Abstract
Programmed cell death (apoptosis) is involved in glomerular injuries leading to glomerulonephritis. Bcl-2 and Fas are proteins that promote cell survival and death, respectively. This study tests the hypothesis that lupus nephritis is associated with alterations of Bcl-2 and Fas protein expression. Thirty-six patients with lupus nephritis and 10 controls (normal individuals) were included in this study. Bcl-2 and Fas positive cells were examined in kidney biopsies by immunohistochemistry. Bcl-2 and Fas serum levels were evaluated by enzyme-linked immunosorbent assay (ELISA). In the glomeruli of normal kidneys, Bcl-2 and Fas proteins were completely absent. In lupus nephritis patients, glomerular expression of Bcl-2 and Fas was seen in mesangial cells (1·3 ± 0·1 and 2·0 ± 0·1 for Bcl-2 and Fas, respectively). Similarly, a statistically significantly higher Bcl-2 (217·1 ± 85·9) and Fas (767·9 ± 271) serum levels were found in lupus patients compared to controls (148·6 ± 87, 550·3 ± 91 for Bcl-2 and Fas, P < 0·05). A direct correlation between serum Bcl-2 and Fas and chronicity index was also found. Compared to normal controls, lupus nephritis is associated with glomerular expression and elevated serum levels of Bcl-2 and Fas proteins. These findings suggest possible roles for Bcl-2 and Fas in glomerular injury during evolution of lupus nephritis. The diagnostic, prognostic and therapeutic ramifications of our findings are open to further investigation.
Keywords: Bcl-2, Fas, kidney, lupus, nephritis
Introduction
Programmed cell death (apoptosis) is a remodelling mechanism involved in both embryonic and adult kidneys [1]. Defective regulation of apoptosis plays a role in development of autoimmune diseases such as systemic lupus erythematosus (SLE) [2,3]. The hallmarks of SLE are polyclonal B cell activation and production of autoantibodies. Autoreactive B cells are found in healthy individuals and their numbers are regulated by apoptosis. In SLE, dysfunction of apoptosis can result in inappropriate longevity of autoreactive B cells and high autoantibody levels. Recent studies have revealed a link between autoimmunity and two molecules with central roles in apoptosis [2,4]. These are Bcl-2, which promotes cell survival, and Fas, a cell surface molecule that induces apoptosis. Bcl-2 protein contributes to checkpoints between cell surface and internal death signals, formation of apoptosome and activation of caspase cascade. Fas induces apoptosis when it binds to Fas ligand (Fas-L) [5,6].
The central role of Bcl-2 and Fas in SLE is supported by several observations: (i) transgenic mice over-expressing Bcl-2 show polyclonal B cell expansion and development of SLE-like autoimmune syndrome; (ii) mice carrying lpr disorders have defects in the Fas gene, do not express functional Fas molecules and suffer from an SLE-like autoimmune syndrome; and (iii) glomerular cells with apoptosis and positive Fas immunoreactivity are seen in proliferative glomerulonephritis [2,7]. Thus altered expression of Bcl-2 and Fas can result in SLE-like autoimmune disease in mice [7]. To date, our knowledge about alterations of apoptotic and survival proteins in lupus nephritis is still incomplete. Here, we hypothesize that lupus nephritis is associated with alterations of Bcl-2 and Fas protein expression. To test this hypothesis, we examined Bcl-2 and Fas proteins in lupus nephritis.
Materials and methods
Tissue specimens
This retrospective study included 36 renal biopsy specimens obtained from 36 patients with systemic lupus erythematosus. An additional 10 normal renal biopsies were obtained (control group, with comparable age and sex). Full clinical data were obtained from the patients' files. The classification of lupus nephritis, including chronicity index, was performed following World Health Organization (WHO) guidelines [8].
Evaluation of serum Bcl-2 and Fas levels
Renal function tests (blood urea and creatinine levels, creatinine clearance and serum beta 2 microglobulin (β2M) as well as 24-h protein excretion were determined. Serum Bcl-2 and Fas levels were assayed by quantitative enzyme-linked immunosorbent assay (ELISA) (IBL Hamburg ELISA kit for Bcl-2 and Apo 1/Fas/CD95 for Fas; Biosource International Inc., California, USA).
