Abstract
Vaccinia virus (VV) has been tested as oncolytic virus against malignant melanoma in clinical trials for more than 40 years. Until now, mainly strains comparable to viral strains used for smallpox vaccination have been probed for anti-tumoral therapy. We have shown recently that the wild-type strain Western Reserve (WR) can interfere with crucial functions of monocyte-derived dendritic cells (DCs). Our aim was to examine whether viral immune evasion mechanisms might be responsible for the ineffectiveness of WR-based vaccination strategies and whether the highly attenuated strain modified virus Ankara (MVA) differs from WR with respect to its possible immunostimulatory capacity after intratumoral injection. Using in vitro experiments, we compared the effect of both strains on melanoma cells and on local bystander DCs. We found that both VV-strains infected melanoma cells efficiently and caused disintegration of the actin cytoskeleton, as shown by fluorescence microscopy. In addition, both VV-strains caused apoptotic cell death in melanoma cells after infection. In contrast to MVA, WR underwent a complete viral replication cycle in melanoma cells. Bystander DCs were consecutively infected by newly generated WR virions and lost their capacity to induce allogeneic T cell proliferation. DCs in contact with MVA-infected melanoma cells retained their capacity to induce T cell proliferation. Immature DCs were capable of phagocytosing MVA-infected melanoma cells. Priming of autologous CD8+ T cells by DCs that had phagocytosed MVA-infected, MelanA positive melanoma cells resulted in the induction of T cell clones specifically reactive against the model antigen MelanA as shown by enzyme-linked immunospot (ELISPOT) analysis. We conclude that the clinical trials with oncolytic wild-type VV failed probably because of suppression of bystander DCs and consecutive suppression of T cell-mediated anti-melanoma immunity. The attenuated VV-strain MVA facilitates the generation of tumour associated antigen (TAA)-specific T cell response as it is oncolytic for melanoma cells, but non-toxic for DC, and should be a promising candidate for intralesional metastatic melanoma therapy.
Keywords: cross-presentation, dendritic cells, immunotherapy, melanoma, oncolysis, vaccinia virus
Introduction
Malignant melanoma has always been regarded as an immunogenic tumour, as regression zones within tumoral lesions can be observed frequently together with a dense infiltration of T helper cells [1] that results from recognition of tumour-associated antigens either on antigen-presenting cells or on the surface of tumour cells by T lymphocytes [2], and spontaneous remissions of primary melanoma have been reported [3]. Furthermore, an inverse correlation between prognosis and the degree of lymphocytic infiltration of the primary tumour [4] suggest that the activation of anti-tumoral immunity might be beneficial in attempts to induce the regression of established tumours or to prevent recurrence. Numerous strategies have been probed aiming for the induction or augmentation of anti-melanoma immunity. Although melanoma cells express a whole bundle of tumour-associated antigens (TAAs), which could serve as targets for specific T cells [2], the immunogenicity of melanoma cells is generally low. Thus, strategies to augment the immunogenicity of melanoma cells in vitro before reinjection or directly in situ are of potential benefit [5]. For the induction of anti-tumoral immunity, viruses have been used in three technically different approaches: (1) the preparation of ex vivo oncolysates as described for Newcastle disease virus [6], influenza virus [7] and, in the vicinity of the efficient poxvirus eradiation campaign, vaccinia virus (VV) [8,9]; (2) in situ tumour transfection was probed for the effect on melanoma beginning with reported prolonged remissions of stage IV melanoma after multiple injections of wild-type VV [10] and later with recombinantly modified poxviruses which express immune-enhancing cytokines and immune co-stimulatory antigens [11]; and (3) vaccination with viruses which recombinantly express TAAs or TAAs together with some co-stimulatory molecules either as single agent [12,13] or for the ex vivo transduction of dendritic cells (DCs) [14]. It should be kept in mind that all three approaches rely ultimately on the capacity of DCs to induce anti-tumoral T cell immunity and that viruses [14] and especially poxviruses [15] interfere with DC function by numerous mechanisms; e.g. the Western Reserve (WR) strain of VV blocks the capacity of mature DC to induce allogeneic T cell proliferation and the expression of the co-stimulatory molecule CD80 [16].
In this study we were able to demonstrate that the highly modified VV strain modified virus Ankara (MVA), which has the capacity to induce anti-TAA T cell immunity [17], infects melanoma cells and induce apoptosis. While WR replicates in melanoma cells and blocks any capacity of bystander DCs to induce T cell proliferation, MVA-infected apoptotic melanoma cells are phagocytosed without negative effects on DCs functionality. Apoptotic melanoma cells have been shown to be a suitable loading agent for DCs in vitro in order to induce anti-tumoral T cell immunity [18,19]. Thus we compared the capacity of apoptotic melanoma cells generated with UV-B radiation and apoptotic melanoma cells infected with MVA to serve as loading agent for DCs. We were able to show that the stimulation of autologous T cells with DCs that phagocytosed MVA-infected apoptotic melanoma cells was more efficient than using UV-irradiated melanoma cells in the induction of TAA-specific T cells.
These data favour the in situ application of MVA, probably recombinantly altered to express multiple immunodominant TAA epitopes, co-stimulatory molecules and immune-enhancing cytokines, for the treatment of stage IV melanoma patients.
Materials and methods
Virus strains and viral infection
Two different strains of recombinant viruses expressing green fluorescent protein (GFP) under the control of a strong synthetic early promoter were used. The wild-type strain Western Reserve (WR) was prepared and titred on HeLa cells monolayers according to standard procedures. The multiplicity of infection (MOI) gives the virion counts per cell during infection and is based on these titres.
The second strain deployed is the attenuated MVA, which was obtained from Bavarian Nordic (Munich, Germany). The GFP coding region of the MVA strain was subcloned into a recombination vector that matched the deletion site in the MVA genome. The homologous recombination was performed as described previously [20]. Recombinant MVA was selected by five consecutive rounds of plaque purification on CEF cells and analysed by polymerase chain reaction (PCR). The correct insertion of the GFP gene into the MVA genome was confirmed by sequencing. Stocks of MVA were grown on pathogen-free primary CEF cells (Charles River). The virus was released from the infected cells by several rounds of mechanical and ultrasonic homogenization. Infection of cells was performed at given MOI in RPMI-1640 for 90 min at 37°C. During the first 30 min, cells were slightly vortexed three times. Subsequently, cells were washed once and resuspended in adequate culture medium.
