 ng/ml GM-CSF and IL-4 (R&D Systems) at 1 × 106/ml in low-endotoxin RPMI-1640 (Biochrom KG, Berlin, Germany) supplemented with 10% fetal calf serum (FCS), 100 mm l-glutamine, and 1% antibiotic–anti-mycotic (all from Gibco brl, Gaithersburg, ML, USA) for 6 days at 37°C and 5% CO2 as described previously [26]. To obtain macrophages, the monocytes were cultured at 1 × 106/ml in the presence of 100 ng/ml M-CSF (R&D Systems) for 6 days at 37°C and 5% CO2, as described previously [27]. All plastic ware and culture reagents were tested for the presence of endotoxin with the Limulus amebocytes lysate multiple test. Endotoxin levels were always < 10 pg/ml.
Proliferation assays
Allogeneic mixed lymphocyte reactions (MLRs) were conducted in 96-well round-bottomed microtitre plates by adding different amounts of irradiated (3000 rad) DC on day 6 to 1 × 105 allogeneic naive T cells, which were obtained with a CD4 positive isolation kit (Dynal) and subsequent negative selection in combination with anti-CD45RO mAb (BD PharMingen), plus goat anti-mouse IgG mAb-conjugated immunomagnetic beads (Dynal). After 4 days at 37°C, cell proliferation was assessed by the uptake of [3H]-thymidine (1·25 µCi/well present for 16 h; Amersham, Little Chalfont, UK).
Flow cytometry
Surface and intracellular molecular labelling was performed as reported previously [26]. The relative fluorescence intensity (rFI) was assessed as follows: rFI = (mean fluorescence intensity (MFI) (surface molecule) − MFI (control)/MFI (control). Intracellular staining for cytokines was carried out as described previously [26]. Phagocytic activity was tested for using fluorescence-labelled latex beads (Polysciences, Wallington, PA, USA). Briefly, cultured cells in the absence or presence of 5-HT on day 5 were incubated with the beads for 3 h at 37°C or 0°C. After washing three times, the cells were fixed with 4% paraform–aldehyde, and then the percentage of cells that performed phagocytosis was analysed by using flow cytometry.
Statistical analysis
Data are expressed as the means ± standard deviation (s.d.). Statistical differences were determined by using paired and unpaired t-tests, and a P-value of less than 0·05 was considered to be statistically significant.
Results
5-HT influences phenotype of MoDC
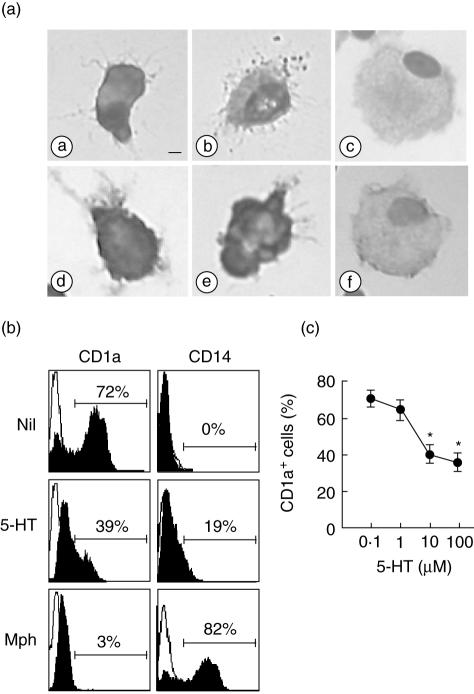
At the start of culturing, the cells used to generate DC in a typical experiment consisted of 95% monocytes, as indicated by CD14 expression and a typical light-scattering profile. They were induced to differentiate into immature DC with IL-4 and GM-CSF over a period of 6 days. The cells rapidly became non-adherent to culture plates and formed large clusters. When monocytes were cultured in the presence of 10 µm 5-HT in addition to IL-4 and GM-CSF, the cells were also non-adhered and formed clusters after 6 days. They were both irregularly shaped, had extended veils and small dendrites and showed similar dendritic morphologies (Fig. 1a). This concentration of 5-HT is relevant in physiological or pathological events and thus was used in the following experiments, because the release of 5-HT during platelet aggregation may reach local concentrations of 100 µM at the immediate site of release [21]. In contrast, M-CSF-induced macrophages were adherent, roundly shaped cells with many vacuoles.