Immunohistochemical evaluation of Bcl-2 and Fas protein expression in renal tissue
Bcl-2 and Fas immunostaining was carried out as described previously [9]. Briefly, sections mounted on glass slides were deparaffinized and rehydrated through graded alcohols to water. Endogenous peroxidase activity was blocked with 0·6% H202. Sections were then immersed in the retrieval solution (10 mM sodium citrate buffer, pH 6·0) and subjected to heat-induced antigen retrieval for 20 min. The slides, in plastic Coplin jars containing retrieval solution, were microwaved in a microwave set at high (∼ 750 watts) for four cycles of 5 min duration each. Non-specific protein binding was blocked with 10 min exposure to 10% normal goat serum. Sections were then incubated with mouse monoclonal antibodies for 30 min at room temperature (clone 124, IgG1, kappa for Bcl-2; Dako Corporation, CA, USA) and G169 rabbit polyclonal IgG to Fas antigen (Santa Cruz Biotechnology, CA, USA). A catalysed signal amplification system (K1500; Dako) was used according to the manufacturer's instructions. Sections were next treated with peroxidase-labelled streptavidin for 30 min at room temperature and incubated with 14-diaminobenzidine and 0·06% H202 for 5 min. They were counterstained with haematoxylin, dehydrated in alcohol, cleared in xylene and cover-slipped. The slides were evaluated independently by the authors and counts were combined to give a final figure.
Positive controls
Specimens consisted of lymph nodes (reactive lymphoid hyperplasia) [9].
Negative controls
Additional sections, running in parallel but with omission of the primary antibody, served as the negative control [9].
Evaluation of Bcl-2 and Fas staining
Expression of Bcl-2 protein was identified as diffuse golden yellow cytoplasmic staining (Bcl-2). Alternatively, membranous and/or cytoplasmic reactivity identified Fas positive cells. The following strategy was used to evaluate positive cells: (i) cells were counted in serial sections, each section contained at least two glomeruli, and all the glomerular cells that were immunoreactive for Bcl-2 or Fas antigens were counted; (ii) cell counting was performed by two different observers; (iii) the population of positive cells was expressed as the number of reactive cells per glomerular cross-section; and (iv) results were expressed as mean ± standard error of mean (s.e.m.) [10].
Statistical analysis
Analyses of variance were used to evaluate the differences of the means. Linear regression was calculated to establish the correlation coefficients (spss software). The minimum level of significance was set at a P-value 0·05.
Results
Clinicopathological features
The mean age of the patients was 35 ± 6·0 years. The lesions were more common in females than in males (34 females and two males; 17:1·0 ratio). According to WHO classification, there were 22 patients with stages II–III (focal proliferative glomerulonephritis and glomerular mesangial hypercellularity involving centre of lobules away from vascular pole, mesangial matrix expansion but mainly patent capillaries; no significant tubular, interstitial or vascular changes). The remaining 14 cases had stage IV disease (diffuse proliferative glomerulonephritis, global proliferative lesions, necrosis, crescents in > 50% of glomeruli, variable sclerosis, prominent inflammatory interstitial infiltrate and wire loop lesions in thickened capillary walls). The clinical features included hypertension (20 cases); fever (20 cases); arthritis (26 cases); arthralgia (16 cases); and proteinuria (24 cases). The levels of β2M and creatinine were higher in lupus patients compared with the control group. A summary of the clinical features is shown in Table 1.
Table 1.
Clinicopathological features of lupus nephritis and alterations of serum Fas- and Bcl-2 proteins.
| Aspect | Serum Bcl-2 (ng/ml) mean ± s.e.m. | Serum Fas (pg/ml) mean ± s.e.m. |
|---|---|---|
| Blood pressure | ||
| BP < 90/140 (n = 16) | 231 ± 37 | 657 ± 360 |
| BP > 90/140 (n = 20) | 281 ± 99 | 136 ± 43 |
| Clinical features | ||
| Fever (n = 20) | 241 ± 70 | 699 ± 267 |
| Arthritis (n = 26) | 234 ± 70 | 742 ± 307 |
| Arthralgia (n = 16) | 268·9 ± 105 | 772 ± 246 |
| Raynaud (n = 36) | 218 ± 62 | 366 ± 129 |
| Proteinuria | ||
| Protein 24 h in urine | ||
| < 3·5 g/24 h (n = 12) | 185·2 ± 59 | 582·2 ± 284 |
| 3·5 g/24 h (n = 12) | 294·8 ± 62 | 860·8 ± 221 |
s.e.m.: Standard error of the mean.