Cells lines and DC generation
The melanoma cell line MEL526 [human leucocyte antigen (HLA): A2, A3, B50, B62] was used for experiments and was kindly provided by Dr M. T. Lotze (University of Pittsburgh, USA). Mel526 cells express MelanA/MART1, tyrosinase, MAGE-3 and gp-100 [21]. For cultivation, we used RPMI-1640 (Bio-Whittaker, Verviers, Belgium) supplemented with 2 mm l-glutamine (Bio-Whittaker), penicillin–streptomycin mixture with 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco-Invitrogen, Karlsruhe, Germany) and 10% heat-inactivated fetal calf serum (FCS) (PAA Laboratories GmbH, Cölbe, Germany). Cells were subcultured every 3 days after treatment with 1% trypsin-ethylenediamine tetraacetic acid (EDTA) (Sigma-Aldrich Chemie GmbH, Munich, Germany).
HeLa cells for viral replication were cultured in Dulbecco's modified Eagle's medium (DMEM) with 4·5% glucose containing 10% FCS (PAA Laboratories GmbH), 2 mM l-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin (both gibco-Invitrogen).
For DC generation, leukaphereses from healthy donors were obtained according to institutional guidelines with a protocol designed to reduce platelet counts. Peripheral blood mononuclear cells (PBMC) were prepared by repeated density centrifugation on Lymphoprep (Axis Shield, Oslo, Norway). PBMC (5 × 108) were added to 100 ml DC cultivation medium consisting of RPMI-1640 (Bio-Whittaker) supplemented with 1% heat-inactivated autologous plasma, 2 mM l-glutamine (Bio-Whittaker) and 20 µg/ml gentamycin (Sigma-Aldrich), filled in triple-layer bottles (Nunc, Roskilde, Denmark) and incubated for 1 h at 37°C. The non-adherent fraction was removed and the remaining adherent cells were resuspended in DC culture medium. Cells were fed repeatedly with 800 IU/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (Cellogenix Technologie Transfer GmbH, Freiburg, Germany) and 250 IU/ml interleukin (IL)-4 (Strathmann AG, Hamburg, Germany) on days 1, 3 and 5. On day 6 immature DC were either used for experiments or maturation was induced with a cocktail of cytokines and prostaglandin E2 (PGE2), as described previously [22]: 250 IU/ml of IL-4; 10 ng/ml of IL-1β; 1000 IU/ml of IL-6 (Strathmann AG); 800 U/ml of GM-CSF (Cellogenix); 1 µg/ml of minprostin E2 (PGE2) (Pharmacia & Upjohn GmbH, Erlangen, Germany); 10 ng/ml of tumour necrosis factor (TNF)-α (Bender, Vienna, Austria). Maturation was achieved within 24 h and confirmed by fluorescence activated cell sorter (FACS) analyses of CD83, CD80 and CD86.
For the priming assays DCs were generated from the PBMC of peripheral blood drawn from healthy volunteers at two different time-points. PBMC were adhered to cell culture dishes and fed, harvested and matured in similar culture media and cytokine concentrations as described above.
Antibodies and flow cytometric analyses
The following monoclonal antibodies (MoAb) were used: fluorescein isothiocyanate (FITC)-labelled murine CD80 (BB1), and HLA-DR (G46-6) MoAb were purchased from Becton-Dickinson/Pharmingen (Hamburg, Germany); phycoerythrin (PE)-conjugated murine CD86 (IT2·2) MoAb from Becton-Dickinson/Pharmingen; CD83 (Hb15a) MoAb from Immunotech (Marseille, France); CD4 (MT310) from Dako (Glostrup, Denmark); and CD3 (SK7) MoAb from Becton Dickinson/Pharmingen. Purified control IgG1-PE was purchased from Dako and IgG2b-PE, IgG1-FITC and IgG2b-FITC MoAb from Becton-Dickinson/Pharmingen.
For extracellular FACS staining, cultured cells were washed, suspended at 2 × 105 in 100 µl of cold phosphate-buffered saline (PBS) containing 0·1% sodium azide and 10 mg/ml human serum albumin (HAS) (FACS media). Subsequently, staining with labelled MoAb or appropriate isotypic controls was performed for 30 min. Cells were then washed and resuspended in 300 µl of cold PBS, 1% HSA (Octopharma GmbH, Langenfeld, Germany). Stained cells were analysed for immunofluorescence with a FACScan cell analyser (Becton-Dickinson). Data was processed using CellQuest software (Becton-Dickinson).
Measuring apoptosis
Apoptosis levels were measured using an annexin V-PE labelling kit (Becton-Dickinson). Melanoma cells were grown on 24-well plates (Nunc) to a loose monolayer and infected by VV at a MOI of 5:1. From days 0–5 after infection, both adherent and non-adherent melanoma cells were taken out the wells by PBS containing 1% trypsin (Sigma-Aldrich), washed twice and resuspended in FACS medium. After adjusting FACScan parameters, Mel526 cells were stained by 2 µl annexin V–PE conjugate per 100 µl cell suspension for 10 min at room temperature followed by 10 µl 7-amino-actinomycin-D (7-AAD) for discrimination between apoptosis and necrosis. Annexin V-positive and 7-AAD-negative cells qualified as apoptopic cells.