Fig. 1.
Morphology and CD1a/CD14 expression on dendritic cells (DC) generated in the presence of interleukin (IL)-4, granulocyte–macrophage colony-stimulating factor (GM-CSF) and serotonin (5-hydroxytryptamine, 5-HT). Monocytes were cultured for 6 days in the presence or absence of 10 µM of 5-HT in addition to IL-4 and GM-CSF. (a) Cytospins of control monocyte-derived DC (MoDC) (a,d), 5-HT-treated cells (b,e) and control macrophages (c,f) were labelled by May–Grünwald–Giemsa stain (a,b,c) or by immunostaining for human leucocyte antigen D receptor (HLA-DR) (d,e,f), ×400; bar, 2 µm. (b) Expression of the CD1a and CD14 on the cell surface is shown for control DC (Nil) and control M-CSF-induced macrophages (Mph) without 5-HT in comparison with the cells cultured with 5-HT for 6 days. (c) The percentage of CD1a-positive cells cultured in the presence of IL-4 and GM-CSF and various concentrations of 5-HT for 6 days. The data are representative of six independent experiments with different donors. *P < 0·05.
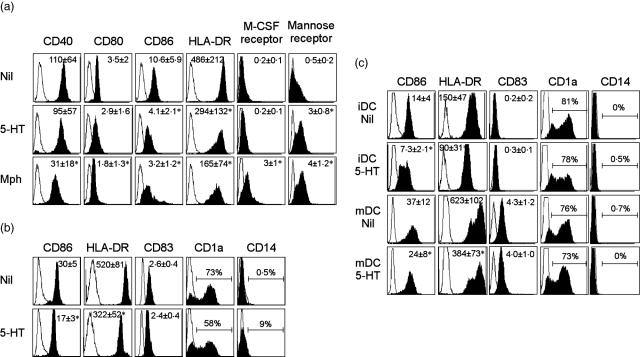
We next examined the effect of 5-HT on the DC phenotype. DC generated under control conditions with GM-CSF and IL-4 expressed high levels of CD1a and low levels of CD14. In contrast, those generated in the presence of 5-HT expressed significantly lower levels of CD1a and higher levels of CD14 (Fig. 1b). The effect of 5-HT on CD1a expression was observed in a dose-dependent manner (Fig. 1c). The expression of HLA-DR and CD86 was reduced significantly with 5-HT, whereas no significant alteration in CD40, CD80, CD83, CD1b, M-CSF receptor, CD11b, CD11c or FXIIIa expression was observed on day 6 (Fig. 2, Table 1). All control DC and 5-HT-treated DC were positive for CD11c. 5-HT induced a significant increase in the mannose receptor. The viability of the observed cells compared to the 5-HT-treated and untreated groups using trypan blue dye exclusion was not significantly different (data not shown). To investigate whether 5-HT could influence the maturation of the DC, IL-4/G-CSF-driven MoDC generated in the presence or absence of 5-HT was stimulated with lipopolysaccharide (LPS) at day 6 for 24 h and their phenotypes were analysed. Although the expression of CD83, a representative marker for maturation of DC, was not altered between them, the expression of CD86 and HLA-DR was significantly lower in 5-HT-treated DC than control DC (Fig. 2b). The effect of 5-HT on the expression of CD1a and CD14 was still observed after LPS stimulation, although the change was smaller when compared with that observed in the cells without LPS stimulation.
Fig. 2.