Glomerular expression of Bcl-2 and Fas proteins in lupus nephritis
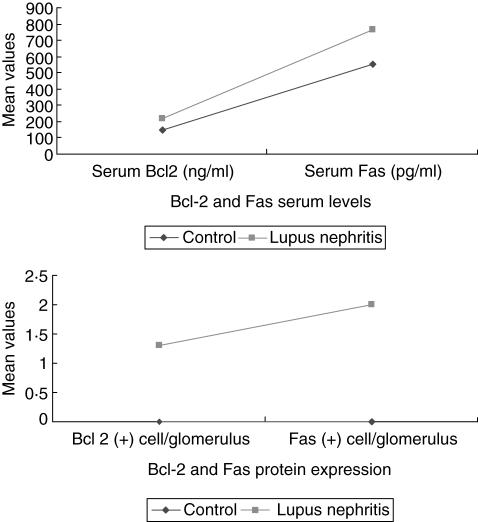
The type of cells that showed positive staining with Fas- and Bcl-2 was evaluated according to their localization or morphology. In normal kidneys (control group), Bcl-2 and Fas proteins were absent in the glomeruli. Tissues from lupus nephritis patients showed various numbers of glomerular cells (mesangial cells, 1·3 ± 0·1) positive for Bcl-2 antigen. Fas-antigen-positive cells were seen in the mesangial area and within the glomerular capillary walls (polymorphs and mesangial cells, 2·0 ± 0·1). Higher Bcl-2 and Fas protein expression values were found in biopsies from patients with increased blood pressure, proteinuria and higher activity indices. A summary of these results is shown in Tables 1 and 2 and Fig. 1.
Table 2.
Renal impairment and alteration of Fas-, Bcl-2 proteins in lupus nephritis.
| Aspect | Control | Lupus nephritis | P-value |
|---|---|---|---|
| Creatinine clearance | 104 ± 1·0 | 103·3 ± 1·7 | 0·05 |
| β2M (ug/ml) | 0·2 ± 0·1 | 4·2 ± 1·9 | < 0·000 |
| 24 h protein in urine g/24 h | 0·5 ± 0·1 | 1·7 ± 0·5 | < 0·000 |
| Serum Bcl-2 (ng/ml) | 148·6 ± 87 | 217·1 ± 85·9 | < 0·05 |
| Serum Fas (pg/ml) | 550·3 ± 91 | 767·9 ± 271 | < 0·05 |
| Bcl 2 (+) cell/glomerulus | 0·0 ± 0·0 | 1·3 ± 0·1 | < 0·000 |
| Fas (+) cell/glomerulus | 0·0 ± 0·0 | 2·0 ± 0·1 | < 0·000 |
Fig. 1.
Alterations of serum Fas- and Bcl-2 proteins in lupus nephritis. Compared to the control group (normal individuals), higher Bcl-2 and Fas protein expression values were found in biopsies from patients with lupus nephritis. Similarly, higher serum Bcl-2 and Fas values were observed in lupus nephritis.
Increased serum levels of Bcl-2 and Fas proteins in lupus nephritis
Compared to the control group, patients with lupus nephritis have significantly higher serum levels of Bcl-2 (148·6 ± 87 versus 217·1 ± 85·9) and Fas (550·3 ± 91 versus 767·9 ± 271). Higher serum Bcl-2 and Fas levels were seen in patients with increased blood pressure and proteinuria. A summary of these results is shown in Tables 1 and 2 and Fig. 1.
Correlation between chronicity index and serum Bcl-2 and Fas proteins
A direct correlation was seen between proteinuria, serum Fas levels and pathogenic mechanisms of glomerular damage according to the WHO classification. Similarly, a direct correlation was found between chronicity index and serum Fas and Bcl-2 protein expression values, β2M, creatinine clearance and proteinuria. A summary of these results is shown in Table 3.
Table 3.
Correlation between the alterations of Fas-, Bcl-2 proteins and pathological features in lupus nephritis.
| Lupus nephritis WHO classification | Lupus nephritis chronicity index | |||
|---|---|---|---|---|
| hr/ | hr/ | |||
| r-value | P-value | r-value | P-value | |
| Creatinine clearance | 0·3 | 0·7 | 0·7 | 0·4 |
| β2M (ug/ml) | 0·3 | 0·1 | 0·7 | 0·009 |
| 24 h protein in urine g/24 h | 0·5 | 0·4 | 0·5 | 0·8 |
| Bcl 2 (+) cell/glomerulus | 0·3 | 0·1 | 0·3 | 0·3 |
| Fas (+) cell/glomerulus | 0·3 | 0·1 | 0·3 | 0·3 |
| Serum Bcl-2 (ng/ml) | 0·3 | 0·2 | 0·6 | 0·007 |
| Serum Fas (pg/ml) | 0·5 | 0·2 | 0·3 | 0·1 |
Discussion
In glomerulonephritis, apoptotic cell death affects mesangial cells, glomerular-tuft endothelial cells, leucocytes and macrophages [1]. Two important molecules are involved in this process: Bcl-2 and Fas, that promote cell survival and death, respectively. To date, our knowledge about the expression pattern of these molecules in lupus nephritis and its correlation with clinicopathological features is still incomplete. In this investigation, we hypothesized that lupus nephritis is associated with alterations of Bcl-2 and Fas protein expression. To test this hypothesis, we carried out this investigation. To accomplish our goals, we examined 36 patients with lupus nephritis and 10 controls (normal individuals). Bcl-2 and Fas-positive cells were examined in renal tissue by immunoperoxidase staining methods. Bcl-2 and Fas serum levels were detected by ELISA. Our study revealed three observations in lupus nephritis patients: (i) glomerular expression of Bcl-2 and Fas proteins; (ii) increased serum levels of Bcl-2 and Fas proteins; and (iii) a direct correlation between chronicity index and serum Bcl-2 and Fas levels.
