Abstract
Myasthenia gravis (MG) is a debilitating and potentially fatal neuromuscular disease characterized by the generation of autoantibodies reactive with nicotinic acetylcholine receptors (AChR) that cause loss of AChR from the neuromuscular endplate with resultant failure of neuromuscular transmission. A role for complement (C) in the pathology of human MG has been suggested based upon identification of C activation products in plasma and deposited at the endplate in MG. In the rat model, experimental autoimmune MG (EAMG), C depletion or inhibition restricts clinical disease, further implicating C in pathology. The mechanisms by which C activation drives pathology in MG and EAMG are unclear. Here we provide further evidence implicating C and specifically the membrane attack complex (MAC) in the Lewis rat passive EAMG model of MG. Rats deficient in C6, an essential component of the MAC, were resistant to disease induction and endplate destruction was reduced markedly compared to C6-sufficient controls. After reconstitution with C6, disease severity and endplate destruction in the C6-deficient rats was equivalent to that in controls. The data confirm the essential role of the MAC in the destruction of the endplate in EAMG and raise the prospect of specific MAC inhibition as an alternative therapy in MG patients resistant to conventional treatments.
Keywords: autoimmunity, complement, inflammation, neuroimmunology, rodent
Introduction
Myasthenia gravis (MG) is a debilitating and potentially life-threatening condition characterized by episodes of profound muscle weakness [1]. MG is a classical ‘organ-specific’ autoimmune disease [2,3]. Autoantibodies against the acetylcholine receptor (AChR) are generated that bind AChR at the neuromuscular junction (endplate) and cause blockade of neuromuscular transmission, resulting in the severe muscle weakness that typifies the condition [4]. The anti-AchR antibodies are not directly blocking, but instead recruit other effectors to cause endplate damage. Complement (C) was implicated over 40 years ago with the demonstration of C consumption in myasthenic patients [5]. Engel and co-workers identified dense localized deposits of C proteins at the endplate in muscle biopsies from MG patients [6], provoking the suggestion that C activation was directly responsible for the destruction of the endplate. The abundance of membrane attack complex (MAC) at this site provoked the suggestion that ‘microlysis’ caused by MAC deposition and lytic pore formation was the cause of the observed destruction [7].
Rodent models of MG have provided a number of important insights into the disease. Experimental autoimmune MG (EAMG) in rats and mice can be induced either actively by immunizing with AChR from the torpedo ray, or passively by administering pre-formed antibodies against the AChR [6,8,9]. In the passive model, several sources of antibody have been used, including MG patient serum, serum from rodents actively induced for EAMG and monoclonal antibodies (mAb) targeting the AChR [10]. Early studies in active and passive EAMG in the rat demonstrated C activation in muscle resembling that found in human MG [6]. Direct evidence implicating C in pathogenesis came from studies utilizing cobra venom factor (CVF) treatment of rats to inactivate C [8]. The CVF-treated rats were resistant to disease whether induced actively or passively, suggesting that C activation was a critical event in the development of pathology. CVF is a crude tool that removes C activity by activation to completion with resultant generation ofproinflammatory mediators that may influence disease induction. However, these data were replicated in EAMG using the specific C inhibitor soluble C receptor 1 (sCR1), confirming the direct role of C in this model [11].
Although a role for the MAC has been suggested for human MG, the evidence is at best circumstantial − the abundance of MAC deposits at the endplate. C activation generates a number of biologically active products, including the anaphylactic peptides C3a and C5a, the opsonic fragments C3b and C4b and the lytic MAC; any or all of these may contribute to pathology in disease [12]. If anti-C therapies are to be maximally effective it is important to know which of the C-derived effectors are driving pathology. Animals deficient in individual C components provide a powerful tool for dissecting out the importance of the various effectors. Rats deficient in C6 were discovered by chance in commercial PVG rat colonies and have been used to provide evidence for a role of the MAC in several disease models [13]. We showed that C6-deficient PVG rats were resistant to disease in a rat model of multiple sclerosis, implicating the MAC in myelin injury and loss [14]. To extend the utility of the C6-deficient rats for use in disease models we have back-crossed eight generations onto Lewis, a strain that has been used extensively in models of autoimmunity. Here we have tested these rats in a mAb-driven passive EAMG model. Whereas C6-sufficient (wild-type) Lewis rats developed a severe clinical disease characterized by profound weakness and weight loss, rats deficient in C6 were completely resistant to disease induction. Endplate destruction and muscle inflammatory cell infiltration were minimal in the absence of C6. C3 deposition at the endplate was unaffected by C6 deficiency, but C9 deposition was seen only in wild-type rats with EAMG. Restoring C activity by administering human C6 rendered C6-deficient rats susceptible to disease. These findings confirm an essential role for the MAC in endplate destruction in EAMG and suggest that targeted inhibition of MAC formation will be of benefit in human MG.
Methods
General reagents and antibodies
All chemicals, unless stated otherwise, were from Sigma Aldrich Chemical Company (Gillingham, UK) or Fisher Scientific UK Ltd (Loughborough, UK).