Serotonin (5-hydroxytryptamine, 5-HT) alters the phenotype of dendritic cells (DC) generated in the presence of interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF). (a) Expression of surface marker is shown for control DC (Nil) and control M-CSF-induced macrophages (Mph) without 5-HT in comparison with the cells cultured with 5-HT for 6 days. The overlay histograms represent the data from six independent experiments with different donors. The values of the of mean relative fluorescence intensity (rFI) ± s.d. from six independent experiments are also demonstrated. Asterisks indicate statistical significance between the control monocyte-derived DC (MoDC) and 5-HT- or M-CSF-treated cells. (b) Monocytes were cultured with IL-4 and GM-CSF for 6 days in the presence or absence of 5-HT. These cells were then stimulated with 100 ng/ml lipopolysaccharide (LPS) for 24 h and their phenotypes were analysed. (c) Monocytes were cultured with IL-4 and GM-CSF for 6 days (iDC) and stimulated with LPS for 24 h (mDC). These cells were treated with 5-HT for 24 h and the phenotypes were analysed. (b,c) Overlay histograms represent the data from six independent experiments with different donors. The values of the of mean rFI ± s.d. from six independent experiments are also demonstrated for CD86, human leucocyte antigen D receptor (HLA-DR) and CD83 expression. Asterisks indicate statistical significance between the control MoDC and 5-HT-treated cells.
Table 1.
Effect of 5-HT on the phenotypes of in vitro-generated dendritic cells.
| CD83 | CD1b | CD11b | CD11c | CD68 | FXIIIa | |
|---|---|---|---|---|---|---|
| Nil | 0·5 ± 0·1 | 0·7 ± 0·1 | 19 ± 4 | 45 ± 3 | 3·9 ± 0·8 | 14 ± 6 |
| 5-HT | 0·4 ± 0·1 | 0·5 ± 0·1 | 22 ± 8 | 46 ± 4 | 4 ± 0·8 | 15 ± 7 |
| Macrophage | 0 ± 0 | 0·1 ± 0·1* | 51 ± 14* | 92 ± 7* | 9·33 ± 3·2* | 13 ± 6 |
The phenotypes of interleukin (IL)-4/G-CSF-driven dendritic cells (DC) (Nil) and control M-CSF-induced macrophages without serotonin (5-hydroxytryptamine, 5-HT) in comparison with the cells cultured with IL-4, granulocyte–macrophage colony-stimulating factor (GM-CSF) and 5-HT for 6 days were fluorescence activated cell sorter (FACS) analysed. The intracytoplasmic staining for CD68 and FXIIIa was performed by permeabilization with saponin. The values of the of mean relative fluorescence intensity (rFI) ± s.d. from six independent experiments with different donors are demonstrated. Asterisks indicate statistical significance between the control monocyte-derived DC (MoDC) and 5-HT- or M-CSF-treated cells.
To examine whether 5-HT could influence the phenotype of already differentiated DC with IL-4 and GM-CSF, 5-HT was added to immature MoDC (day 6) or LPS-matured MoDC (day 7). As shown in Fig. 2c, the expression of CD86 and HLA-DR was also decreased significantly by the addition of 5-HT in both immature and mature MoDC. However, the expression of CD1a, CD14 and CD83 was not altered by the addition of 5-HT. When immature MoDC (day 6) was treated with 5-HT for 60 min, washed and then stimulated with LPS for 24 h, the expression of CD83 was increased as much as those seen in MoDC treated only with LPS (data not shown), further suggesting that 5-HT is not capable of influencing maturation of DC.
5-HT reduces T cell stimulatory capacity in MLR
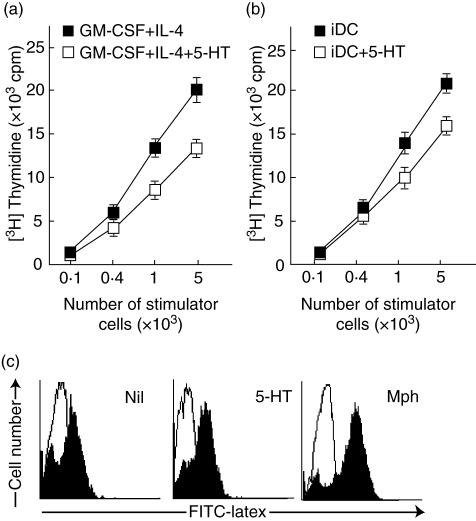
A characteristic feature of DC is their capacity to stimulate naive T cells, as seen in the model of allogeneic MLR. We thus examined whether 5-HT affects their T cell-stimulating capacity in MLR. As shown in Fig. 3a, compared with MoDC generated under control conditions 5-HT-treated DC exhibited significantly reduced stimulatory activity toward allogeneic T cells. To examine whether 5-HT could influence the antigen-presenting capacity of already differentiated DC, immature MoDC (day 6) was treated with 5-HT and then used as stimulatory cells in MLR. Treatment of immature DC at day 6 with 5-HT also reduced the antigen-presenting capacity (Fig. 3b). Intracellular expression of interferon (IFN)-γ and interleukin (IL)-4 in CD4+ naive T cell after incubation with allogeneic 5-HT-treated DC exhibited no difference to those in the control DC (data not shown). The phagocytic activity of these cells was then evaluated using FITC-labelled latex beads. There was no significant difference between both types of DC in the phagocytosis of FITC-latex beads (Fig. 3c).