Actin staining
Mel526 cells were cultivated on coverslips for 2 days. Cells were infected by VV (MOI 5:1) or left uninfected. On days 0–4 after infection, cells were fixed in PBS containing 4% paraformaldehyde (Polysciences Inc., Warrington, PA, USA) for 10 min, washed twice in PBS containing 1% bovine serum albumin (Sigma-Aldrich), permeabilized by 0·005% saponine (Sigma-Aldrich) for 20 min, again washed twice and stained for filamentous actin by 0·2 µm tetramethylrhodamine-B-isothiocyanate (TRITC)-conjugated phalloidine (Sigma-Aldrich) for 30 min, washed three times and mounted on a coverslide with Mowiol (Sigma-Aldrich). Pictures were taken on a Leica fluorescence microscope (DMRB + RD) with an attached CCD-Camera (QImaging, Burnaby, BC, Canada) at least three different spots of the coverslide at low magnification (200 ×) for survey and in detail (up to 1000 ×). Pictures were processed with Openlab software (Improvision, Coventry, UK).
Macs sorting
For magnetic cell sorting, the EasySep kit and procedure from StemCell Technologies (Vancouver, BC, Canada) was used. T cells were diluted at a concentration of 108 cells/ml in PBS containing 2% FCS (PAA Laboratories GmbH) and 1 mm EDTA (Sigma-Aldrich) in a 5-ml polystyrene Falcon tubes (Becton-Dickinson). One hundred µl of EasySep human CD8-positive selection cocktail per 1 ml cell suspension was added, mixed and incubated for 15 min. Thereafter, 50 µl of EasySep magnetic nanoparticles were supplemented for 10 min. Cell suspension was diluted to a total volume of 2·5 ml, put into a magnetic field for 5 min and then the supernatant was poured off; 2·5 ml of fresh medium was added. The last steps were repeated twice for purification.
Phagocytosis assay
Phagocytosis of melanoma cells by immature DCs was measured by PKH staining (Sigma-Aldrich) and consecutive FACS analysis. Immature DCs were labelled by PKHred. Mel526 were stained by PKHgreen, additional to their GFP expression in the case of VV infection. Cells (5 × 106) were added to 250 µl of a special dye buffer for PKHred and for PKHgreen, respectively; 250 µl of dye buffer containing 1 µl of PKHred or PKH green was added. The suspension was stirred gently for 3 min in the case of DCs and 4 min in the case of Mel526, followed by supplementation of 500 µl of 100% FCS for blocking over 1 min. Thereafter, cells were washed three times in pure RPMI and resuspended in the appropriate medium. Immature DCs and melanoma cells were co-cultured for 24 h at 37°C and at 4°C as negative control. Cells were analysed in two-channel flow cytometry using a FACScan flow cytometer (Becton-Dickinson).
Mixed leucocyte reaction
DCs were plated in round-bottomed 96-well plates in 200 µl RPMI-1640 medium containing 5% heat-inactivated pooled human serum (Bio-Whittaker). Allogeneic T cells (1 × 105) were added to each well and the cultures were incubated for 5 days at 37°C. T cell proliferation was determined by adding 1 µCi/well of [3H]-TdR (Amersham, Buckinghamshire, UK) during the last 16 h of culture. Measurement of the incorporated thymidine was performed with a Wallac 1450 Microbeta liquid scintillation counter (PerkinElmer-Wallac GmbH, Freiburg, Germany).
T cell priming and enzyme-linked immunospot (ELISPOT) assays
Immature DCs were generated as described above. Melanoma cells that had been infected with MVA 4 days previously were added for 36 h for phagocytosis. The medium was supplemented with a cytokine cocktail and PGE2 for reliable maturation of the DC [22]. Loaded and matured DCs were than added to autologous T cells at a ratio of 1:20 in 12-well plates in 1 ml RPMI-1640 medium containing 5% heat-inactivated pooled human serum. On days 2 and 4, 20 IU IL-2 were added to the cultures. On day 7, a second round of stimulation with autologous DCs, generated similar to the first stimulation, were added to these T cells and the medium was refreshed. On days 9 and 11, IL-2 was added at a concentration of 20 IU. On days 12–13 T cells were harvested and analysed.
The ELISPOT assay was used as described [23] to quantify antigen-specific interferon (IFN)-γ release of effector T cells; 105 CD8+ T cells and 5 × 103 DCs per well were added in triplicate to nitrocellulose-bottomed 96-well plates (MAHA S4510) precoated with the primary anti-IFN-γ MoAb (1-D1K, Mabtech, Stockholm, Sweden) in 50 µl ELISPOT medium (RPMI-1640, 5% heat-inactivated human serum) per well. After addition of mature DCs pulsed with the HLA-A2 restricted immunodominant peptide corresponding to residues 27–35 (AAGIGILTV) from MelanA/MART1 tumour associated antigen [24] and incubation for 20 h, wells were washed six times, incubated with biotinylated second MoAb to IFN-γ (7-B6-1, Mabtech) for 2 h, washed and stained with Vectastain Elite kit (Vector Laboratories, Burlingame, CA, USA). Spots were evaluated and counted by a computer-assisted video imaging system (Carl Zeiss Vision, Jena, Germany).