Glomerular expression of Bcl-2 and Fas proteins in lupus nephritis
The glomerular expression of Bcl-2 and Fas proteins versus its absence in normal kidneys suggests a possible use of Bcl-2/Fas as diagnostic and prognostic markers. The mesangial cells are modified myofibroblasts that support the glomerular tuft and regulate capillary width and blood flow. These cells are phagocytic in nature and have a remarkable proliferative power. The glomerular expression of Bcl-2 in the mesangial cells of lupus nephritis patients is in agreement with previous investigations [1,10,11]. Bcl-2 protein (prosurvival factor) may play a role in the prolonged proliferation of mesangial cells with subsequent mesangial hypercellularity and matrix expansion, i.e. proliferative glomerulonephritis. Also, Bcl-2 expression may help to maintain glomerular hypercellularity by prevention of mesangial cell death. In support, in crescentic glomerulonephritis, Bcl-2 positive cells are seen frequently [12]. To date the therapeutic interventions directed at Bcl-2 that regulate apoptosis in renal disease are unknown. A hypothesis to be tested is that mesangial cellularity and matrix expansion in glomerulonephritis may be the outcome of interplay between apoptotic and survival molecules.
In our investigation, Fas expression in mesangial cells of lupus nephritis patients concurs with previous reports and suggests involvement of the Fas system in the regulation of renal cell apoptosis. During renal injury, renal cells express the Fas ligand and activation of the Fas receptor promotes apoptosis of these cells [1,10,11]. Fas agonists can induce glomerular injury with subsequent mesangial cell proliferation. In glomerulonephritis, Fas antagonists can regulate the Fas system.
Increased serum levels of Bcl-2 and Fas proteins in lupus nephritis
Increased serum levels of Bcl-2 and Fas proteins in lupus nephritis patients is in agreement with previous studies [13–16]. This increase may be reasoned to be the occurrence of circulating T and B lymphocytes with abnormally high Bcl-2 and Fas expression [13]. In harmony with these findings, examination of both T and B cells from SLE patients showed overexpression of Bcl-2 and Fas messenger RNA [17–19]. The higher serum Bcl-2 and Fas levels in patients with active disease than in those with inactive disease may be due to maintained activity of lymphocytes in the former. Freshly isolated lymphocytes from patients with active SLE expressed more Fas antigen than did lymphocytes from patients with inactive SLE [20]. The direct correlation between Bcl-2 and Fas protein levels and chronicity index suggest a common mechanism controlling the expression of these molecules and the development of pathological changes in lupus nephritis.
Here we report glomerular expression of Bcl-2 and Fas proteins and their elevated serum levels in patients with lupus nephritis. The co-expression of Fas (apoptotic) and Bcl-2 (anti-apoptotic) proteins suggests that both apoptosis and proliferation are contributory processes in lupus nephritis. The possible diagnostic, therapeutic and prognostic ramifications of these findings await further investigation.
