Abstract
Objectives
To estimate the effectiveness, cost‐effectiveness and resource impact of faecal occult blood testing (FOBT) and flexible sigmoidoscopy (FSIG) screening options for colorectal cancer to inform the Department of Health's policy on bowel cancer screening in England.
Methods
We developed a state transition model to simulate the life experience of a cohort of individuals without polyps or cancer through to the development of adenomatous polyps and malignant carcinoma and subsequent death in the general population of England. The costs, effects and resource impact of five screening options were evaluated: (a) FOBT for individuals aged 50–69 (biennial screening); (b) FOBT for individuals aged 60–69 (biennial screening); (c) once‐only FSIG for individuals aged 55; (d) once‐only FSIG for individuals aged 60; and (e) once‐only FSIG for individuals aged 60, followed by FOBT for individuals aged 61–70 (biennial screening).
Results
The model suggests that screening using FSIG with or without FOBT may be cost‐saving and may produce additional benefits compared with a policy of no screening. The marginal cost‐effectiveness of FOBT options compared to a policy of no screening is estimated to be below £3000 per quality adjusted life year gained.
Conclusions
Screening using FOBT and/or FSIG is potentially a cost‐effective strategy for the early detection of colorectal cancer. However, the practical feasibility of alternative screening programmes is inevitably limited by current pressures on endoscopy services.
Keywords: colorectal neoplasms, costs and cost analysis, economics, mass screening
Colorectal cancer (CRC) is the third most common form of cancer in the UK, where approximately 34 500 new cases of CRC are diagnosed each year,1 resulting in around 16 200 CRC‐related deaths annually.2 Recent evidence from randomised controlled trials (RCT) of faecal occult blood testing (FOBT) suggest that population‐based screening for CRC can significantly reduce mortality,3,4,5,6,7 and early results from the UK demonstration pilot suggest that population screening using FOBT is feasible within current healthcare resource constraints.8 Evidence relating to the effectiveness of flexible sigmoidoscopy (FSIG) as a screening option for CRC is less well established, although three RCTs are currently underway.9,10,11
It has been estimated that current annual expenditures on the surgical, adjuvant and palliative treatment of CRC are around £300 million11; this is likely to increase substantially as the National Institute for Health and Clinical Excellence (NICE) issues guidance on newer cytotoxic therapies for the adjuvant and palliative treatment of CRC. While the introduction of a national CRC screening programme would inevitably entail substantial immediate costs, such expenditures may be offset through a reduction in CRC incidence and through the less intensive treatment required for cancers detected earlier. Two mathematical models have been previously developed to estimate the cost‐effectiveness of FOBT screening in the UK, based upon the Nottingham trial12,13,14 and the UK FOBT demonstration pilot.15 Both analyses indicated that FOBT screening may be potentially cost‐effective in comparison to no screening. The aim of this study was to estimate the likely effectiveness, cost‐effectiveness and resource impact resulting from the implementation of alternative CRC screening programmes using FOBT, FSIG or a combination of both tests. The study was undertaken on behalf of the English Bowel Cancer Screening Working Group, to inform the Department of Health's CRC screening policy in England.
Methods
Modelling methodology and structure
We developed a state transition model to simulate the life experience of a cohort of individuals without polyps or cancer through to the development of adenomatous polyps and malignant carcinoma and subsequent death in the general population of England. The health economic model consists of three interlinked sub‐models:
A state transition model which simulates the natural history of CRC;
A model of the screening intervention and subsequent colonoscopic surveillance which interacts directly with the natural history model; and
A mortality model which reflects age‐specific “other‐cause” mortality, mortality due to CRC and mortality resulting from perforation due to endoscopic procedures.
The state transition modelling methodology is particularly useful for modelling diseases or conditions whereby risk is ongoing over time, where events may occur more than once, and where the timing of events is important.16,17 Central to this methodology is the division of the given disease process into a finite number of mutually exclusive health states, and the division of the relevant time horizon for the analysis into equal increments of time (Markov cycles). At any point in time, all patients must exist within one of the defined health states. The distribution of patients across the health states over time is governed by a series of transition matrices which describe the probability of transiting from the current health state to an alternative health state during each model cycle. Costs and utilities are associated with spending time in each health state or with the transition between health states; these are aggregated over the time horizon to provide an estimate of the expected costs and health outcomes of each screening option.
The model estimates the expected costs, life years gained (LYGs) and quality adjusted life years (QALYs) gained associated with five screening options as compared to a policy of no screening:
Biennial FOBT for individuals aged 50–69;
Biennial FOBT for individuals aged 60–69;
Once‐only FSIG for individuals aged 55;
Once‐only FSIG for individuals aged 60; and
Once‐only FSIG for individuals aged 60, followed by biennial FOBT for individuals aged 61–70.
The structure of the health economic model was informed by a critical review of existing CRC screening models,18 in particular the earlier work of Frazier and colleagues,19 and was developed alongside methodological guidelines for modelling screening interventions20 (for details of parametric and structural assumptions used within previous CRC screening models see supplementary appendix available at http://gut.bmj.com/supplemental).