Results
Changes in morphology and altered actin cytoskeleton of VV-infected melanoma cells
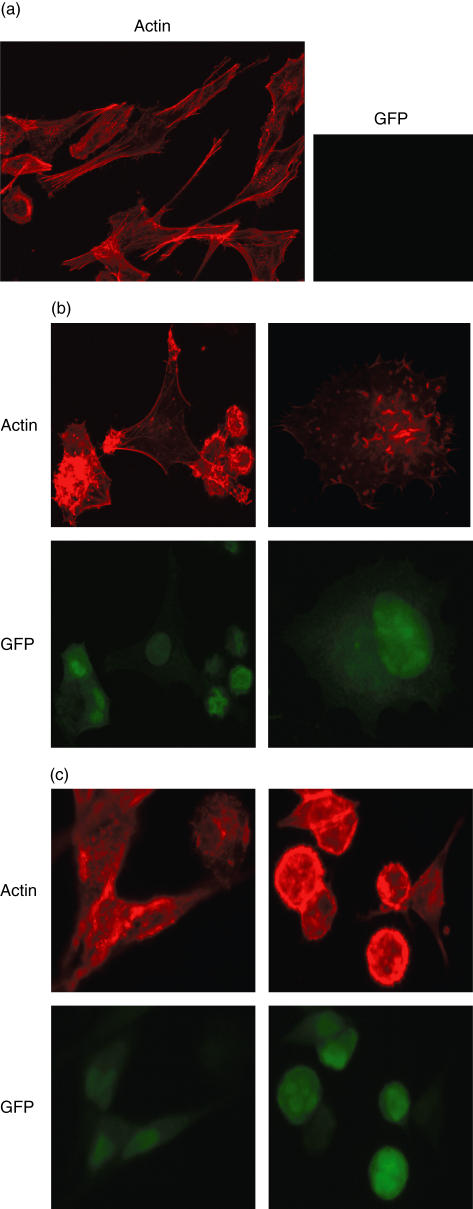
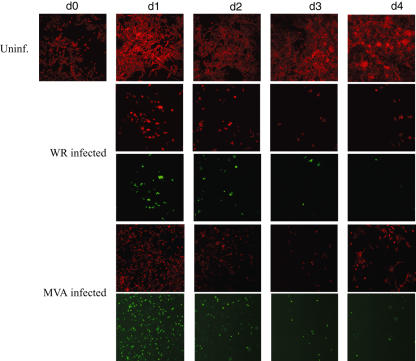
We first observed the behaviour of Mel526 cells upon VV-infection by brightfield microsocopy. Uninfected Mel526 cells grew rapidly, multi-layered and adherent with a fusiform morphology. In comparison, VV-infected Mel526 cells appeared rounded and lost intercellular contact. We found an explanation for this by a detailed view of the actin cytoskeleton by phalloidin–TRITC staining of VV-infected Mel526 and fluorescence microscopy (Fig. 1a–c). At up to 1000-fold magnification, the disintegration of actin filaments and an aggregation of actin, and in a few cells a rearrangement of actin to so-called comet-like tails, can be seen after 1 day of VV-infection. Comet-like tails have already been described in VV-infected HeLa cells and are used by VV virions for the active spread to neighboured cells [25]. GFP-coding sequences that were transduced by VV were used as infection marker. At 200-fold magnification, we followed the growth and infection of VV-infected Mel526 over 4 days (Fig. 2). In the case of WR-infection, all remaining Mel526 cells are affected after 4 days and cell growth ceases, whereas MVA-infected Mel526 recover on day 4 and growth of uninfected, unaltered cells can be observed.
Fig. 1.
Melanoma cells were cultivated on coverslips for 2 days. Cells were infected by vaccinia virus [multiplicity of infection (MOI) 5:1] or left uninfected. Staining for filamentous actin was performed with tetramethylrhodamine-B-isothiocyanate (TRITC)-conjugated phalloidine. Pictures were taken on a Leica fluorescence microscope (DMRB + RD) at low magnification (200 ×) for survey and in detail (up to 1000 ×). (a) Uninfected melanoma cells with a fusiform morphology and strong actin filaments. (b) Western Reserve (WR)-infected melanoma cells: rounded cells, actin clumps and comet-like tails appear. (c) Modified virus Ankara (MVA)-infected melanoma cells: similarly rounded cells, actin clumps and comet-like tails are visible.
Fig. 2.
Melanoma cell cultures stained for filamentous actin with corresponding pictures of green fluorescence (vaccinia virus infection) are shown. (a) Uninfected melanoma cells (first row) grow continuously and with adhesion to the tissue culture plate, Western Reserve (WR)-infected melanoma cells (second row) lose contact soon after infection and undergo apoptotic cell death. The infection with WR can be seen in the last row as a green spot in row 3. Similarly, melanoma cells are infected with modified virus Ankara (MVA) (fifth row) and lose the contact with the tissue culture plate. When MVA was used, a reappearance of uninfected melanoma cells can be traced (fourth row, last picture).
Induction of apoptosis in infected Mel526 cells
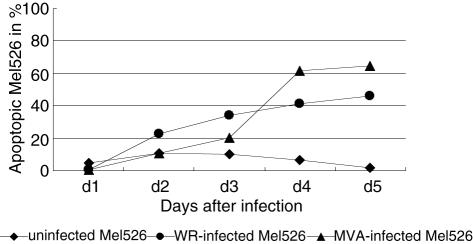
As demonstrated above, both VV strains caused severe alterations in infected melanoma cells which led finally to cell death of the melanoma cells. To discriminate between apoptosis and necrosis, we used annexin V and 7-AAD staining. Figure 3 shows the ratio of apoptopic cells to the total amount of living cells measured over 5 days in cultures of uninfected, WR- and MVA-infected Mel526 cells. WR-infected Mel526 cells showed a steady increase up to 46% on day 5. Initially, MVA-infected Mel526 presented smaller levels of apoptosis, but on day 3 a strong rise occurred resulting in 65% apoptotic cells on day 5. Uninfected Mel526 cells showed average apoptosis levels of 7% ± 3·5%.
Fig. 3.
Melanoma cells were infected with both viral strains and harvested from days 0–5 after infection. Both adherent and non-adherent melanoma cells were removed from the tissue culture plates and stained with annexin V–phycoerythrin (PE) conjugate and 7-amino-actinomycin-D (7-AAD) for discrimination between apoptosis and necrosis. Annexin V-positive and 7-AAD-negative cells were measured and depicted in percentage of total cells. The experiment shown represents three experiments with similar results.
Replication of WR in Mel526
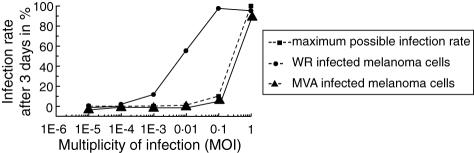
For further studies, it seemed important to us to look for the replication capacity of VV in Mel526 cells. We infected Mel526 cells with increasing MOIs of VV and measured the infection rate of the melanoma cells after 3 days by FACS analysis. As Fig. 4 shows, MVA infects Mel526 cells very efficiently but does not exceed the maximum infection rate of a non-replicative virus, stringent with the general inabililty of MVA to replicate in human cells [26]. The level of infection achieved by WR exceeds the maximum infection level by far, indicating the generation of new WR virions in Mel526 cells. Even at a MOI of 1 virion to 100 Mel526 cells, an infection rate of 57% can be measured after 3 days of infection. Further, we could demonstrate the infectiousness of supernatant of WR-infected Mel526 cells in contrast to supernatant of MVA-infected Mel526 cells (data not shown). These observations have important consequences for the co-cultivation of DC with VV-infected Mel526 cells in our experimental assays and, moreover, for local bystander DC in anti-tumoral, intralesional vaccination strategies in vivo.