Polyclonal goat anti-rat C3c was from Nordic Laboratories (Copenhagen, Denmark) and was used at a dilution of 1:400; polyclonal sheep anti-human C9 was generated in-house and was used at a dilution of 1:400. Mouse anti-rat CD68 mAb was from Serotec (Oxford, UK) and was used at a final concentration of 2 µg/ml. Donkey anti-mouse IgG fluorescein isothiocyanate (FITC) and donkey anti-goat/sheep IgG FITC were from Jackson Immunoresearch (Luton, UK) and were used at 1:200. α-Bungarotoxin–rhodamine conjugate (T-1175) was from Molecular Probes (Invitrogen, Paisley, UK).
The rat hybridoma TIB-175, secreting the anti-AChR mAb35 [15], was obtained from the American Tissue Culture Collection (ATCC). The hybridoma was maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal calf serum (FCS, heat inactivated), 2 mm l-glutamine and 1 mm sodium pyruvate. The mAb35, produced against Electrophorus electricus electric organ muscle-type nicotinic AChR, binds the main immunogenic region (MIR) of the alpha subunit of the AChR and cross-reacts with chicken, rat, mouse and human AChR. The IgG1 mAb was purified from tissue culture supernatant on Protein A Sepharose using a protocol modified for purification of rat IgG1 with minimal bovine IgG contamination. Rat IgG1 is known to be a C-activating isotype and this was confirmed in our laboratory for mAb35 (unpublished data).
Human C6 was purified from plasma using an affinity column comprising an in-house mAb against C6 immobilized on sepharose. Purity and function were confirmed using standard methods.
Animals
C6-deficient PVG rats were originally obtained from Banting and Kingman Universal Inc. (Fremont, CA, USA) and a colony maintained in-house. C6-deficient PVG rats were back-crossed onto Lewis for eight generations. Control C6-sufficient rats were either littermates from the final back-cross or were obtained from Bantin & Kingman (Hull, UK). All animals were maintained according to Home Office guidelines within the Biomedical Services Unit (BSU) at Cardiff University.
Passive induction of EAMG
All animal studies were reviewed and approved by the UK Home Office. To assess the dose of mAb35 required for disease induction, groups of two female wild-type Lewis rats (160–200 g) were given 0·1 mg/kg, 0·5 mg/kg or 1 mg/kg mAb35 intraperitoneally (i.p.) in phosphate-buffered saline (PBS) under restraint. Animals were assessed for onset of EAMG at frequent intervals over 48 h and scored for weakness and weight loss as described below.
For testing effects of C6 deficiency, six female Lewis rats (160–200 g) were obtained from Bantin & Kingman and allowed to acclimatize for 1 week. Five female C6-deficient Lewis rats (160–200 g) were obtained from BSU. All animals were injected with 1 mg/kg mAb35 in PBS (i.p.) on day 0, and assessed for changes in weight and clinical score as described below.
To assess the effects of adding back C6 in C6-deficient rats, two separate experiments were performed, In the first, two matched groups, each comprising six female C6-deficient rats, were injected with 1 mg/kg mAb35 in PBS (i.p.); one group were additionally administered human C6 (8 mg/kg in PBS) in the same i.p. injection. In the second, matched groups of six female C6-deficient rats were injected with mAb35 as in the first experiment and human C6 (10 mg/kg in PBS) was given with the initiating mAb and a second dose i.p. after 12 h. Animals were assessed for weight loss and clinical score as described below. Animals were scored according to their ability to grasp and lift the lid of a mouse cage: 0, no disease; 1, reduced grip strength in front paws (can grip but cannot lift); 2, loss of grip in front paws; 3, loss of grip and hind limb weakness and wasting; 4, loss of grip and hind limb paralysis. Half scores were given for intermediate symptoms. Animals were also weighed at frequent intervals and were killed by a Schedule 1 method when weight loss was equal to or exceeded 20% of original body weight, or when clinical score reached 4. After the onset of clinical symptoms, rats were given pre-wetted food daily and assessed every 12 h.
Assessment of haemolytic C in C6-deficient rats reconstituted with human C6
C6-deficient rats in each of the two C6 add-back experiments were bled by tail tipping before and at intervals after i.p. administration of human C6. Serum was promptly separated and stored at −40°C until assay. Antibody-sensitized sheep erythrocytes (EA) were prepared using standard protocols and haemolytic sensitivity titred using normal rat serum. C6-deficient rat serum was without haemolytic activity in this assay. To assess the C activity in sera from rats given human C6, the minimum dose of normal rat serum causing 90–100% lysis of EA was identified from the titration curve and the test sera used at this dilution in the assay. The percentage haemolysis for each serum sample was measured and results expressed as a percentage of the haemolysis caused by normal rat serum in the same assay.
Collection and processing of tissues for immunohistochemical analysis
Rats were euthanized and the soleus muscle removed and snap-frozen in isopentane on dry ice and stored at −80°C. Frozen tissues were embedded in octreotide (OCT) medium (Agar Scientific, Stanstead, Essex, UK), sectioned longitudinally in a cryostat at 8–10 µm and transferred to Superfrost slides (Surgipath, Peterborough, UK). Sections were fixed in acetone for 5 min before storing at −20°C.