CRC natural history model
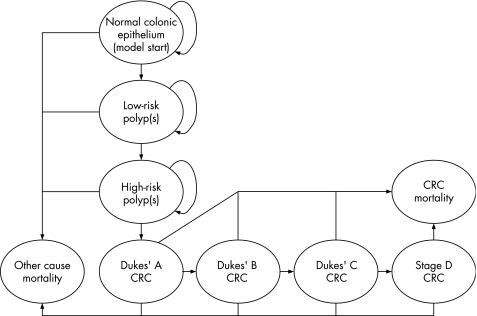
The natural history of CRC was modelled as a series of transitions between mutually exclusive health states (fig 1). The model uses age‐dependent transition matrices to calculate the number of individuals in each health state for each model cycle through a process of iterative matrix multiplication. Transitions between model health states are calculated using an annual cycle length until the entire model cohort has been absorbed into the dead states. Health states were defined according to the true underlying histological state of the individual. Health states describing the presence of adenomas and pre‐clinical cancers were defined in terms of the “index” lesion; that is, the greatest malignant potential of the adenoma or the most advanced cancer present. Individuals with adenomas were defined as “low‐risk” or “high‐risk” to reflect current guidelines for endoscopic surveillance following adenoma removal.21 Individuals with pre‐clinical or diagnosed CRC were modelled according to the Turnbull modification of Dukes' staging.22,23 Owing to the lack of direct evidence concerning the rate at which de novo cancers develop, the model assumes that all cancers arise from pre‐existing adenomas. Separate health states were assigned to the presence of distal and proximal polyps and cancers, as FSIG can only detect neoplasia in the distal portion of the colon. Incidence rates for adenomas and cancers are assumed to be higher in the distal colon than in the proximal colon. Owing to a lack of evidence to the contrary, the model assumes identical disease progression rates in both the distal and proximal colon.
Figure 1 Simplified colorectal cancer (CRC) natural history model progression diagram.
At the beginning of the simulation, the model cohort is aged 30, at which point the prevalence of polyps and CRC is assumed to be zero. Subsequently, the cohort is filtered through the health states according to transition probabilities based on relevant literature24,25,26,27,28,29,30,31 and through the calibration of model outputs against published CRC incidence1 and mortality rates.32
Screening and surveillance intervention model
We applied a screening intervention and surveillance model to the natural history model to estimate the potential impact of the earlier detection and removal of polyps through endoscopy, the detection and treatment of CRC, and the ongoing surveillance of high‐risk individuals in whom adenomas are detected. The test characteristics of FOBT, FSIG and colonoscopy were defined in terms of the probability of achieving a positive or negative test result given an individual's true underlying histological state. The impact of the screening test, follow‐up colonoscopy and treatment of detected polyps and cancers was modelled by re‐distributing the model cohort across the health states at the point of screening and surveillance. Individuals in whom adenomas are found are assumed to undergo polypectomy and are subsequently assigned a higher risk of adenoma recurrence.33 Individuals identified as high‐risk at screening are assumed to enter into a 3‐yearly colonoscopic surveillance programme. Individuals with pre‐clinical detectable CRC may present symptomatically during any model cycle; the probability of clinical presentation was estimated through model calibration and is assumed to increase according to cancer stage. Individuals in whom previously undetected CRC is identified enter into a clinical management state. Individuals in whom neither adenomas nor cancer are detected are assumed to be re‐invited to attend CRC screening during the next round.
Mortality model
The model includes three types of mortality: other cause mortality, CRC‐specific mortality and death following endoscopic perforation. The probability of dying from other causes was modelled as an age‐dependent probability depending on the age of the cohort during each model cycle, based on UK life tables.34 Dukes' stage‐specific mortality rates were estimated within the model calibration process through fitting the model against published Office for National Statistics (ONS) incidence1 and mortality data.32 The probability of death due to endoscopic perforation was modelled according to the experience of existing screening studies.11,35
Screening participation
Within the base case analysis, constant participation rates were assumed for screening and colonoscopic surveillance, based upon evidence from UK CRC screening trials and the UK FOBT pilot.11,15,36 Participation rates during each screening round were assumed to be independent of the individual's previous participation in screening or surveillance for CRC.
Health outcome valuation
For the cost‐effectiveness analysis, health outcomes were valued in terms of LYGs. For the cost‐utility analysis, LYGs were adjusted according to the level of health‐related quality of life associated with each health state, thus giving an estimate of the number of QALYs gained. Spending one model cycle in a particular model health state is associated with a state‐specific utility score (whereby 0 = “dead” and 1 = “perfect health”). A constant utility score was applied to all non‐cancers states. Lower utility scores were assumed for clinically diagnosed CRC states to reflect the severity of the disease and the invasiveness of treatment. These estimates were derived from a standard gamble health utility study in individuals who had previously undergone removal of colorectal adenoma.37
Resources and costs
The model incorporates two groups of costs: costs associated with the screening programme and costs associated with the diagnosis, treatment and follow‐up of CRC. The cost of FOBT was assumed to include the cost of two tests and an additional administration cost. The cost of FSIG was derived from Whynes et al.38 The cost of colonoscopy was taken from the NHS Reference Costs.39 Resources and costs associated with the diagnosis, treatment and follow‐up of patients with CRC were sourced from the literature and using expert clinical opinion. Key assumptions underpinning these cost estimates and the treatment pathways they are intended to reflect are detailed within the full study report.18 In line with current UK recommendations,40 all costs and health outcomes were discounted at 3.5% per year.
Model calibration methods
Several model parameters, in particular transition probabilities from adenoma health states to CRC, and probabilities of transiting through the pre‐clinical cancer states cannot be empirically observed.19 Where possible, likely ranges for uncertain or unknown parameters were derived from the available literature. Each model parameter was then assigned a wide uniform distribution; Monte Carlo sampling was undertaken whereby all unknown parameters were simultaneously sampled and propagated through the model over 60 000 iterations. Those parameter combinations which generated the minimum mean squared errors between the model predictions and published incidence,1 mortality,32 stage distribution data41 and prevalence estimates26,27,28,29,30,31 were retained for inclusion in the model. The mean values of these samples were used to produce the central estimates of cost‐effectiveness. Screening outcomes estimated by the model were validated against UK trial data.3,11
Table 1 presents a list of all parameter values assumed within the model, together with the sources of evidence used to inform each parameter.