Fig. 4.
To analyse whether vaccinia virus replicates in melanoma cells we infected Mel526 cells with different multiplicities of infection (MOIs) of Western Reserve (WR) and modified virus Ankara (MVA) viruses. The infection rate after 3 days was measured by fluorescence activated cell sorter (FACS) analysis of green fluorescent protein (GFP)-expressing cells. Without replication only one melanoma cell can be infected with one virus particle (depicted as maximum possible infection rate).
Stimulatory capacity of DCs that have phagocytosed VV-infected Mel526 cells
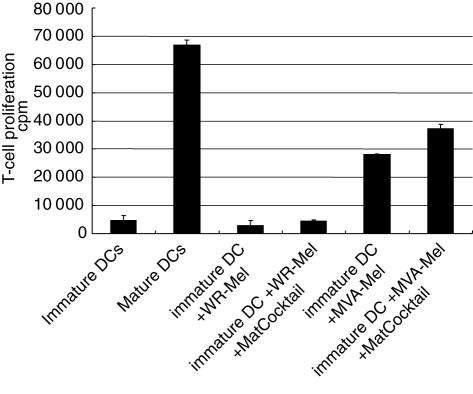
The next step was to examine changes in the immunogenic capacity of DCs that have been in contact with VV-infected Mel526 cells. Immature DCs were co-cultured with VV-infected Mel526 cells after three rounds of washes to exclude the presence of virus particles. Co-incubation was set up for 18 h at 37°C at a ratio of two immature DCs to one melanoma cell. After repeated washing of these cultures, DCs were tested for their immunostimulatory capacity in mixed leucocyte reactions (MLRs) over 5 days. Figure 5 shows that in the presence of WR-infected Mel526, DCs cannot induce any allogeneic T cell proliferation. Furthermore, addition of the maturation-inducing cytokine cocktail together with the infected melanoma cells does not show any augmentation of the proliferative capacity of DCs cultured with WR infected melanoma cells. When immature DCs were co-cultured with MVA-infected Mel526 cells and used for a MLR, significant T cell proliferation could be measured, reaching about half the quantity compared to positive control of uninfected, mature DCs in a MLR. In the case of MVA, the addition of DC maturation cocktail could enhance T cell proliferation. T cells that have been in contact with WR-infected DCs over 5 days show a state of anergy and are resistant to further stimulation by mature DCs (Greiner et al. submitted). Thus we performed the following experiments with only MVA-infected melanoma cells.
Fig. 5.
Immature dendritic cells (DCs) were cultured with either Western Reserve (WR) (WR-Mel) or modified virus Ankara (MVA)A (MVA-Mel)-infected melanoma cells for 18 h at 37°C at a ratio of 2:1 and tested thereafter for their capacity to induce allogeneic T cell proliferation in mixed leucocyte reactions (MLRs). Furthermore, a maturation inducing cocktail of cytokines was added when indicated to analyse whether the vaccinia virus-infected melanoma cells inhibit the maturation of immature DCs. T cell proliferation without DCs was below 3000 counts per million. The figure represents one of three experiments with similar results.
Uptake of MVA-infected melanoma cells by immature DCs
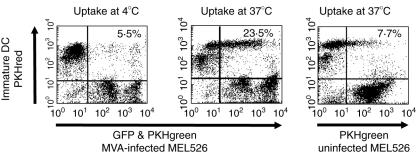
We demonstrated that immature DCs are still capable of inducing T cell proliferation after contact with MVA-infected Mel526 cells. As apoptopic melanoma cells are known to be phagocytosed efficiently by immature DCs and are suitable as loading material for DCs in order to induce anti-tumoral T cell immunity [18,27], we measured the phagocytosis of MVA-infected Mel526 by immature DCs in phagocytosis assays as described previously [18]. We found that 23·5% of the immature DC had incorporated MVA-infected Mel526 cells at least partially (Fig. 6) after 24 h. Phagocytosis levels were only 7% when uninfected Mel526 cells were used and 4% when phagocytosis assays were carried out at 4°C.
Fig. 6.
To test whether modified virus Ankara (MVA)-infected melanoma cells are phagocytosed by immature dendritic cells (DCs) we labelled both cell populations with PKH staining (immature DCs were labelled with PKHred and melanoma cells were stained with PKHgreen, additionally to their green fluorescent protein (GFP) expression in the case of MVA infection). After 24 h of incubation at either 4°C or 37°C cells were analysed by two-channel flow cytometry. Uninfected melanoma cells with a low rate of apoptotic cells served as control.
Generation of MelanA-specific reactive T cells by priming with autologous DCs that had phagocytosed MVA-infected Mel526 cells
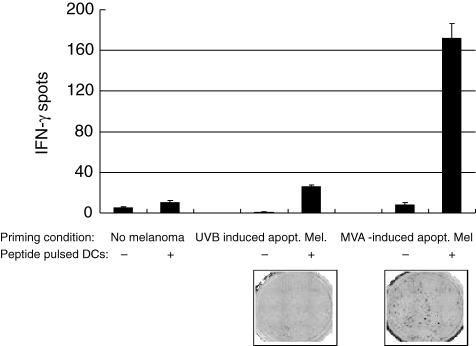
We performed stimulation experiments in order to prove the capacity of DCs to cross-present TAA after the uptake of MVA-infected melanoma cells. Immature DCs were exposed to MVA-infected melanoma cells over a period of 24 h in the presence of the cytokine cocktail known to induce maturation. Autologous T cells were added and cultured as described. After 1 week a restimulation of the primed T cells was performed by using autologous DCs generated in the same manner as for the first priming. In parallel, apoptotic melanoma cells in which apoptosis had been induced by UV-B radiation were used to load another batch of DCs. Unloaded DCs, otherwise treated similarly, served as negative control. After a total period of 2 weeks, the primed T cells were measured for specific IFN-γ production after contact with DCs loaded with the peptide of MelanA presented in the HLA-A2 context by ELISPOT analysis.