Fig. 3.
Suppression of the stimulatory activity of allogeneic T cells by dendritic cells (DC) generated in the presence of serotonin (5-hydroxytryptamine, 5-HT). (a) Monocytes were cultured with (open square) or without 5-HT (closed square) in addition to interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 6 days. These cells were washed three times and then co-cultured with purified responder allogeneic naive CD4+ T cells (1 × 105cells/100 µl) for 4 days. Results are expressed as the mean ± s.d. for triplicate cultures. Similar results were obtained when the experiment was repeated three additional times. (b) Monocytes were cultured for 6 days in the presence of IL-4 and GM-CSF. These immature DC were treated with 5-HT for 60 min or untreated, washed and then co-cultured with purified responder allogeneic naive CD4+ T cells for 4 days. Results are expressed as the mean ± s.d. for triplicate cultures. Similar results were obtained when the experiment was repeated two additional times. (c) Effects of 5-HT on the phagocytic activity of DC were tested for phagocytic capacity at 0°C (white histogram) and 37°C (black histogram) using fluorescein isothiocyanate (FITC)-labelled latex beads and flow cytometry. Representative histograms from four independent experiments with different donors are shown.
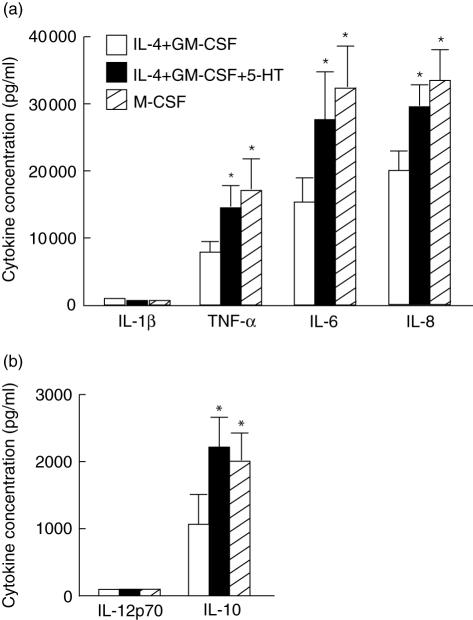
Next, we examined their capacity to produce cytokines by stimulating them with LPS after 6 days of culturing. The ability to produce pro- and anti-inflammatory cytokines including IL-10 was significantly elevated in 5-HT-treated DC except IL-12 and IL-1β (Fig. 4a,b). Endogenous production of M-CSF was not detected in control MoDC and 5-HT-treated DC using enzyme-linked immunosorbent assay (ELISA) (data not shown).
Fig. 4.
Capacity for producing proinflammatory cytokines of serotonin (5-hydroxytryptamine, 5-HT)-treated dendritic cells (DC). (a,b) Monocytes were cultured with (closed square) or without 5-HT (open square) in addition to interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 6 days. Alternatively, monocytes were cultured in the presence of M-CSF alone for 6 days (hatched square). After washing, the cells (1 × 106/ml) were stimulated with 100 ng/ml lipopolysaccharide (LPS) for 24 h. The cytokine concentrations of the culture supernatants were estimated by enzyme-linked immunosorbent assay (ELISA). The data are the means ± s.e.m. of four independent experiments with different donors.