Table 1 Parameter values used within the base case model analysis.
| Model parameter | Parameter estimate | Sources used to inform model parameter |
|---|---|---|
| Test characteristics | ||
| FOBT sensitivity for polyps | 5.00% | Assumption |
| FOBT sensitivity for CRC | 40.58% | Allison et al42,43 |
| FOBT specificity | 98.50% | Allison et al41,43 |
| FSIG and COL sensitivity for low‐risk distal polyps | 76.00% | Bressler et al,44 Hixson et al,45 Rex et al46 |
| COL sensitivity for low‐risk proximal polyps | 76.00% | Bressler et al,44 Hixson et al,45 Rex et al46 |
| FSIG and COL sensitivity for high‐risk distal adenomas and CRC | 97.00% | Bressler et al,44 Hixson et al,45 Rex et al46 |
| COL sensitivity for high‐risk proximal polyps and CRC | 94.00% | Bressler et al,44 Hixson et al,45 Rex et al46 |
| COL and FSIG specificity | 100.00% | Assumption |
| Natural history parameters | ||
| Probability of distal polyp given proximal cancer | 28.00% | Dinning et al47 |
| Normal epithelium to low‐risk polyp (men and women) | 1.60% | Eide et al,26 Rickert et al,27 Williams et al,28 |
| Blatt,29 Vatn et al,30 | ||
| Arminski et al,31 model calibration | ||
| Low‐risk polyp to high‐risk polyp | 2.12% | Knoernschild,25 model calibration |
| High‐risk polyp to Dukes' A | 3.26% | Stryker et al,24 model calibration |
| Dukes' A to Dukes' B | 58.29% | Model calibration |
| Dukes' B to Dukes' C | 65.55% | Model calibration |
| Dukes' C to stage D | 86.48% | Model calibration |
| Probability of recurrence given history of low‐risk polyp (year 1) | 18.00% | Winawer et al33 |
| Probability of recurrence given history of low‐risk polyp (year 2+) | 5.00% | Winawer et al33 |
| Probability of recurrence given history of high‐risk polyp (year 1) | 25.00% | Winawer et al33 |
| Probability of recurrence given history of high‐risk polyp (year 2+) | 6.00% | Winawer et al33 |
| Probability of presenting symptomatically with Dukes' A | 7.00% | Model calibration |
| Probability of presenting symptomatically with Dukes' B | 32.00% | Model calibration |
| Probability of presenting symptomatically with Dukes' C | 49.00% | Model calibration |
| Probability of presenting symptomatically with stage D | 85.40% | Model calibration |
| Annual CRC‐specific mortality rate (Dukes' A) | 0.00% | Model calibration |
| Annual CRC‐specific mortality rate (Dukes' B) | 1.00% | Model calibration |
| Annual CRC‐specific mortality rate (Dukes' C) | 6.02% | Model calibration |
| Annual CRC‐specific mortality rate (stage D) | 38.67% | Model calibration |
| Harm parameters | ||
| COL probability of perforation (without polypectomy) | 0.08% | Atkin et al11 |
| COL probability of perforation (with polypectomy) | 0.17% | Atkin et al11 |
| COL probability of death following perforation | 5.82% | Gatto et al35 |
| FSIG probability of perforation (without polypectomy) | 0.0025% | Atkin et al11 |
| FSIG probability of perforation (with polypectomy) | 0.0025% | Atkin et al11 |
| FSIG probability of death following perforation | 5.82% | Gatto et al35 |
| Probability of bleeding following FSIG | 0.0295% | Atkin et al11 |
| Probability of bleeding following COL | 0.439% | Atkin et al11 |
| Screening participation parameters | ||
| FOBT participation rate | 60.00% | Hardcastle,3 UK Colorectal Cancer Screening Pilot Group |
| FSIG compliance | 60.00% | Assumption based on Atkin et al11 |
| COL compliance | 80.00% | Lund et al36 |
| Health‐related quality of life parameters | ||
| Utility cancer free | 0.91 | Ness et al37 |
| Utility Dukes' A | 0.74 | Ness et al37 |
| Utility Dukes' B | 0.70 | Ness et al37 |
| Utility Dukes' C | 0.50 | Ness et al37 |
| Utility stage D | 0.25 | Ness et al37 |
| Resource use parameters | ||
| FSIG probability of inadequate bowel preparation | 5.26% | Atkin et al11 |
| COL probability of inadequate bowel preparation | 10.00% | Assumption |
| Cost of FSIG (with/without polypectomy) | £51.60 | Whynes et al38 |
| Cost of FOBT (2 tests) | £11.74 | Personal communication* |
| Cost of COL | £188.40 | NHS Reference Costs39 |
| Cost of treating bowel perforation (major surgery) | £5407.74 | NHS Reference Costs39 |
| Cost of admittance for bleeding | £250.21 | NHS Reference Costs39 |
| Pathology cost for adenoma | £30.00 | Personal communication† |
| Pathology cost for cancer | £250.00 | Personal communication† |
| Lifetime cost of Dukes' A | £8299.24 | Tappenden et al18 |
| Lifetime cost of Dukes' B | £12 441.41 | Tappenden et al18 |
| Lifetime cost of Dukes' C | £19 076.90 | Tappenden et al18 |
| Lifetime cost of stage D | £11 945.78 | Tappenden et al18 |
*Personal communication, Dr Julietta Patnick, Director of NHS Cancer Screening Programmes; †personal communication, Professor Neil Shepherd, Consultant Pathologist, Royal Gloucestershire Hospital.