α-Bungarotoxin–rhodamine conjugate (T-1175) was used to identify the AChR, and provided a tool with which to evaluate the numbers of α-bungarotoxin-reactive AChR from comparable sections from each animal. α-Bungarotoxin–rhodamine was diluted 1:200 in block buffer [PBS, 1% bovine serum albumin (BSA)], applied to sections and incubated for 40 min at room temperature in a humid chamber. Sections were washed in PBS and mounted using VectorShield for analysis using a fluorescent microscope.
The area occupied by α-bungarotoxin-reactive AChR in each section was measured using density slicing in an image analysis system (Openlab Improvision, Coventry, UK). Twenty fields were captured from comparable regions of muscle in each sample at the same exposure and magnification.
To detect C deposition, frozen sections were stained for 1 h at room temperature with primary antibodies at the stated dilutions in block buffer. Sections were washed and incubated with appropriate secondary antibodies for 40 min at room temperature. To detect macrophage infiltration, frozen sections were stained for CD68 using clone ED1. Numbers of ED1-positive cells were counted in three fields from each animal and mean cell number per field calculated. Sections were washed and mounted in VectorShield before analysis.
For haemotoxylin and eosin (H&E) staining, muscle was post-fixed in 10% formaldehyde in PBS and embedded for sectioning and staining. Inflammatory cell infiltration was scored blind in H&E-stained sections using a semiquantitative method: 0, no inflammatory infiltrates; 1, infiltrates < 1 per low power field; 2, infiltrates 1–5 per field; 3, infiltrates 6–10 per field; 4, infiltrates > 10 per field.
Statistical analysis
For analysis of differences between two groups, Student's t-test was used. Where more than two groups were compared, the analysis of variance (anova) test was applied with post-hoc analysis using the t-test with Bonferroni correction. Statistical analysis utilized the InStat package (GraphPad, San Diego, CA, USA). Results were considered significantly different when P < 0·05.
Results
Induction of EAMG and requirement for C6
In dosing studies, rats administered mAb35 at 0·1 mg/kg developed only very mild clinical disease and no weight loss; in contrast, rats given 0·5 mg/kg mAb35 developed moderate weakness with a maximum mean clinical score of 1·5, while animals given 1 mg/kg mAb35 developed severe weakness and weight loss (data not shown). As the anticipated effect of C6 deficiency was protection from disease, the 1 mg/kg dose was chosen for subsequent studies.
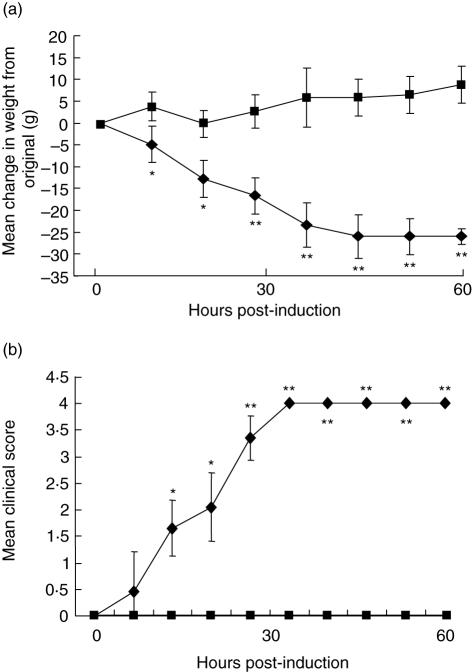
In the definitive experiment, all wild-type rats began to lose weight consistently by 20 h post-induction; in contrast, C6-deficient animals were stable or continued to gain weight over the time-course of the experiment; weight differences were significantly different (P < 0·05–P < 0·005) between the groups at all time-points (Fig. 1a). In this experiment, wild-type animals began to display clinical symptoms comprising limp tails, pilo-erection and reduced grip strength by 20 h post-induction, and all exhibited severe disease with hind limb weakness and/or partial paralysis by 41 h; in contrast, all C6-deficient animals were protected from any clinical manifestations of EAMG for the duration of the experiment, there was no measurable decrease in grip strength and the general condition remained unaltered; clinical score was significantly different (P < 0·05–P < 0·005) between the groups at all time-points except at 6 h (Fig. 1b).
Fig. 1.
C6 deficiency protects from clinical disease in the passive experimental autoimmune MG (EAMG) model. Age- and weight-matched groups of C6-deficient (n = 5; squares) and wild-type (n = 6; diamonds) female rats were administered anti-acetylcholine receptor (AChR) at time 0. Weight at time 0 was 230 ± 5 g for the C6-deficient rats (mean ± s.d.) and 234 ± 6·5 g (mean ± s.d.) for wild-type controls. Animals were assessed for change in weight (a) and clinical score (b) as described in Methods. Bars represent ± 1 s.d. Asterisks indicate significant differences between the groups (*P < 0·05; **P < 0·01).