Figure 7 shows the amount of specifically reacting T cells. Compared to Mel526 cells that underwent apoptosis due to UV-B radiation, the use of MVA-infected Mel526 cells showed a sixfold increase in the number of IFN-γ producing MelanA-peptide-reactive T cells. When T cells were primed by mature DC lacking phagocytosis of Mel526 cells, no significant specific IFN-γ production could be measured in response to the MelanA peptide-loaded DC which served as targets. Thus these priming experiments demonstrate the superiority of melanoma cells as loading agent for theinduction of anti-tumoral immunity in which apoptosis has been induced by MVA compared to a non-viral method of apoptosis induction.
Fig. 7.
To test whether modified virus Ankara (MVA)-infected apoptotic melanoma cells can be used as loading agent for the priming of tumour associated antigen (TAA)-specific T cells melanoma cells infected with MVA were added to immature dendritic cells (DCs) to allow 36 h of phagocytosis. The medium was supplemented with the maturation inducing cytokine cocktail and prostaglandin E2. Loaded and matured DCs were added to autologous T cells. After 1 week of priming a second round of stimulation was introduced with similar conditions. At day 13 T cells were harvested and analysed in an enzyme-linked immunospot assay to identify the generation of T cells reactive against the human leucocyte antigen (HLA)-A2 restricted immunodominnt peptide from MelanA/MART1 TAA pulsed on mature DCs. Numbers of interferon (IFN)-γ producing T cells generated with either UV-B radiated or MVA-infected apoptotic melanoma cells are depicted. One of three experiments with similar results is shown.
Discussion
Early clinical virotherapy approaches were inspired by occasional tumour regressions observed in vaccinated or virus-infected patients [28]. These studies were restricted mainly to wild-type viruses. Today, recombinant viruses modified to exhibit a certain tumour cell specificity [29] or to express a bundle of TAAs and T cell stimulatory molecules such as co-stimulatory molecules can be introduced in virotherapeutic approaches [30]. Since 1995 oncolytic viruses from five different species have been taken into to phases I and II clinical trials in over 300 cancer patients [30]. Furthermore, epidemiological studies revealed that vaccinations with VV significantly reduced the risk of subsequently developing melanoma [31], indicating an association of anti-viral and anti-tumoral immunity for VV and melanoma.
Viral therapy, or viral oncolysis, aims at inducing cell death selectively in tumour cells in order to interfere with tumour growth and organization of the tumour [32], and consequently to induce or augment anti-tumoral T cell immunity.
VV is a double-stranded enveloped lytic DNA virus with a large DNA size capacity (up to 25 kb). It has several advantages over other lytic viruses as it is easy to manipulate, has no potential capacity to integrate in foreign DNA, and has been proved to exhibit an excellent safety profile in the vaccination campaign to eradicate smallpox [14,30,33]. Several poxvirus based vaccinations are currently under investigation for anti-cancer immunotherapy [34–37,37], highlighting the relevance of his virus for anti-cancer immunotherapy.
In the study presented here we were able to demonstrate that two VV strains (MVA and WR) have the capacity to cease the growth of melanoma cells after infection and that infected melanoma cells undergo apoptopic cell death. When WR is used as infectious agent the infection proceeds until almost all melanoma cells are infected. In contrast, when the non-replicative strain MVA is used as infectious agent, tumour growth reappears at day 4 and growth of uninfected melanoma cells can be observed. According to clinical trials, in which melanoma lesions could be dissolved by local injection of WR [10], the use of a relatively small number of MVA virions compared to the number of melanoma cells would result in a renewed local tumour growth. Thus, in clinical settings the regular reinjection of MVA particles would be required.
Beyond growth behaviour, changes in the actin cytoskeleton of the melanoma cells were observed by fluorescence microscopy only few hours after infection. Thereby, melanoma cells lose their ability for intercellular adhesion. The degradation of the actin cytoskeleton and the rearrangement of so-called comet-like tails observed VV-infected melanoma cells has already been described in VV-infected HeLa cells [25]. The loss of intracellular integrity may also be the reason for inhibition of cell growth and the apoptopic cell death of the melanoma cells, as changes in the actin cytoskeleton interfere with the cell cycle [38]. One important point associated with the disintegration of the actin cytoskeleton must be mentioned: the (reversible) loss of intercellular adhesion is crucial for the spread of tumour metastasis [39,40]. Altogether it can be summarized that both VV strains, the wild-type WR strain and the attenuated MVA strain, effectively cause oncolysis in melanoma cells while only the WR strain replicated in the melanoma cells.
The viral oncolysis achieved by VV is obviously not specific for melanoma cells, as VV can also infect other cells. A relative specificity of VV could, however, be secured by the intralesional injection of a limited number of virions of this non-replicative virus.