The effect of 5-HT on the DC phenotype and function was mediated by 5-HTR1 or 5-HTR7
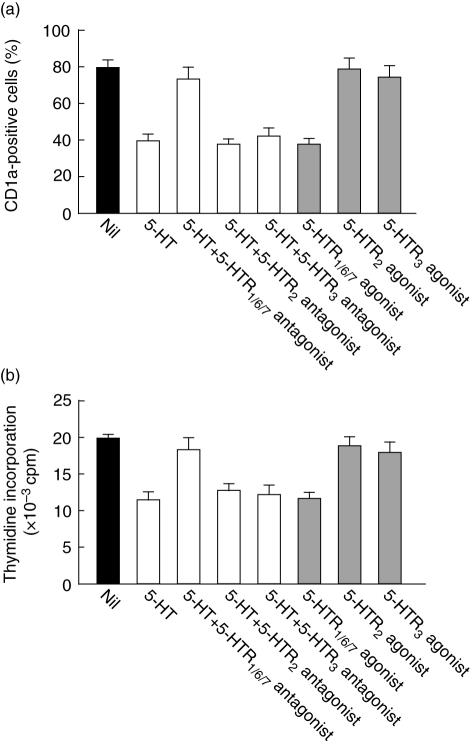
In the pharmacological characterization of receptor subtypes using 5-HT receptor agonists and antagonists, 15 receptor subtypes were shown [28]. A recent study suggested that human monocytes and immature DC express 5-HTR1E, 5-HTR2, 5-HTR3, 5-HTR4 and 5-HTR7 mRNAs [29,30]. Therefore, to determine which receptor was responsible for the 5-HT-induced alteration in phenotype and function, we preincubated monocytes with 5-HT receptor antagonists, including 5-HTR1/6/7 antagonist, 5-HTR2A antagonist and 5-HTR3 antagonist, before adding 5-HT. The 5-HT-induced alteration of their phenotype and reduction in antigen-presenting capacity was blocked by incubation of the monocytes with 5-HTR1/6/7 antagonist but not with 5-HTR2A or 5-HTR3 antagonists (Fig. 5a,b). In addition, a 5-HTR1/6/7 agonist mimicked the effect of 5-HT on the alteration of MoDC in phenotype and function, whereas 5-HTR 2 agonist or 5-HTR 3 agonist showed no effect on it. These results indicate that the 5-HT-induced alteration of their phenotype and reduction in antigen presenting capacity are mediated via 5-HTR1E or 5-HTR7.
Fig. 5.
Serotonin (5-hydroxytryptamine, 5-HT) affects the phenotype and function of dendritic cells (DC) via HTR1/7. Monocytes were incubated with 10 µM of 5-HTR antagonists for 30 min, washed three times and then cultured in the presence of 5-HT, interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) for 6 days. Alternatively, monocytes were each incubated with 10 µM of 5-HTR agonist for 6 days in the presence of IL-4 and GM-CSF. Then, their CD1a expression was analysed by flow cytometry (a) and their stimulatory capacity for allogeneic T cells was measured by mixed lymphocyte reactions (MLRs) (b). The data are representative of four independent experiments with different donors.
Discussion
5-HT is one of the major chemical mediators secreted by activated platelets [21,22]. Although the 5-HT-induced Ca2+ influx in monocytes and immature MoDC has been reported [29,30], the effect of 5-HT on monocytic differentiation into DC has not been studied. Because DC can be derived from monocytes and play critical roles in immune responses and inflammation, it is of interest to understand how 5-HT affects the differentiation of monocytes into DC, including their phenotypical and functional characteristics. The results of our study demonstrate that 5-HT down-modulates the expression of CD86 and HLA-DR. In contrast to IL-4/G-CSF-driven MoDC, those generated in the presence of 5-HT expressed significantly lower levels of CD1a and higher levels of CD14. However, these cells were negative for the macrophage marker M-CSFR. Microscopic observation demonstrates that 5-HT-treated cells have dendritic morphology and are non-adherent to plastic dishes, suggesting that the cells that developed in the presence of GM-CSF, IL-4 and 5-HT were DC.