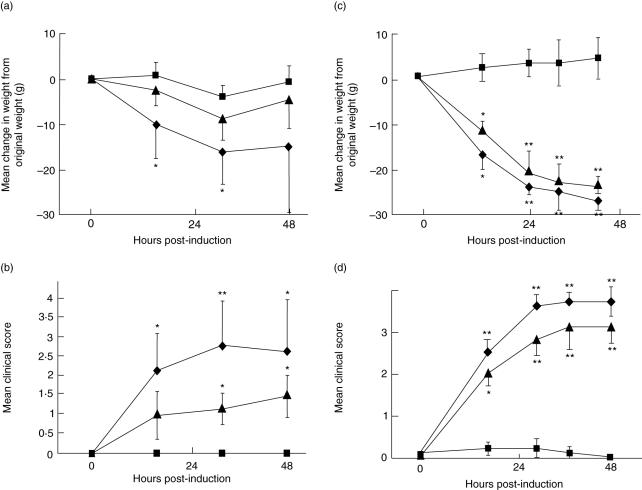
In the first C6 add-back experiment, rats deficient in C6 were again refractory to disease induction, while rats given a single dose of human C6 at the time of disease induction developed clinical disease that closely resembled that in wild-type rats, albeit less severe, with progressive weakness and weight loss (Fig. 2a,b). Statistical analysis confirmed that, for both parameters, there were significant differences between C6 reconstituted rats and C6-deficient controls at all times post-induction (P < 0·05–P < 0·01). Weight loss was not significantly different between C6 reconstituted and wild-type rats (P > 0·05), but clinical scores were significantly higher in the latter group at all time-points post-induction (P < 0·05). Because clinical disease was less severe in reconstituted rats than in wild-types, a second experiment was performed with an increased dose of C6 at induction and a second dose given after 12 h. In this experiment, clinical disease in C6-deficient rats given this higher dose of human C6 was even more severe and not different from that in wild-type rats (P > 0·05 at all time-points; Fig. 2c,d).
Fig. 2.
Administration of human C6 renders C6-deficient rats susceptible to disease. In two separate experiments, age- and weight-matched groups of six wild-type and C6-deficient female Lewis rats were administered anti-acetylcholine receptor (AChR). In each experiment, wild-type (diamonds), C6-deficient rats without supplementary C6 (squares) and C6-deficient rats with supplementary C6 (triangles) were compared. Animals were assessed for weight change (a,c) and clinical score (b,d) as described in Methods. In experiment 1 (a,b), a single dose (8 mg/kg) of human C6 was given at t = 0; in experiment 2 (c,d), two doses (10 mg/kg each) of humans C6 were given at t = 0 and t = 12 h. Bars represent ± 1 s.d. Where error bars overlapped, only unidirectional bars are shown for clarity. Asterisks indicate significant differences between experimental and control groups (*P < 0·05; **P < 0·01).
Haemolytic activity of C6-deficient rats following C6 reconstitution
C6-deficient rats had no measurable C haemolytic activity in the assay. Administration of human C6 at 8 mg/kg p.i. at the time of induction restored serum haemolytic activity to an average of 53 ± 7% of wild-type control at 24 h and 48 ± 8·5% at 48 h, demonstrating that this dose of C6 caused a partial but long-lived restoration of activity. Administration of human C6 at 10 mg/kg p.i. at time of induction, repeated at 12 h, caused essentially complete restoration of haemolytic activity compared to controls (mean 87 ± 4% at 24 h; 81% ± 5·5% at 48 h). All values are means ± standard deviation (s.d.) from six animals. Differences in haemolytic activities in C6 reconstituted groups compared to C6-deficient controls were highly significant at both time-points in both experiments (P < 0·01). In the second experiment, haemolytic activities in C6 reconstituted rats were not significantly different to those in wild-type controls (P > 0·05).
C6 deficiency inhibits endplate destruction in EAMG
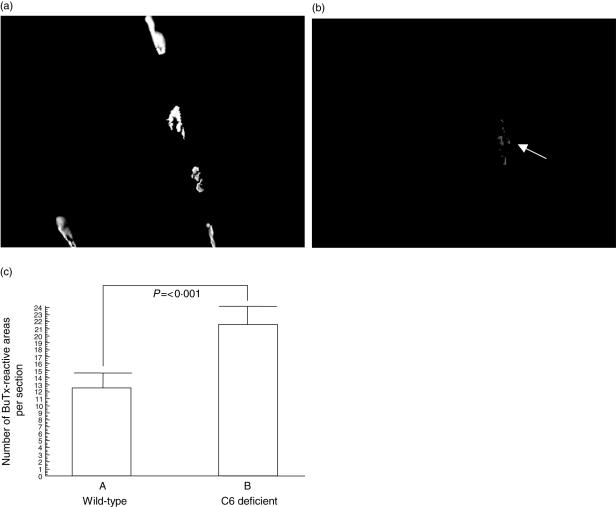
Endplates were quantified by measuring α-bungarotoxin–rhodamine staining in muscle sections. At time of killing in the EAMG studies, wild-type animals had fewer α-bungarotoxin-reactive endplates compared with C6-deficient animals, and this difference was highly significant (P < 0·0001) (Fig. 3a–c).
Fig. 3.