COL, colonoscopy; CRC, colorectal cancer; FOBT, faecal occult blood test; FSIG, flexible sigmoidoscopy.
Uncertainty analysis
Sensitivity analysis was undertaken to explore the impact of alternative parameter values on the marginal cost‐effectiveness of each of the screening options. One‐way sensitivity analysis was undertaken to explore the impact of changing individual values for cost and participation parameters on central estimates of cost‐effectiveness. Probabilistic sensitivity analysis was undertaken using the potentially valid parameter sets identified within the calibration process, together with uniform probability distributions for the sensitivity and specificity of the screening and surveillance tests. The model was run 2000 times in order to generate a distribution of expected costs and health outcomes; the results of these data are presented as marginal cost‐effectiveness planes.
Results
Central estimates of effectiveness, cost‐effectiveness and cost‐utility
Table 2 presents marginal health outcomes for each the five core screening options compared to a policy of no screening. The model indicates that a policy offering FOBT screening to individuals aged 50–69 would result in the greatest number of screen‐detected cancers. However, the model suggests that offering FSIG at age 60 followed by biennial FOBT at age 61–70 may result in the greatest reduction in both CRC incidence and mortality.
Table 2 Expected health outcomes for alternative screening options for a population of 100 000 individuals invited to attend screening.
| Strategy | Screen‐detected cancers | Symptomatic cancers | CRC deaths | Cases of CRC avoided (% reduction) | CRC deaths avoided (% reduction) |
|---|---|---|---|---|---|
| Biennial FOBT at 50–69 years | 715.88 | 3029.68 | 1655.00 | 354.70 (8.65%) | 506.05 (23.42%) |
| Biennial FOBT at 60–69 years | 531.73 | 3407.88 | 1852.79 | 160.80 (3.92%) | 308.26 (14.26%) |
| FSIG once at 55 years | 150.83 | 3146.55 | 1662.04 | 802.61 (19.57%) | 499.01 (23.09%) |
| FSIG once at 60 years | 240.22 | 3032.97 | 1636.60 | 826.54 (20.16%) | 524.45 (24.27%) |
| FSIG once at 60 years and | 581.96 | 2586.75 | 1439.60 | 930.85 (22.70%) | 721.45 (33.38%) |
| biennial FOBT at 61–70 years | |||||
| No screening | – | 4100.70 | 2161.05 |
FOBT, faecal occult blood test; FSIG, flexible sigmoidoscopy.
The corresponding marginal estimates of cost‐effectiveness and cost‐utility for each of the five core screening options versus the no screening policy are presented in table 3. While the expected health gains for each individual offered screening are small, ranging from 4.59 to 9.89 days of life gained, the average cost per person invited to attend screening is also expected to be low. Offering FSIG once to individuals aged 60 followed by FOBT screening from age 61 to 70 is predicted to have the greatest impact on both survival and quality‐adjusted survival (9.89 and 10.28 days, respectively), although this is not the least expensive strategy. Offering FOBT to individuals aged 60–69 is expected to offer the smallest survival and quality‐adjusted survival gain over no screening (4.59 and 3.79 days, respectively). Offering FSIG to all individuals aged 55 or 60 appears to be the most cost‐effective option compared to a strategy of no screening; under the base case scenario these options dominate the no screening policy (ie, they are less expensive and more effective).
Table 3 Marginal cost‐effectiveness and cost‐utility estimates for alternative screening options.
| Screening option | Biennial FOBT at 50–69 years | Biennial FOBT at 60–69 years | FSIG once at 55 years | FSIG once at 60 years | FSIG once at 60 years, biennial FOBT at 61–70 years |
|---|---|---|---|---|---|
| Marginal cost | £66.95 | £24.53 | −£28.77 | −£28.51 | −£1.92 |
| Marginal LYGs | 0.026 | 0.0126 | 0.0237 | 0.0197 | 0.0271 |
| Marginal QALYs gained | 0.0227 | 0.0104 | 0.027 | 0.0221 | 0.0282 |
| Marginal cost per LYG | £2576.72 | £1950.29 | Dominates | Dominates | Dominates |
| Marginal cost per QALY gained | £2949.64 | £2364.99 | Dominates | Dominates | Dominates |
LYG, life year gained; QALY, quality adjusted life year.
Uncertainty analysis
Table 4 presents the results of the one‐way sensitivity analysis; these results show the impact of alternative parametric assumptions on the marginal cost‐utility estimate for each screening option as compared to no screening. Each scenario describes alternative assumptions concerning between one and four model parameters, as described in the table. The final two rows show the impact of alternative sets of natural history parameter values, as estimated through the calibration of the model, on the marginal cost‐utility of each screening option.
Table 4 One‐way sensitivity results for marginal cost‐utility analysis.