References
- 1.Takemura T, Murakami K, Miyazato H, et al. Expression of Fas antigen and Bcl-2 in human glomerulonephritis. Kidney Int. 1995;48:1886–92. doi: 10.1038/ki.1995.487. [DOI] [PubMed] [Google Scholar]
- 2.Teh SJ, Dutz JP, Motyka B, et al. Fas (CD95)-independent regulation of immune responses by antigen-specific CD4-CD8+ T cells. Int Immunol. 1996;8:675–81. doi: 10.1093/intimm/8.5.675. [DOI] [PubMed] [Google Scholar]
- 3.Sano H, Asano K, Minatoguchi S, et al. Plasma soluble fas and soluble fas ligand in chronic glomerulonephritis. Nephron. 1998;80:153–61. doi: 10.1159/000045159. [DOI] [PubMed] [Google Scholar]
- 4.Fournie G. Ann Med Interne (Paris. 1996;147:472–9. Cell death and lupus. [PubMed] [Google Scholar]
- 5.Hussein MR, Haemel AK, Wood GS. Apoptosis and melanoma: molecular mechanisms. J Pathol. 2003;199:275–88. doi: 10.1002/path.1300. [DOI] [PubMed] [Google Scholar]
- 6.Hussein MR. Apoptosis in the ovary: molecular mechanisms. Hum Reprod Update. 2005;11:162–77. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- 7.Rose LM, Latchman DS, Isenberg DA. Bcl-2 and Fas, molecules which influence apoptosis. A possible role in systemic lupus erythematosus. Autoimmunity. 1994;17:271–8. doi: 10.3109/08916939409010667. [DOI] [PubMed] [Google Scholar]
- 8.Kewalramani R, Singh AK. Immunopathogenesis of lupus and lupus nephritis: recent insights. Curr Opin Nephrol Hypertens. 2002;11:273–7. doi: 10.1097/00041552-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hussein MR, Bedaiwy MA, Falcone T. Analysis of apoptotic cell death, Bcl-2, and p53 protein expression in freshly fixed and cryopreserved ovarian tissue after exposure to warm ischemia. Fertil Steril. 2006;85(1):1082–92. doi: 10.1016/j.fertnstert.2005.10.020. Suppl. [DOI] [PubMed] [Google Scholar]
- 10.Uguz A, Gonlusen G, Ergin M, et al. Expression of Fas, Bcl-2 and p53 molecules in glomerulonephritis and their correlations with clinical and laboratory findings. Nephrology (Carlton. 2005;10:311–6. doi: 10.1111/j.1440-1797.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 11.Uda S, Yoshimura A, Sugenoya Y, et al. Mesangial proliferative nephritis in man is associated with increased expression of the cell survival factor, Bcl-2. Am J Nephrol. 1998;18:291–5. doi: 10.1159/000013353. [DOI] [PubMed] [Google Scholar]
- 12.Nitta K, Horita S, Honda K, et al. Glomerular expression of cell-cycle-regulatory proteins in human crescentic glomerulonephritis. Virchows Arch. 1999;435:422–7. doi: 10.1007/s004280050420. [DOI] [PubMed] [Google Scholar]
- 13.Aringer M, Wintersberger W, Steiner CW, et al. High levels of bcl-2 protein in circulating T lymphocytes, but not B lymphocytes, of patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37:1423–30. doi: 10.1002/art.1780371004. [DOI] [PubMed] [Google Scholar]
- 14.Baima B, Sticherling M. Apoptosis in different cutaneous manifestations of lupus erythematosus. Br J Dermatol. 2001;144:958–66. doi: 10.1046/j.1365-2133.2001.04182.x. [DOI] [PubMed] [Google Scholar]
- 15.Miret C, Font J, Molina R, et al. Relationship of oncogenes (sFas, Bcl-2) and cytokines (IL-10, alfa-TNF) with the activity of systemic lupus erythematosus. Anticancer Res. 2001;21:3053–9. [PubMed] [Google Scholar]
- 16.Kacprzyk F. Pol Arch Med Wewn. 2002;108:843–7. Serum level and urinary excretion of soluble Fas (sFas) in patients with primary glomerulopathies. [PubMed] [Google Scholar]
- 17.Huang QR, Morris D, Manolios N. Evaluation of the BCL-2 gene locus as a susceptibility locus linked to the clinical expression of systemic lupus erythematosus (SLE. Rheumatol Int. 1996;16:121–4. doi: 10.1007/BF01409984. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Konuma T, Yanagisawa N, et al. Fas-Fas ligand system in the peripheral blood of patients with renal diseases. Nephron. 2000 doi: 10.1159/000045642. [DOI] [PubMed] [Google Scholar]
- 19.Yang B, Johnson TS, Thomas GL, et al. A shift in the Bax/Bcl-2 balance may activate caspase-3 and modulate apoptosis in experimental glomerulonephritis. Kidney Int. 2002;62:1301–13. doi: 10.1111/j.1523-1755.2002.kid587.x. [DOI] [PubMed] [Google Scholar]
- 20.Ohsako S, Hara M, Harigai M, et al. Expression and function of Fas antigen and bcl-2 in human systemic lupus erythematosus lymphocytes. Clin Immunol Immunopathol. 1994;73:109–14. doi: 10.1006/clin.1994.1176. [DOI] [PubMed] [Google Scholar]