Loss of acetylcholine receptor (AChR) following experimental autoimmune MG (EAMG) induction requires C6. Soleus muscles were harvested from each rat, frozen sections cut and stained with α-bungarotoxin–rhodamine to identify AChR. Plates show representative fields from C6-deficient (a) and wild-type (b) rats. Using OpenLab imaging software, intensity limits were set, corresponding to positively stained AChR, and the image was density-sliced. The number of discrete areas was measured automatically on the density-sliced image. Twenty fields from each rat were analysed as described in Methods and the mean number of discrete areas was obtained for each rat (c). Columns represent averages for the wild-type (n = 6) and C6-deficient (n = 5) rats and bars show standard deviation for each group. The significance of the difference between groups is shown.
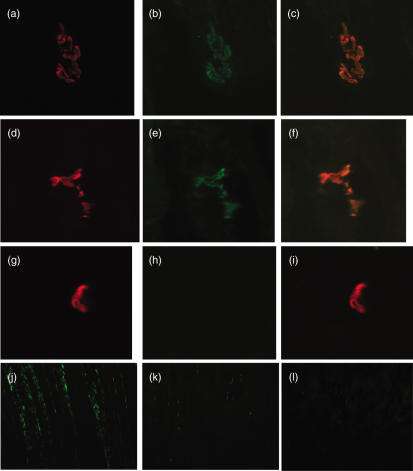
C activation at the endplate was assessed by measuring deposition of C3 and C9, the latter a surrogate marker of the MAC. C3 deposition was found co-localized with α-bungarotoxin-positive endplates in all animals subjected to induction of EAMG, whether wild-type or C6-deficient (Fig. 4a–f). In contrast, α-bungarotoxin-positive endplates in naive rats were negative for C3 staining (Fig. 4g–i).
Fig. 4.
Assessment of C3 deposition at the acetylcholine receptor (AChR) and muscle inflammation in wild-type, C6-deficient and naive Lewis rats. Soleus muscle from wild-type (a–c) and C6-deficient (d–f) Lewis rats induced for experimental autoimmune MG (EAMG) and a naive rat (g–i) was harvested and flash-frozen in isopentane as described. Ten μm thin sections were cut and stained for AChR (a, d, g) using rhodamine-conjugated α-bungarotoxin (red staining). Sections were double-stained for activated C3 (C3c; b, e, h) using goat anti-rat C3c, and detected with donkey anti-goat-Ig-fluorescein isothiocyanate (FITC) conjugate (green staining). Sections were mounted in VectorShield and analysed under an inverted fluorescent microscope. Images were merged for detection of co-localization (orange staining; c, f, i). Images were taken at 400× magnification and magnified a further twofold electronically. For assessment of inflammatory cell infiltration, frozen soleus muscle sections from wild-type (j) and C6-deficient (k) Lewis rats induced for EAMG and a naive rat (l) were stained with mouse anti-rat CD68 (ED1) to identify macrophages within the muscle. Images were taken at 200× magnification
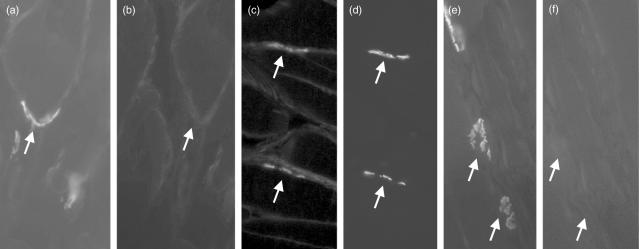
C9/MAC staining was absent from naive rat muscle (Fig. 5a,b). In EAMG, endplates in wild-type rats were strongly and universally stained for C9/MAC (Fig. 5c,d), whereas endplates from C6-deficient rats were negative (Fig. 5e,f), confirming that staining represented MACdeposition at this site. Sections from C6-deficient EAMG rats supplemented with human C6 were positive for C9/MAC staining in the same distribution as in wild-types.
Fig. 5.
Assessment of C9/membrane attack complex (MAC) deposition at the acetylcholine receptor (AChR) in wild-type, C6-deficient and naive Lewis rats. Soleus muscle from naive rats (a,b) and wild-type (c,d) or C6-deficient (e,f) Lewis rats induced for experimental autoimmune MG (EAMG) were harvested, and flash-frozen in isopentane as described. Ten μm thin sections were cut and stained for AChR (a,c,e) using rhodamine-conjugated bungarotoxin. Sections were double-stained for rat C9/MAC (b,d,f) using rabbit anti-rat C9, and detected with anti-rabbit-Ig-fluorescein isothiocyanate (FITC) conjugate. Sections were mounted in VectorShield and analysed under an inverted fluorescent microscope. Images were taken at 800× magnification. Arrows in paired images show identical regions stained in one or both.