It is considered that platelets are the major source of 5-HT in the inflammatory skin lesions of human. Human mast cells may also release 5-HT [31]. Allergen challenge has been shown to induce pulmonary recruitment and the activation of platelets in the murine model of asthma [32] and asthmatic patients [33], suggesting that platelets would also be accumulated and activated in AD skin lesions. Additionally, in AD scratching due to severe itch often results in excoriation and subsequent platelet aggregation at the lesion. The engagement of IgE receptors on platelets due to environmental allergens (e.g. dust mites and pollen) may also take place on these lesions. These events promote platelets to release 5-HT. In AD individuals, monocytes could thus be exposed to 5-HT in the skin. Ammon et al. [34] have demonstrated that intact platelets strongly prompted monocyte differentiation into macrophages in vitro. However, 5-HT does not seem to contribute to this effect because they showed that lipid fractions of platelet membranes are possible inducers of monocyte maturation. 5-HT should be added as another platelet-derived modifier of monocytic differentiation.
DC developed in the presence of GM-CSF, IL-4 and 5-HT showed the reduced expression of CD86 and HLA-DR molecules. In addition, 5-HT down-regulate the expression of CD86 and HLA-DR in already differentiated DC with IL-4 and GM-CSF. In accordance with this, 5-HT-treated DC exhibited significantly reduced stimulatory activity toward allogeneic T cells. Interestingly, skin migratory CD1a+CD14– DC shows a mature phenotype with the high expression of HLA-DR, CD54, CD80 and CD86, and potent stimulatory function for allogeneic CD4+ T cells [35–37]. By contrast, CD1a–CD14+ skin migratory DC were weak for allogeneic CD4+ T cells with the low expression of HLA-DR and co-stimulatory molecules. Recently, we showed that monocytes cultured in the presence of IL-4, GM-CSF and histamine differentiated into CD1a–CD14+ DC, in which histamine-induced endogenous production of IL-10 by monocytes plays a certain role [12]. 5-HT also induces CD1a–CD14+ DC, although its effect on monocytic differentiation into CD1a–CD14+ DC was weaker than that of histamine. 5-HT did not induce the significant endogenous production of IL-10 in our study (data not shown), which may explain the difference in the effect on down-regulation of CD1a expression.
Our results indicate that the effect of 5-HT on monocytic differentiation into DC is mediated via 5-HTR1 or 5-HTR7. Idzko et al. have demonstrated clearly that the signal transduction pathway and subsequent events evoked by each receptor subtype are different in monocytes and MoDC [29,30]. In human monocytes, activation of the Gs-coupled 5-HTR4 and 5-HTR7 subtypes increases the intracellular cyclic adenosine monophosphate and secretion of IL-1β, IL-6, IL-12p40 and IL-8/CXCL8 [30]. Interestingly, 5-HTR1 and 5-HTR2 agonists do not modulate the LPS-induced cytokine production in human monocytes [30]. Although the precise mechanism by which the phenotype and function of DC are regulated remains unknown, recent studies suggest that mitogen-activated protein kinase signalling pathways, such as extracellular signal-regulated kinases, c-Jun N-terminal kinases and p38 mitogen-activated protein kinases, differentially regulate all aspects of phenotypic maturation, cytokine production and the functional maturation of MoDC [38]. For example, the extracellular signal-regulated kinase signalling pathway appears to regulate negatively the phenotypic maturation of MoDC to some degree [38,39]. Using 5-HTR-selective agonists and antagonists in combination with such kinase-specific inhibitors will shed light on the signalling pathway of 5-HT in modulating DC differentiation.
Recently, accumulating evidence has suggested the role of platelets in inflammation [40,41]. Platelets are involved in the initiation of inflammation by releasing chemokines [40] and proinflammatory cytokines [41], and in the tissue remodelling in allergen-induced asthma [32]. Thus, the regulation of platelet activation and/or blocking the activity of platelet-derived mediators should be one of the probable strategies to control allergic inflammation. In the present study, we provide insight into new immunomodulatory effects of 5-HT on DC differentiation and function. DC developed in the presence of IL-4, GM-CSF and 5-HT show a different phenotype from that seen in control immature MoDC, enhanced cytokine-producing capacity and reduced antigen-presenting capacity. These results imply that 5-HT-treated DC might play certain regulatory roles in immune responses and inflammatory reactions.