| Scenario | Marginal cost per QALY gained versus no screening | ||||
|---|---|---|---|---|---|
| Biennial FOBT at 50–69 years | Biennial FOBT at 60–69 years | FSIG once at 55 years | FSIG once at 60 years | FSIG once at 60 years, biennial FOBT at 61–70 years | |
| Base case scenario | £2949.64 | £2364.99 | Dominates | Dominates | Dominates |
| Undiscounted costs and effects | £1161.22 | £975.96 | Dominates | Dominates | Dominates |
| 40% of individuals never participate in | £2949.64 | £2364.99 | – | – | Dominates |
| FOBT screening | |||||
| 60% compliance with follow‐up COL | £4015.46 | £3301.71 | Dominates | Dominates | Dominates |
| Doubled CRC treatment costs | £1433.75 | £850.42 | Dominates | Dominates | Dominates |
| 20% lower sensitivity for FOBT and | £4257.3 | £3657.48 | Dominates | Dominates | £426.37 |
| FSIG screening | |||||
| 10% FOBT sensitivity for all adenomas | £1758.42 | £1369.49 | Dominates | Dominates | Dominates |
| 5% FOBT sensitivity for low‐risk | £1891.75 | £1362.24 | Dominates | Dominates | Dominates |
| adenomas, 10% FOBT | |||||
| sensitivity for high‐risk adenomas | |||||
| Double adenoma recurrence rates | £3203.04 | £2506.07 | Dominates | Dominates | £155.82 |
| following polypectomy | |||||
| Utility for all cancer states = 0.50 | £4351.17 | £4058.72 | Dominates | Dominates | Dominates |
| FSIG cost = COL cost (£188.40) | – | – | Dominates | Dominates | £252.02 |
| Double cost of FOB test | £6519.84 | £5358.51 | – | – | £961.46 |
| Best case scenario for calibrated natural | £551 | £15 | Dominates | Dominates | Dominates |
| history and sensitivity parameters | |||||
| Worst case scenario for calibrated natural | £7992 | £6111 | Dominates | Dominates | £263 |
| history and sensitivity parameters | |||||
COL, colonoscopy; CRC, colorectal cancer; FOBT, faecal occult blood test; FSIG, flexible sigmoidoscopy.
Table 4 suggests that the model is relatively insensitive to changes in most of the groups of parameter values. The greatest impact on cost‐utility results from the use of alternative sets of calibrated transition probabilities; these clearly represent an important area of uncertainty within the current evidence base.
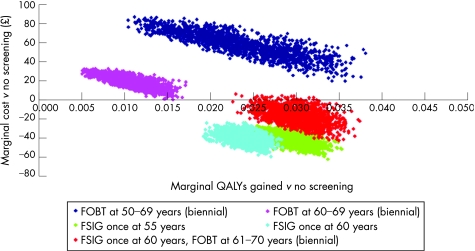
Figure 2 presents the results of the probabilistic sensitivity analysis using a marginal cost‐effectiveness plane; this shows the distribution of marginal costs and QALYs resulting from the use of alternative screening options in comparison to a policy of no screening. The uncertainty analysis demonstrates wide variability in marginal costs and effects for all five screening options, in particular the policy of offering FOBT biennially to all individuals aged 50–69. While the plane suggests a high degree of clustering of costs and effects, there is some overlap between the once‐only FSIG options when compared to no screening. Despite the uncertainty surrounding the natural history of the disease and the true sensitivity of the screening tests, all of the screening options appear to be economically attractive when compared to a policy of no screening. In particular, FSIG offered to individuals aged 55 or 60 is always expected to dominate the no screening policy. Figure 2 clearly suggests that the FOBT 60–69 policy is always expected to be more expensive and less effective than the once‐only FSIG options.
Figure 2 Marginal cost‐effectiveness plane for screening options versus no screening. FOBT, faecal occult blood test; FSIG, flexible sigmoidoscopy.
Additional resource use estimates
An assessment of the impact of each screening strategy upon endoscopy services was undertaken alongside the cost‐effectiveness analysis. This involved identifying the relevant age cohort of screen‐eligible patients for each screening strategy and propagating them through the model to determine the expected total number of colonoscopies and FSIGs, in addition to the number of patients being treated with surgery, chemotherapy and radiotherapy (both for primary disease and subsequent relapse). This analysis considered the expected impact in the 5 year period following the implementation of each screening strategy, and enabled the generation of estimates of additional resource requirements in terms of nurse‐trained endoscopists, gastrointestinal consultants and endoscopy units. There are currently around 240 hospital‐based endoscopy units in England, at which approximately one third of all activity is concerned with CRC. It is assumed that 80% of these units have two endoscopy rooms, with the remainder having either one room or more than two rooms. Table 5 outlines the results of the resource use analysis for the first year following implementation of a screening programme. These figures relate to the additional resource use requirements of each screening option over a policy of no screening for the entire population of England.
Table 5 Predicted additional resources required for alternative screening options.
| Treatment option | Biennial FOBT at 50–69 years | Biennial FOBT at 60–69 years | FSIG once at 55 years | FSIG once at 60 years | FSIG once at 60 years, biennial FOBT at 61–70 years |
|---|---|---|---|---|---|
| Additional number of FSIGs* | 0 | 0 | 344 605 | 274 969 | 274 969 |
| Additional number of colonoscopies* | 83 373 | 39 176 | 7093 | 8638 | 47 967 |
| Additional number of WTE nurse‐ | 0 | 0 | 110 | 88 | 88 |
| trained endoscopists* | |||||
| Additional number of WTE | 53 | 25 | 4–5 | 5–6 | 31 |
| gastrointestinal consultants* | |||||
| Additional endoscopy units required* | 20 | 9 | 40 | 33 | 38 |
| Annual cost following roll‐out over 5 years* | £94.5–128.5 m | £54.4–78.1 m | £25.7–27.5 m | £24.8–27.4 m | £26.3–84.5 m |
*Additional resource requirements over no screening policy. FSIG, flexible sigmoidoscopy; WTE, whole time equivalent.
Discussion
Health economic results
The CRC screening model suggests that screening using FSIG or FOBT, or a combination of both, is likely to have a cost‐effectiveness profile which is better than many interventions which are currently funded on the NHS. The health economic model suggests that once‐only screening using FSIG is more effective and less expensive than a policy of no screening. While the lifetime costs of FOBT screening may be more expensive than those for FSIG screening, the model suggests that biennial screening using this test may produce health gains at a cost which is currently considered acceptable to health care policy‐makers.