The generation of an anti-tumoral immunity depends largely on the capacity of DCs to present and cross-present phagocytosed material in an immunogenic context [41]. Thus we wanted to determine the interaction of such virally infected apoptotic melanoma cells with immature DCs which are able to uptake (and cross-present) apoptotic melanoma cells [18]. First, as WR in contrast to MVA replicates in melanoma cells we also found a strong viral infection of the DCs when WR was used to infect the melanoma cells. We have shown in earlier work that DCs are infected by WR and consecutively lose their capacity to induce T cell proliferation [16]. This functional incapacity is due most probably to the down-regulation of the co-stimulatory molecule CD80 on the surface of infected and uninfected bystander DCs and the subsequent incomplete activation of the T cells. We have analysed this effect further on T cells and were able to show that WR-infected but not MVA-infected DCs induce the generation of non-proliferative, IL-10-producing suppressor T cells (Greiner et al. submitted). In this context it was not surprising to find that DCs in contact with WR-infected apoptotic melanoma cells and WR virus particles had lost their capacity to induce antigen-driven T cell proliferation. These in vitro findings make it extremely unlikely for this virus to induce an anti-tumoral immunity against melanoma antigens and, indeed, clinical studies confirm this experimental in vitro finding [10]. We thus focused on the effects of MVA-infected apoptotic melanoma cells on immature DCs. We were able to demonstrate that the phagocytosis of MVA-infected, apoptopic melanoma cells by immature DCs was efficient. Compared to uninfected melanoma cells, even significantly higher levels of phagocytosis were found when MVA-infected melanoma cells were used. Surprisingly, MVA-infected melanoma cells induced an enhancement of the DCs capacity to induce T cell proliferation. As we never saw the activation of immature DCs by MVA infection, this maturation has to be ascribed to the virally infectedmelanoma cells. The maturation of the DCs in contact with MVA-infected melanoma cells was enhanced further by adding a cocktail of cytokines known to induce substantial maturation in immature DCs.
Apoptotic melanoma cells were shown to be suitable agents to load DCs in order to achieve the MHC-I and MHC-II restricted presentation of tumoral antigen and to induce anti-tumoral T cell immunity [18,42]. Thus, we wanted to test the ability of DCs, which have phagocytosed MVA-infected, apoptotic melanoma cells to induce a specific cytotoxic immune response by CD8+ T cells. We were able to show that the induction of TAA-specific T cells by DCs loaded with MVA-infected apoptotic melanoma cells exceeded the T cell response generated with DCs loaded with UV-B radiated, apoptopic melanoma cells. The T cell response was tested for MelanA specificity only, but due to our approach with whole MVA-infected melanoma cells preparations priming and activation of many more T cell clones is to be expected. This will be the subject of further studies. Further sets of experiments will also be required to elucidate why MVA-induced apoptosis appears more immunogenic than UVB-induced apoptosis.
Recapitulating, it ca be said that by using the attenuated MVA-strain instead of the WR strain it was possible to achieve efficient oncolysis of melanoma cells. Instead of suppressing a subsequent T cell response as in the case of WR, the application of the attenuated MVA strain showed an enhanced TAA-specific, anti-tumoral T cell response. The attenuated VV-strain MVA strain thus appears to us as a promising candidate for viral oncolysis with consecutive induction of a T cell anti-tumoral immunity, which could be enhanced even further by incorporation of TAA- and T cell-enhancing molecules [13].
Acknowledgments
We are grateful for the excellent technical assistance of Christiane Schwank. This work was sponsored by the Research Training Grant GK592 of the Deutsche Forschungsgemeinschaft (DFG), by intramural funding from the ELAN programme of the University Hospital Erlangen, and in part by the German Research Foundation (DFG) (project SCHU 1538 and SFB643-C1) and the European Union (DCVACC, contract no. 503037).
References
- 1.Lowes MA, Bishop GA, Crotty K, Barnetson RS, Halliday GM. T helper 1 cytokine mRNA is increased in spontaneously regressing primary melanomas. J Invest Dermatol. 1997;108:914–19. doi: 10.1111/1523-1747.ep12292705. [DOI] [PubMed] [Google Scholar]
- 2.van BP, Zhang Y, Chaux P, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 3.Bodurtha AJ, Berkelhammer J, Kim YH, Laucius JF, Mastrangelo MJ. A clinical histologic, and immunologic study of a case of metastatic malignant melanoma undergoing spontaneous remission. Cancer. 1976;37:735–42. doi: 10.1002/1097-0142(197602)37:2<735::aid-cncr2820370221>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Day CL, Jr, Lew RA, Mihm MC, Jr, et al. A multivariate analysis of prognostic factors for melanoma patients with lesions greater than or equal to 3.65 mm in thickness. The importance of revealing alternative Cox models. Ann Surg. 1982;195:44–9. doi: 10.1097/00000658-198201001-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon T, Coulie PG, Eynde BJ, Bruggen PV. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 6.Voit C, Kron M, Schwurzer-Voit M, Sterry W. Intradermal injection of Newcastle disease virus-modified autologous melanoma cell lysate and interleukin-2 for adjuvant treatment of melanoma patients with resectable stage III disease. J Dtsch Dermatol Ges. 2003;1:120–5. doi: 10.1046/j.1610-0387.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindenmann J, Klein PA. Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med. 1967;126:93–108. doi: 10.1084/jem.126.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim EM, Sivanandham M, Stavropoulos CI, Bartolucci AA, Wallack MK. Overview analysis of adjuvant therapies for melanoma − a special reference to results from vaccinia melanoma oncolysate adjuvant therapy trials. Surg Oncol. 2001;10:53–9. doi: 10.1016/s0960-7404(01)00020-2. [DOI] [PubMed] [Google Scholar]
- 9.Hersey P, Coates AS, McCarthy WH, et al. Adjuvant immunotherapy of patients with high-risk melanoma using vaccinia viral lysates of melanoma: results of a randomized trial. J Clin Oncol. 2002;20:4181–90. doi: 10.1200/JCO.2002.12.094. [DOI] [PubMed] [Google Scholar]