Acknowledgments
This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports and Culture.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Hart DN. Dendritic cells: unique leukocytes populations which control the primary immune responses. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 3.Buelens C, Verhasselt V, De Grote D, Thielemans K, Goldman M, Willems F. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage colony-stimulating factor. Eur J Immunol. 1997;27:756–62. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 4.Palucka KA, Taquet N, Sanchez-Chapuis F, Gluckman JC. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–95. [PubMed] [Google Scholar]
- 5.Novak N, Bieber T, Katoh N. Engagement of FcεRI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J Immunol. 2001;167:797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- 6.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–8. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 7.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk A. Induction of IL-10-producing, nonproliferating CD4+ cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 10.Vieweg J, Jackson A. Modulation of antitumor responses by dendritic cells. Springer Semin Immunopathol. 2005;26:329–41. doi: 10.1007/s00281-004-0175-1. [DOI] [PubMed] [Google Scholar]
- 11.Novak N, Haberstok J, Geiger E, Bieber T. Dendritic cells in allergy. Allergy. 1999;54:792–803. doi: 10.1034/j.1398-9995.1999.00101.x. [DOI] [PubMed] [Google Scholar]
- 12.Katoh N, Soga F, Nara T, Masuda K, Kishimoto S. Histamine induces the generation of monocyte-derived dendritic cells that express CD14 but not CD1a. J Invest Dermatol. 2005;125:753–60. doi: 10.1111/j.0022-202X.2005.23891.x. [DOI] [PubMed] [Google Scholar]
- 13.Woltman AM, Massacrier C, de Fijter JW, Caux C, van Kooten C. Corticosteroids prevent generation of CD34+-derived dermal dendritic cells but do not inhibit Langerhans cell development. J Immunol. 2002;168:6181–8. doi: 10.4049/jimmunol.168.12.6181. [DOI] [PubMed] [Google Scholar]
- 14.Furman MI, Benoit SE, Barnard MR, et al. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352–8. doi: 10.1016/s0735-1097(97)00510-x. [DOI] [PubMed] [Google Scholar]
- 15.Rinder CS, Bonan JL, Rinder HM, Mathew J, Hines R, Smith BR. Cardiopulmonary bypass induces leukocyte–platelet adhesion. Blood. 1992;79:1201–5. [PubMed] [Google Scholar]
- 16.Page CP. Platelets as inflammatory cells. Immunopharmacology. 1989;17:51–9. doi: 10.1016/0162-3109(89)90008-8. [DOI] [PubMed] [Google Scholar]
- 17.Kasperska-Zając A, Nowakowski M, Rogala B. Enhanced platelet activation in patients with atopic eczema/dermatitis syndrome. Inflammation. 2004;28:299–302. doi: 10.1007/s10753-004-6054-z. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa S, Pawankar R, Suzuki K, et al. Functional expression of the high affinity receptor for IgE (FcεRI) in human platelets and its intracellular expression in human megakaryocytes. Blood. 1999;93:2543–51. [PubMed] [Google Scholar]
- 19.Matsuda H, Ushio H, Geba GP, Askenase PW. Human platelets can initiate T cell-dependent contact sensitivity through local serotonin release mediated by IgE antibodies. J Immunol. 1997;158:2891–7. [PubMed] [Google Scholar]
- 20.De Clerck F. The role of serotonin in thrombogenesis. Clin Physiol Biochem. 1990;8(Suppl. 3):40–9. [PubMed] [Google Scholar]
- 21.Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol. 1997;272:L795–806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ. Histamine and serotonin. Pulm Pharmacol Ther. 2001;14:329–39. doi: 10.1006/pupt.2000.0318. [DOI] [PubMed] [Google Scholar]
- 23.Cazzola I, Matera MG. 5-HT modifiers as a potential treatment of asthma. Trends Pharmacol Sci. 2000;21:13–6. doi: 10.1016/s0165-6147(99)01408-x. [DOI] [PubMed] [Google Scholar]