C6 deficiency inhibits inflammatory cell infiltration of muscle in EAMG
In muscle sections from wild-type animals with EAMG, massive inflammatory cell infiltration was evident throughout the soleus muscle when assessed by ED1 staining for macrophages in unfixed tissue (Fig. 4j); in contrast, ED1-positive inflammatory infiltrates were minimal in C6-deficient EAMG animals (Fig. 4k), and absent from naive animals (Fig. 4l). Repletion of C6 (10 mg/kg p.i. at times 0 and 12 h) in EAMG animals markedly enhanced the infiltration of ED1-positive inflammatory cells (Table 1, P < 0·05 for a difference between these groups). Post-fixed sections were stained with H&E to identify inflammatory infiltrates; results fully supported the ED1 staining data with large infiltrates seen only in wild-type animals with EAMG (Table 1).
Table 1.
Inflammatory cell infiltration in experimental autoimmune MG (EAMG) muscle.
| Rat group | Inflammatory score | ED1+ cells per field |
|---|---|---|
| Naive (n = 1) | 0 | < 10 |
| w-t; EAMG (n = 6) | 3·5 ± 0·55 | 120 ± 35 |
| C6d; EAMG (n = 6) | 0·5 ± 0·22 | 19 ± 12 |
| C6d/C6; EAMG (n = 6) | 3·2 ± 0·48 | 135 ± 24 |
EAMG was induced in wild-type (w-t), C6-deficient (C6d) and C6-deficient administered C6 (10 mg/kg at time 0 and 12 h; C6d/C6) rats. Inflammation in muscle was assessed in two ways. Inflammatory infiltrates in haematoxylin and eosin-stained sections were assessed in a semiquantitative manner and scored blind on a scale from 0, no inflammatory infiltrates; 1, infiltrates < 1 per low power field; 2, infiltrates 1–5 per field; 3, infiltrates 6–10 per field; 4, infiltrates > 10 per field. Scores represent means for the stated groups ± s.d. Infiltration of ED1+ cells was measured in frozen sections by staining with specific antibody. Positive cells were counted in three sections per animal to obtain mean scores. Numbers shown represent mean of these means for each experimental group ± s.d.
Discussion
There is substantial evidence that C activation is a central component in the neuromuscular endplate destruction that typifies MG and the rodent model EAMG. In MG, C activation is demonstrable in serum and the levels of C activation products correlate with anti-AChR titres [16]. C activation products are also found at the neuromuscular endplate; indeed, the MAC has been implicated by association in endplate destruction in MG and EAMG by virtue of its abundance at the endplate [6,17]. The presence of C activation products at the endplate has even been suggested as an aid to diagnosis in MG [18]. This abundance of evidence implicating C has provoked the suggestion that anti-C therapies may prove effective in the treatment of MG resistant to conventional therapies [19,20]. The rational use of anti-C therapies requires an understanding of the C products involved in the pathology. Given the known involvement of anti-AChR antibody, it would be predicted that the classical pathway drives disease. Knock-out mice deficient in individual components of the C activation pathways have been used to confirm this prediction at the genetic level [21]. Mice deficient in either C3 or C4, the latter specific for the classical (and lectin) pathway(s), were resistant to disease induced by immunization with AChR, provoking the authors to suggest that therapies targeting the classical pathway might be beneficial in MG. Evidence for a role of MAC in passive EAMG in the rat came from a study of the effects of administering an anti-C6 polyclonal immunoglobulin fraction on the course of disease [22]. Clinical disease was markedly inhibited in the treated rats and neuromuscular conduction was preserved. This single study has implicated MAC formation in endplate damage in the model. However, the conclusions of this important study can be questioned because the large doses of rabbit IgG administered might have had other consequences in a disease known to respond to intravenous immunoglobulin administration [9,11,23].
To dissect out the contribution of the MAC to tissue injury in C-mediated diseases, we and others have utilized PVG rats deficient in C component C6 [14,24,25]. In the absence of C6, production of the opsonic and chemotactic products of C activation is unimpaired but generation of the MAC is ablated. C6-deficient rats were protected from disease in experimental demyelination, providing strong evidence that MAC formation is required for myelin loss in this model of multiple sclerosis [14]. Here we have used C6-deficient Lewis rats to explore the role of MAC in the passively induced EAMG model [8,26]. Whereas wild-type rats developed marked weakness and weight loss within hours of administering anti-AChR mAb, C6-deficient rats were completely resistant to disease induction. Histological analysis demonstrated a highly significant reduction in the loss of α-bungarotoxin-positive endplates in the absence of C6; C3 was deposited at the endplate in both wild-type and C6-deficient rats with EAMG, but C9/MAC was detected at the endplate only in wild-types. Heavy and widespread infiltration of muscle with macrophages and other inflammatory cells was seen in wild-type rats with EAMG but inflammation was minimal in C6-deficient rats, demonstrating that inflammation was, at least in part, driven by MAC formation. Inflammation was not restricted to areas around the endplates as has been reported in other studies [17], due probably to the acute nature of the disease in the passive EAMG model used here. Given that there is abundant C3 activation at the endplate in EAMG in the C6-deficient rats, and C-derived anaphylatoxins will be produced, the lack of inflammatory cell infiltration is surprising. We have found previously, in the experimental autoimmune encephalomyelitis model, that inflammation was much decreased in the central nervous system (CNS) in C6-deficient rats [14], and treatment with a C5a receptor antagonist in this model did not affect inflammation or clinical disease [27]. The cell activation and damage caused by the MAC must trigger the local production of chemotactic factors in both these models.