The resource use analysis indicates that there are considerable differences between the screening strategies in terms of endoscopy staffing and capital requirements. If total endoscopy services are constrained, the favoured option is likely to be the programme of biennial FOB testing between the ages of 60 and 69. However, this option is estimated to be the least effective in terms of quality adjusted survival and is estimated to have a total first year cost in the middle of the estimated range. If financial resources are constrained, the FSIG options may be considered the most appropriate, with lower costs due to a smaller cohort of people being screened than with FOBT strategies. The two FSIG strategies have a similar impact upon the marginal QALYs gained compared to a policy of no screening, and minimise the requirement for consultant gastroenterologists, though relying on a greater number of nurse endoscopists. The cost and resource analysis suggests that the most costly option in terms of screening and cancer management costs in the first year of the screening programme would be for the FOBT age 50–69 strategy, owing to the high number of people who would be offered screening each year. The higher number of cancers detected under the FOBT age 50–69 option would have significant cost implications, particularly in terms of the increase in surgery volume of around 5500 in the first year. Significant investment would be needed to meet the requirements of the implementation of any of the screening strategies on a nationwide basis.
Limitations of the model
This model covers a broader scope than existing UK models,12,13,14,15 which have considered only the expected costs and health outcomes resulting from FOBT screening. In addition, the surveillance model is more representative of current UK endoscopic practice, and up‐to‐date cancer stage‐specific utility data are used. Importantly, cost and ethical considerations would prohibit a trial‐based evaluation of all potential CRC screening options. Model‐based evaluations allow the available evidence to be brought to bear on policy decisions. As with any health economic model, our analysis incorporates both structural and parametric assumptions which influence the estimated costs and consequences of alternative screening options. It is important that these cost‐effectiveness results are interpreted in the light of the uncertainties within the existing evidence base.
Evidence concerning the true prevalence of colorectal adenomas within the general population of England is limited. Age‐specific adenoma prevalence estimates were drawn from six autopsy studies26,27,28,29,30,31; however these studies are dated and may not reflect current prevalence rates in England. This is an important gap in the existing evidence base.
The absence of direct evidence on rates of transition between disease states means that several of the model parameters had to be fitted to published data. However, there are several potentially valid solutions which fit the data. This is a critical point; few previous economic analyses of CRC screening have reported the way in which model parameters have been fitted against published incidence and mortality data. In turn, such analyses are unlikely to have allowed for the impact of the joint uncertainty in these parameter values on resulting cost‐effectiveness estimates. Given the uncertainty surrounding the underlying natural history of CRC, alternative combinations of these parameter values have the ability to produce favourable or unfavourable estimates of cost‐effectiveness for any CRC screening modality. Our analysis attempts to comprehensively and explicitly model the uncertainty surrounding the natural history of CRC through the probabilistic calibration of model outputs against published incidence and mortality, as well as the use of Monte Carlo sensitivity analysis to describe the impact of this uncertainty on the resulting cost‐effectiveness estimates.
It is broadly accepted that most CRCs arise from pre‐existing adenomatous polyps; while there is some indirect evidence which suggests the possibility that a small proportion of CRC arises de novo, this is subject to considerable uncertainty. Our model assumes that all cancers derive from pre‐existing adenomas; this assumption favours all screening options. In particular, the impact of this assumption is that those screening strategies which have a high sensitivity for detecting adenomas (ie, FSIG) will be favoured by the model. Notably, this assumption has also been applied within several existing health economic models of CRC screening.15,19,48,49,50 Further, probabilities of cancer progression are assumed to be equivalent in both the distal and proximal colon.
Evidence concerning the true differential sensitivity of FOBT in detecting small low‐risk adenomas, high‐risk adenomas and CRC is generally weak. Some previous models have assumed that the sensitivity of FOBT in detecting small and large adenomas is the same,15,19,51,52 while others have assumed different sensitivities for small and large adenomas.48 The absence of good quality empirical evidence makes it difficult to justify the values that these parameters should take. We assumed a single 5% sensitivity value for all adenomas, which broadly reflects the findings of the Nottingham RCT3; this assumption was based upon the advice of the English Bowel Cancer Screening Working Group. However, the impact of this assumption on the cost‐effectiveness of FOBT is minimal.
Further research
The uncertainties surrounding the health economic model presented here are a result of the paucity of direct evidence concerning the natural history of CRC and the operating characteristics of the screening tests. The central uncertainty concerns the true prevalence of undiagnosed asymptomatic CRC in England; inevitably, this is highly influential in determining the effectiveness and cost‐effectiveness of any CRC screening programme. While the natural history of the disease cannot be observed using standard study designs, there are two potential designs that could provide information.
Firstly, valuable information could be obtained through undertaking a large autopsy series in England. Given a sufficiently large sample size, such a study could be used to obtain better estimates of adenoma incidence rates and natural history parameters, and to determine the underlying prevalence of pre‐clinical CRC. Secondly, an analysis of existing screening trials is warranted. The problem with the screening trials, and conventional methods of analysis, is that the results confound the natural history and the characteristics of the screening test. Analytical methods which synthesise data from other sources (for example ONS incidence and mortality, stage distribution at diagnosis, and survival estimates from audit studies) allow information concerning natural history and test characteristics contained within the trial data to be drawn out. Bayesian synthesis analysis of existing trial data, based upon an underlying model of disease natural history and incorporating data from a range of available sources, should be undertaken.