- 24.Sternberg EM, Trial J, Parker CW. Effect of serotonin on murine macrophages: suppression of Ia expression by serotonin and its reversal by 5-HT2 serotonergic receptor antagonists. J Immunol. 1986;137:276–82. [PubMed] [Google Scholar]
- 25.Arzt E, Costas M, Finkelman S, Nahmod VE. Serotonin inhibition of tumor necrosis factor-α synthesis by human monocytes. Life Sci. 1991;48:2557–62. doi: 10.1016/0024-3205(91)90612-f. [DOI] [PubMed] [Google Scholar]
- 26.Katoh N, Kraft S, Weßendorf JHM, Bieber T. The high-affinity IgE receptor (FcεRI) blocks apoptosis in normal human monocytes. J Clin Invest. 2000;105:183–90. doi: 10.1172/JCI6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonov AS, Munn DH, Kolodgie FD, Virmani R, Gerrity RG. Aortic endothelial cells regulate proliferation of human monocytes in vitro via a mechanism synergistic with macrophage colony-stimulating factor. Convergence at the cyclin E/p27 (Kip1) regulatory checkpoint. J Clin Invest. 1997;99:2867–76. doi: 10.1172/JCI119480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyer D, Clarke DE, Fozard JR, et al. VII. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 29.Idzko M, Panther E, Stratz C, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172:6011–19. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 30.Dürk T, Panther E, Müller T, et al. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol. 2005;17:599–606. doi: 10.1093/intimm/dxh242. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre H, Compagnon P, Contesse V, et al. Production and metabolism of serotonin (5-HT) by the human adrenal cortex: paracrine stimulation of aldosterone secretion by 5-HT. J Clin Endocrinol Metab. 2001;86:5001–7. doi: 10.1210/jcem.86.10.7917. [DOI] [PubMed] [Google Scholar]
- 32.Pitchford SC, Riffo-Vasquez Y, Sousa A, et al. Platelets are necessary for airway wall remodelling in a murine model of chronic allergic inflammation. Blood. 2004;103:639–47. doi: 10.1182/blood-2003-05-1707. [DOI] [PubMed] [Google Scholar]
- 33.Lupinetti MD, Sheller JR, Catella F, Fitzgerald GA. Thromboxane biosynthesis in allergen-induced bronchospasm. Evidence for platelet activation. Am Rev Respir Dis. 1989;140:932–5. doi: 10.1164/ajrccm/140.4.932. [DOI] [PubMed] [Google Scholar]
- 34.Ammon C, Kreuts M, Rehli M, Krause SW, Andreesen R. Platelets induce monocyte differentiation in serum-free coculture. J Leukoc Biol. 1998;63:469–76. doi: 10.1002/jlb.63.4.469. [DOI] [PubMed] [Google Scholar]
- 35.Larregina AT, Morelli AE, Spencer LA, et al. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2:1151–8. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- 36.Morelli AE, Rubin JP, Erdos G, et al. CD4+ T cell response elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–15. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- 37.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–45. [PubMed] [Google Scholar]
- 38.Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa S, Ohtani T, Mizuashi M, et al. p38 mitogen activated protein kinases mediates dual role of ultraviolet B radiation in induction of maturation and apoptosis of monocyte-derived dendritic cells. J Invest Dermatol. 2004;123:361–70. doi: 10.1111/j.0022-202X.2004.23238.x. [DOI] [PubMed] [Google Scholar]
- 40.Fujisawa T, Fujisawa R, Kato Y, et al. Presence of high contents of thymus and activation-regulated chemokine in platelets and elevated plasma levels of thymus and activation-regulated chemokine and macrophage-derived chemokine in patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:139–46. doi: 10.1067/mai.2002.126079. [DOI] [PubMed] [Google Scholar]
- 41.Massberg S, Vogt F, Dickfeld T, Brand K, Page S, Gawaz M. Activated platelets trigger an inflammatory response and enhance migration of aortic smooth muscle cells. Thromb Res. 2003;110:187–94. doi: 10.1016/s0049-3848(03)00342-6. [DOI] [PubMed] [Google Scholar]