- 10.Roenigk HH, Jr, Deodhar S, St Jacques R, Burdick K. Immunotherapy of malignant melanoma with vaccinia virus. Arch Dermatol. 1974;109:668–73. [PubMed] [Google Scholar]
- 11.Mastrangelo MJ, Lattime EC. Virotherapy clinical trials for regional disease: in situ immune modulation using recombinant poxvirus vectors. Cancer Gene Ther. 2002;9:1013–21. doi: 10.1038/sj.cgt.7700538. [DOI] [PubMed] [Google Scholar]
- 12.Meyer RG, Britten CM, Siepmann U, et al. A phase I vaccination study with tyrosinase in patients with stage II melanoma using recombinant modified vaccinia virus Ankara (MVA-hTyr) Cancer Immunol Immunother. 2005;54:453–67. doi: 10.1007/s00262-004-0616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oertli D, Marti WR, Zajac P, et al. Rapid induction of specific cytotoxic T lymphocytes against melanoma-associated antigens by a recombinant vaccinia virus vector expressing multiple immunodominant epitopes and co-stimulatory molecules in vivo. Hum Gene Ther. 2002;13:569–75. doi: 10.1089/10430340252809856. [DOI] [PubMed] [Google Scholar]
- 14.Jenne L, Schuler G, Steinkasserer A. Viral vectors for dendritic cell-based immunotherapy. Trends Immunol. 2001;22:102–7. doi: 10.1016/s1471-4906(00)01813-5. [DOI] [PubMed] [Google Scholar]
- 15.Humrich J, Jenne L. Viral vectors for dendritic cell-based immunotherapy. Curr Top Microbiol Immunol. 2003;276:241–59. doi: 10.1007/978-3-662-06508-2_11. [DOI] [PubMed] [Google Scholar]
- 16.Jenne L, Hauser C, Arrighi JF, Saurat JH, Hugin AW. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 2000;7:1575–83. doi: 10.1038/sj.gt.3301287. [DOI] [PubMed] [Google Scholar]
- 17.Drexler I, Antunes E, Schmitz M, et al. Modified vaccinia virus Ankara for delivery of human tyrosinase as melanoma-associated antigen: induction of tyrosinase- and melanoma-specific human leukocyte antigen A*0201-restricted cytotoxic T cells in vitro and in vivo. Cancer Res. 1999;59:4955–63. [PubMed] [Google Scholar]
- 18.Jenne L, Arrighi JF, Jonuleit H, Saurat JH, Hauser C. Dendritic cells containing apoptotic melanoma cells prime human CD8+ T cells for efficient tumor cell lysis. Cancer Res. 2000;60:4446–52. [PubMed] [Google Scholar]
- 19.Larsson M, Fonteneau JF, Bhardwaj N. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 2001;22:141–8. doi: 10.1016/s1471-4906(01)01860-9. [DOI] [PubMed] [Google Scholar]
- 20.Sutter G, Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA. 1992;89:10847–51. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuting T, Wilson CC, Martin DM, et al. Autologous human monocyte-derived dendritic cells genetically modified to express melanoma antigens elicit primary cytotoxic T cell responses in vitro: enhancement by cotransfection of genes encoding the Th1- biasing cytokines IL-12 and IFN-alpha. J Immunol. 1998;160:1139–47. [PubMed] [Google Scholar]
- 22.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 23.Schuler-Thurner B, Schultz ES, Berger TG, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–88. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valmori D, Fonteneau JF, Lizana CM, et al. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–8. [PubMed] [Google Scholar]
- 25.Ploubidou A, Way M. Viral transport and the cytoskeleton. Curr Opin Cell Biol. 2001;13:97–105. doi: 10.1016/S0955-0674(00)00180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoine G, Scheiflinger F, Dorner F, Falkner FG. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998;244:365–96. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- 27.Thumann P, Moc I, Humrich J, et al. Antigen loading of dendritic cells with whole tumor cell preparations. J Immunol Meth. 2003;277:1–16. doi: 10.1016/s0022-1759(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 28.Webb HE, Smith CE. Viruses in the treatment of cancer. Lancet. 1970;1:1206–8. doi: 10.1016/s0140-6736(70)91790-3. [DOI] [PubMed] [Google Scholar]
- 29.Ries SJ. Elucidation of the molecular mechanism underlying tumor-selective replication of the oncolytic adenovirus mutant ONYX-015. Fut Oncol. 2005;1:763–6. doi: 10.2217/14796694.1.6.763. [DOI] [PubMed] [Google Scholar]
- 30.Aghi M, Martuza RL. Oncolytic viral therapies − the clinical experience. Oncogene. 2005;24:7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 31.Pfahlberg A, Kolmel KF, Grange JM, et al. Inverse association between melanoma and previous vaccinations against tuberculosis and smallpox: results of the FEBIM study. J Invest Dermatol. 2002;119:570–5. doi: 10.1046/j.1523-1747.2002.00643.x. [DOI] [PubMed] [Google Scholar]
- 32.Donahue JM, Mullen JT, Tanabe KK. Viral oncolysis. Surg Oncol Clin N Am. 2002;11:661–80. doi: 10.1016/s1055-3207(02)00025-x. [DOI] [PubMed] [Google Scholar]
- 33.Thorne SH, Kirn DH. Future directions for the field of oncolytic virotherapy: a perspective on the use of vaccinia virus. Exp Opin Biol Ther. 2004;4:1307–21. doi: 10.1517/14712598.4.8.1307. [DOI] [PubMed] [Google Scholar]
- 34.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–8. [PubMed] [Google Scholar]
- 35.Horig H, Lee DS, Conkright W, et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother. 2000;49:504–14. doi: 10.1007/s002620000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Mehren M, Arlen P, Tsang KY, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6:2219–28. [PubMed] [Google Scholar]
- 37.Yang S, Kittlesen D, Slingluff CL, Jr, Vervaert CE, Seigler HF, Darrow TL. Dendritic cells infected with a vaccinia vector carrying the human gp100 gene simultaneously present multiple specificities and elicit high-affinity T cells reactive to multiple epitopes and restricted by HLA-A2 and -A3. J Immunol. 2000;164:4204–11. doi: 10.4049/jimmunol.164.8.4204. [DOI] [PubMed] [Google Scholar]
- 38.Woodring PJ, Hunter T, Wang JY. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci. 2003;116:2613–26. doi: 10.1242/jcs.00622. [DOI] [PubMed] [Google Scholar]
- 39.Friedl P, Wolf K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem Soc Symp. :277–85. doi: 10.1042/bss0700277. [DOI] [PubMed] [Google Scholar]
- 40.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 41.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–4. doi: 10.1016/s0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 42.Berard F, Blanco P, Davoust J, et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J Exp Med. 2000;192:1535–44. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]