Conclusions
Screening for CRC using either FOBT, FSIG or a combination of both strategies may provide health gains at a cost which is currently considered acceptable to NHS policy‐makers. The introduction of any of these screening options will inevitably require significant investment; consideration of the specific nature of current resource constraints should be considered prior to the roll‐out of any of these screening options. In particular, the practical feasibility of the alternative screening programmes in the UK will be limited by current pressures on endoscopy services.
Supplementary Material
Acknowledgements
This study was commissioned by the Department of Health in England, on behalf of the Bowel Cancer Screening Working Group. We would like to acknowledge the input of members of the Bowel Cancer Screening Working Group in providing support and direction for this work, in particular, Professor Wendy Atkin, Mr Ron Parker and Professor Bob Steele. We are also indebted to Dr Sue Moss and Professor John Scholefield for providing data from the Nottingham RCT, and to Professor Wendy Atkin for providing early data from the UK flexible sigmoidoscopy trial.
Abbreviations
CRC - colorectal cancer
FOBT - faecal occult blood test
FSIG - flexible sigmoidoscopy
LYG - life year gained
ONS - Office for National Statistics
QALY - quality adjusted life year
RCT - randomised controlled trial
Footnotes
Competing interests: None.
Ethics approval: Ethical approval was not required for this study.
References
- 1.Office for National Statistics Cancer registration statistics 2000. Series MB1 No 31, 2003. Available from http://www.statistics.gov.uk (accessed 24 January 2007)
- 2.Cancer Research U K. CancerStats Mortality ‐ UK 2005. Available from http://info.cancerresearchuk.org:8000/cancerstats/ (accessed 24 January 2007)
- 3.Hardcastle J D. Randomised controlled trial of faecal‐occult‐blood screening for colorectal cancer. Lancet 19963481472–1477. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J.et al Randomised study of screening for colorectal cancer with faecal‐occult‐blood test. Lancet 19963481467–1471. [DOI] [PubMed] [Google Scholar]
- 5.Mandel J S, Bond J H, Church T R.et al Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 19933281365–1371. [DOI] [PubMed] [Google Scholar]
- 6.Kewenter J, Brevinge H, Engaras B.et al Results of screening, rescreening, and follow‐up in a prospective randomized study for detection of colorectal cancer by fecal occult blood testing. Results for 68,308 subjects. Scand J Gastroenterol 199329468–473. [DOI] [PubMed] [Google Scholar]
- 7.Towler B P, Glasziou P, Weller D.et al Screening for colorectal cancer using the faecal occult blood test, hemoccult. Cochrane Database Syst Rev 2000(2)CD001216. [DOI] [PubMed]
- 8.UK Colorectal Cancer Screening Pilot Group Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ 2004329133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Senore C, Segnan N, Rossini F.et al Screening for colorectal cancer by once only sigmoidoscopy: a feasibility study in Turin, Italy. J Med Screen 1996372–78. [DOI] [PubMed] [Google Scholar]
- 10.Prorok P, Andriole G, Bresalier R.et al Design of the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials 200021273S–309S. [DOI] [PubMed] [Google Scholar]
- 11.Atkin W S. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet 20023591291–1300. [DOI] [PubMed] [Google Scholar]
- 12.Neilson A R, Whynes D K. Cost‐effectiveness of screening for colorectal cancer: a simulation model. IMA J Math Appl Med Biol 199512(3–4)355–367. [DOI] [PubMed] [Google Scholar]
- 13.Whynes D K. Cost‐effectiveness of faecal occult blood screening for colorectal cancer: results of the Nottingham trial. Crit Rev Oncol Hematol 199932155–165. [DOI] [PubMed] [Google Scholar]
- 14.Whynes D K, Neilson A R, Walker A R.et al Faecal occult blood screening for colorectal cancer: is it cost‐effective? Health Econ 1998721–29. [DOI] [PubMed] [Google Scholar]
- 15.UK CRC Screening Pilot Evaluation Team Evaluation of the UK Colorectal Cancer Screening Pilot, 2003. Available from http://www.cancerscreening.nhs.uk/bowel/finalreport.pdf (accessed 24 January 2007)
- 16.Sonnenberg F A, Beck J R. Markov models in medical decision making: a practical guide. Med Decis Making 199313322–338. [DOI] [PubMed] [Google Scholar]
- 17.Briggs A, Sculpher M. Introducing Markov models for economic evaluation. PharmacoEconomics 199813(4)397–409. [DOI] [PubMed] [Google Scholar]
- 18.Tappenden P, Eggington S, Nixon R.et al Colorectal cancer screening options appraisal: cost‐effectiveness, cost‐utility and resource impact of alternative screening options for colorectal cancer. Report to the English Bowel Cancer Screening Working Group, September 2004. Available from http://www.cancerscreening.nhs.uk/bowel/scharr.pdf (accessed 24 January 2007)
- 19.Frazier A L, Colditz G A, Fuchs C S.et al Cost‐effectiveness of screening for colorectal cancer in the general population. JAMA 2000284(15)1954–1961. [DOI] [PubMed] [Google Scholar]
- 20.Karnon J. A review and critique of modelling in prioritising and designing screening programmes. 2005. Report to the National Co‐ordinating Centre for Research Methodology. Available from http://www.pcpoh.bham.ac.uk/publichealth/nccrm/PDFs%20and%20documents/Publications/JHO2_Karnon_FR_%20April_2006.pdf (accessed 28 February 2007) [DOI] [PubMed]