To confirm that the observed protection was due solely to the absence of C6, deficient rats were reconstituted with human C6. In the first experiment, serum haemolytic activity was restored to approximately 50% of that in wild-type rats and when given anti-AChR, C6-deficient rats reconstituted with C6 developed disease of a severity approaching that seen in wild-types and with muscle pathology indistinguishable from wild-types. In a second experiment, where serum haemolytic activity was restored completely, clinical disease and pathology were indistinguishable from that in wild-type rats, confirming that the absence of C6 was solely responsible for the lack of disease in C6 deficiency. Taken together, the data demonstrate conclusively that MAC formation is necessary for endplate damage and paralysis in passive EAMG in the rat.
The EAMG model closely resembles the human disease, both of which are driven by antibodies against the neuromuscular endplate and both associated with systemic C activation and local C deposition. Blocking assembly of the MAC would therefore be predicted to be protective in MG. Specific blockade of MAC assembly offers significant advantages over other C inhibitory strategies as it spares the important anti-bacterial activities provided by C opsonins and chemotaxins [20]. Several agents specifically targeting MAC assembly are in development and may fit this role [28,29]. Currently, anti-C5 agents have taken centre stage in C therapeutics; these agents, derived from anti-C5 antibodies, inhibit C5 cleavage, preventing formation of C5a and the MAC. The humanized anti-C5, pexeluzimab, is already in clinical trials for several inflammation and reperfusion-related injuries [30,31]. The time is ripe for testing this agent in MG.
Acknowledgments
We thank the staff of Biomedical Services, Cardiff University, for help with animal care. This work was supported by The Wellcome Trust (Programme Grant no. 068590 to B. P. M. and University Award no. 068823 to C. L. H.) and a MRC Industrial Collaborative Studentship (G 78/7562).
References
- 1.Hughes BW, De Casillas MLM, Kaminski HJ. Pathophysiology of myasthenia gravis. Semin Neurol. 2004;24:21–30. doi: 10.1055/s-2004-829585. [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom J. Acetylcholine receptors and myasthenia. Muscle Nerve. 2000;23:453–77. doi: 10.1002/(sici)1097-4598(200004)23:4<453::aid-mus3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Lindstrom J. Nicotinic acetylcholine receptors of muscles and nerves. Comparison of their structures, functional roles and vulnerability to pathology. Ann NY Acad Sci. 2003;998:41–52. doi: 10.1196/annals.1254.007. [DOI] [PubMed] [Google Scholar]
- 4.Newsom-Davis J, Pinching AJ, Vincent A, Wilson SG. Function of circulating antibody to acetylcholine receptor in myasthenia gravis: investigation by plasma exchange. Neurology. 1978;28:266–72. doi: 10.1212/wnl.28.3.266. [DOI] [PubMed] [Google Scholar]
- 5.Nastuk WL, Plescia OJ, Osserman KE. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. 1960;105:177–84. doi: 10.3181/00379727-105-26050. [DOI] [PubMed] [Google Scholar]
- 6.Nakano S, Engel AG. Myasthenia gravis − quantitative immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology. 1993;43:1167–72. doi: 10.1212/wnl.43.6.1167. [DOI] [PubMed] [Google Scholar]
- 7.Sahashi K, Engel AG, Lambert EH, Howard FM. Ultrastructural localisation of the terminal and lytic ninth complment component (C9) at the motor endplate in myasthenia gravis. J Neuropathol Exp Neurol. 1980;39:160–72. doi: 10.1097/00005072-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Tang H, Miller SM, Ermilov LG, Lennon VA, Brimijoin SA. Complement-mediated lesion of sympathetic ganglia in vitro with acetylcholinesterase antibodies. J Neuroimmunol. 1999;97:86–93. doi: 10.1016/s0165-5728(99)00060-0. [DOI] [PubMed] [Google Scholar]
- 9.Lindstrom J, Einarson BL, Lennon VA, Seybold ME. Pathological mechanisms in experimental autoimmune myasthenia gravis I Immunogenicity of syngeneic muscle acetylcholine receptor and quantitative extraction of receptor and antibody-receptor complexes from muscles of rats with experimental autoimmune myasthenia gravis. J Exp Med. 1976;144:726–38. doi: 10.1084/jem.144.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozrzymas JW, Lorenzon P, Riviera AP, Tedesco F, Ruzzier F. An electrophysiological study of the effects of myasthenia-gravis sera and complement on rat isolated muscle fibers. J Neuroimmunol. 1993;45:155–62. doi: 10.1016/0165-5728(93)90176-y. [DOI] [PubMed] [Google Scholar]
- 11.Piddlesden SJ, Jiang SS, Levin JL, Vincent A, Morgan BP. Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol. 1996;71:173–7. doi: 10.1016/s0165-5728(96)00144-0. [DOI] [PubMed] [Google Scholar]