- 21.Atkin W S, Saunders B P. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 200251v6–v9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnbull R B, Kyle K, Watson F R.et al Cancer of the colon: the influence of the no touch isolation technique on survival rates. Ann Surg 1967166420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dukes C E. The classification of cancer of the rectum. J Pathol Bacteriol 193235332 [Google Scholar]
- 24.Stryker S J, Wolff B G, Culp C E.et al Natural history of untreated colonic polyps. Gastroenterology 1987931009–1013. [DOI] [PubMed] [Google Scholar]
- 25.Knoernschild M D. Growth rate and malignant potential of colonic polyps: early results. Surg Forum 196314137–138. [PubMed] [Google Scholar]
- 26.Eide T J, Stalsberg H. Polyps of the large intestine in northern Norway. Cancer 1978422839–2848. [DOI] [PubMed] [Google Scholar]
- 27.Rickert R R, Auerbach O, Garfinkel L.et al Adenomatous lesions of the large bowel: an autopsy survey. Cancer 1979431847–1857. [DOI] [PubMed] [Google Scholar]
- 28.Williams A R, Balasooriya B A W, Day D W. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut 198223835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blatt L J. Polyps of the colon and rectum: incidence and distribution. Dis Colon Rectum 19614277–282. [Google Scholar]
- 30.Vatn M H, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer 198249819–925. [DOI] [PubMed] [Google Scholar]
- 31.Arminski T C, McLean D W. Incidence and distribution of adenomatous polyps of the colon and rectum based on 1,000 autopsy examinations. Dis Colon Rectum 19647249–261. [DOI] [PubMed] [Google Scholar]
- 32.Office for National Statistics Mortality statistics: Review of the Registrar General on deaths by cause, sex and age in England and Wales, 2002. Series DH2 No. 29. Available from http://www.statistics.gov.uk (accessed 24 January 2007)
- 33.Winawer S J, Zauber A G, O'Brien M J.et al Randomised comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med 1993328901–906. [DOI] [PubMed] [Google Scholar]
- 34.Government Actuary's Department Interim Life Tables 2003–05. Available from http://www.gad.gov.uk (accessed 24 January 2007)
- 35.Gatto N M, Frucht H, Sundararajan V.et al Risk of perforation after colonoscopy and sigmoidoscopy: a population‐based study. J Natl Cancer Inst 200395230–236. [DOI] [PubMed] [Google Scholar]
- 36.Lund J N, Scholefield J H, Grainge M J.et al Risks, costs, and compliance limit colorectal adenoma surveillance: lessons from a randomised trial. Gut 20014991–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ness R M, Holmes A M, Klein R.et al Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol 1999941650–1657. [DOI] [PubMed] [Google Scholar]
- 38.Whynes D K, Frew E, Edwards R.et al Costs of flexible sigmoidoscopy screening for colorectal cancer in the United Kingdom. Int J Technol Assess Health Care 200319384–395. [DOI] [PubMed] [Google Scholar]
- 39.Department of Health NHS reference costs 2003 and National tariff 2004 (Payment by results core tools 2004). Available from http://www.dh.gov.uk (accessed 24 January 2007)
- 40.National Institute for Clinical Excellence Guide to the methods of technology appraisal. Report N0515, i‐47. 2004. London: NICE, Available from http://www.nice.nhs.uk (accessed 24 January 2007)
- 41.South West Cancer Intelligence Service Data held on file, 1995: Audit study of patients with colorectal cancer undertaken in the Wessex Region 1991–1995. Winchester, UK: South West Public Health Observatory,
- 42.Allison J E, Tekawa I S, Ransom L J.et al A comparison of fecal occult‐blood tests for colorectal‐cancer screening. N Engl J Med 1996334155–159. [DOI] [PubMed] [Google Scholar]
- 43.Allison J E, Feldman R, Tekawa I S. Hemoccult screening in detecting colorectal neoplasm: sensitivity, specificity, and predictive value. Ann Intern Med 1990112328–333. [DOI] [PubMed] [Google Scholar]
- 44.Bressler B, Paszat L, Vinden C.et al Colonoscopic miss rates for colorectal cancer: a population based analysis. Gastrointest Endosc 200459AB110. [DOI] [PubMed] [Google Scholar]
- 45.Hixson L J, Fennerty M B, Sampliner R E.et al Prospective blinded trial of the colonoscopic miss‐rate of large colorectal polyps. Gastrointest Endosc 199137125–127. [DOI] [PubMed] [Google Scholar]
- 46.Rex D K, Cutler C S, Lemmel G T.et al Colonoscopic miss rates of adenomas determined by back‐to‐back colonoscopies. Gastroenterology 199711224–28. [DOI] [PubMed] [Google Scholar]
- 47.Dinning J P, Hixson L J, Clark L C. Prevalence of distal colonic neoplasia associated with proximal colon cancers. Arch Intern Med 1994154853–856. [PubMed] [Google Scholar]
- 48.Khandker R K, Dulski J D, Kilpatrick J B.et al A decision model and cost‐effectiveness analysis of colorectal cancer screening and surveillance guidelines for average‐risk adults. Int J Technol Assess Health Care 200016799–810. [DOI] [PubMed] [Google Scholar]
- 49.Loeve F. Endoscopic colorectal cancer screening: a cost‐saving analysis. J Natl Cancer Instit 200092557–563. [DOI] [PubMed] [Google Scholar]
- 50.Ness R M, Holmes A M, Klein R.et al Cost‐utility of colonoscopic screening for colorectal cancer at various ages. Am J Gastroenterol 2000971800–1811. [DOI] [PubMed] [Google Scholar]
- 51.Vijan S, Hwang E W, Hofer T P.et al Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med 2001111593–601. [DOI] [PubMed] [Google Scholar]
- 52.Ladabaum U, Chopra C L, Huang G.et al Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer: a cost effectiveness analysis. Ann Intern Med 2001135769–781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.