- 12.Morgan BP, Gasque P, Singhrao SK, Piddlesden SJ. The role of complement in disorders of the nervous system. Immunopharmacology. 1997;38:43–50. doi: 10.1016/s0162-3109(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 13.Leenaerts PL, Stad RK, Hall BM, Van Damme BJ, Vanrenterghem Y, Daha MR. Hereditary C6 deficiency in a strain of PVG/c rats. Clin Exp Immunol. 1994;97:478–82. doi: 10.1111/j.1365-2249.1994.tb06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mead RJ, Singhrao SK, Neal JW, Lassman H, Morgan BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol. 2002;168:458–65. doi: 10.4049/jimmunol.168.1.458. [DOI] [PubMed] [Google Scholar]
- 15.Tzartos SJ, Hochschwender S, Vasquez P, Lindstrom J. Passive transfer of experimental autoimmune myasthenia gravis by monoclonal antibodies to the main immunogenic region of the acetylcholine receptor. J Neuroimmunol. 1987;15:185–94. doi: 10.1016/0165-5728(87)90092-0. [DOI] [PubMed] [Google Scholar]
- 16.Romi F, Kristoffersen EK, Aarli JA, Gilhus NE. The role of complement in myasthenia gravis: serological evidence of complement consumption in vivo. J Neuroimmunol. 2005;158:191–4. doi: 10.1016/j.jneuroim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Sahashi K, Engel AG, Lindstrom J, Lambert EH, Lennon VA. Ultrastructural localisation of immune complexes (IgG and C3) at the endplate in experimental autoimmune myasthenia gravis. J Neuropathol Exp Neurol. 1978;37:212–23. doi: 10.1097/00005072-197803000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Zander T, Schwab S, Laufenberg I, Sieb JP. Immunohistochemical detection of complement factors. a reliable method for the diagnosis of myasthenia gravis. Nervenarzt. 2000;71:666–9. doi: 10.1007/s001150050644. [DOI] [PubMed] [Google Scholar]
- 19.Asghar SS, Pasch MC. Therapeutic inhibition of the complement system. Y2K update. Frontiers Biosci. 2000;5:E63–82A. doi: 10.2741/asghar. [DOI] [PubMed] [Google Scholar]
- 20.Morgan BP, Harris CL. Complement therapeutics: history and current progress. Mol Immunol. 2003;40:159–70. doi: 10.1016/s0161-5890(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 21.Tuzun E, Scott BG, Goluszko E, Higgs S, Christadoss P. Genetic evidence for involvement of classical complement pathway in induction of experimental autoimmune myasthenia gravis. J Immunol. 2003;171:3847–54. doi: 10.4049/jimmunol.171.7.3847. [DOI] [PubMed] [Google Scholar]
- 22.Biesecker G, Gomez CM. Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J Immunol. 1989;142:2654–9. [PubMed] [Google Scholar]
- 23.Albuquerque EX, Eldefrawi AT. Myasthenia gravis. London: Chapman & Hall; 1983. [Google Scholar]
- 24.Brauer RB, Baldwin WM, Daha MR, Pruitt SK, Sanfilippo F. Use of C6-deficient rats to evaluate the mechanism of hyperacute rejection of discordant cardiac xenografts. J Immunol. 1993;151:7240–8. [PubMed] [Google Scholar]
- 25.Leenaerts PL, Hall BM, Van Damme BJ, Daha MR, Vanrenterghem YF. Active Heymann nephritis in complement component C6 deficient rats. Kidney Int. 1995;47:1604–14. doi: 10.1038/ki.1995.224. [DOI] [PubMed] [Google Scholar]
- 26.Lindstrom J, Engel AG, Seybold ME, Lennon VA, Lambert EH. Pathological mechanisms in experimental autoimune myasthenia gravis II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine receptor antibodies. J Exp Med. 1976;144:739–53. doi: 10.1084/jem.144.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan BP, Griffiths M, Khanom H, Taylor SM, Neal JW. Blockade of the C5a receptor fails to protect against experimental autoimmune encephalomyelitis in rats. Clin Exp Immunol. 2004;138:430–8. doi: 10.1111/j.1365-2249.2004.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris CL, Fraser DA, Morgan BP. Tailoring anti-complement therapeutics. Biochem Soc Trans. 2002;30:1019–26. doi: 10.1042/bst0301019. [DOI] [PubMed] [Google Scholar]
- 29.Fraser DA, Harris CL, Williams AS, et al. Generation of a recombinant, membrane-targeted form of the complement regulator CD59: activity in vitro and in vivo. J Biol Chem. 2003;278:48921–7. doi: 10.1074/jbc.M302598200. [DOI] [PubMed] [Google Scholar]
- 30.Fleisig AJ, Verrier ED. Pexelizumab − a C5 complement inhibitor for use in both acute myocardial infarction and cardiac surgery with cardiopulmonary bypass. Expert Opin Biol Ther. 2005;5:833–9. doi: 10.1517/14712598.5.6.833. [DOI] [PubMed] [Google Scholar]
- 31.Verrier ED. Terminal complement blockade with pexelizumab during coronary artery bypass graft surgery requiring cardiopulmonary bypass: a randomized trial. JAMA. 2006;295:164–5. doi: 10.1001/jama.291.19.2319. [DOI] [PubMed] [Google Scholar]