Abstract
Background
Liver cirrhosis, which is caused by the accumulation of extracellular matrix materials, is a serious clinical problem that can progress to hepatic failure. Transforming growth factor‐β (TGFβ) plays a pivotal role in extracellular matrix production, but bone morphogenetic protein (BMP)‐7, a member of the TGFβ superfamily, can antagonise the fibrogenic activity of TGFβ.
Aim
In this study, we examined whether adenovirus‐mediated overexpression of BMP‐7 (Ad‐BMP‐7) antagonised the effect of TGFβ in vitro and in vivo.
Methods and results
In primary cultured rat stellate cells and the LX‐2 human stellate cell line, induction of BMP‐7 by Ad‐BMP‐7 infection decreased the expression of collagen 1A2 mRNA and smooth muscle α‐actin in the presence or absence of TGFβ, via Smad 1/5/8 phosphorylation. BMP‐7 triggered the mRNA expression of inhibitors of differentiation 2 (Id2) in LX‐2. Although endogenous expression of BMP‐7 was hardly detectable, Smad1 and Id2 overexpression increased BMP‐7 expression in LX‐2. A liver fibrosis model was induced by the repetitive intraperitoneal injection of thioacetamide (200 mg/kg body weight) twice per week for up to 7 weeks. In rats administered Ad‐BMP‐7 via the tail vein, hydroxyproline content and the areas stained by Sirius red dye in the liver were significantly reduced compared to controls. Ad‐Id2 also reduced fibrosis.
Conclusion
These data demonstrate that BMP‐7, Smad 1/5/8 and Ids interact to antagonise hepatic fibrogenesis.
Keywords: BMP‐7, hepatic stellate cells, Id2, liver fibrosis, TGFβ
Hepatic stellate cells (HSC) are included in the group of sinusoidal constituent cells that play multiple roles in the liver. In the normal liver, HSC in the space of Disse surround the sinusoids as pericytes and store vitamin A‐associated lipid droplets.1 When the liver parenchyma suffers from chronic injury due to disease, elimination of damaged hepatocytes leads HSC to become activated by multiple peptide, lipid and gaseous mediators that are released from hepatocytes, Kupffer cells, endothelial cells and infiltrating inflammatory cells.2
HSC activation accompanies phenotypic transformation into myofibroblast‐like cells, as represented by the expression of smooth muscle α‐actin (αSMA) and by the production of extracellular matrix materials (ie, collagens, fibronectin, laminin and proteoglycans), thereby contributing to the progression of fibrosis in the chronically damaged liver.3,4 HSC activation is driven particularly by transforming growth factor‐β (TGFβ).5 Activated HSC produce TGFβ1 which stimulates their own collagen gene expression in an autocrine loop. TGFβ1 also upregulates tissue inhibitors of metalloproteinases6 that exhibit anti‐apoptotic effects towards myofibroblast‐like cells.7 In addition, a contribution of TGFβ1 to organ fibrogenesis has recently been suggested through induction of epithelial‐mesenchymal transition (EMT) in the injured kidney, which process was suppressed by bone morphogenic protein (BMP)‐7.8
BMPs, which belong to the TGFβ superfamily, are 30–35 kDa hetero‐ or homodimeric proteins originally identified as signals capable of inducing ectopic cartilage and bone upon non‐systemic injection in mammals.9 Several studies have since demonstrated that these proteins play essential roles during embryonic development10,11 and some BMP KO mice exhibit embryonic lethality.12,13 However, the functions of BMPs in adult organ tissues have not been fully elucidated. Recently, BMP‐7 was reported to counteract some of the profibrogenic action of TGFβ in renal mesangial cells14 and in the injured kidney.8 BMPs usually do not affect the growth of epithelial cells,15 although they exhibit weak antiproliferative effects towards thyroid carcinoma cells.16 The activation of BMP receptors by their ligands leads to the phosphorylation and activation of Smad1, Smad5 and Smad8 and to their heterodimerisation with Smad4, followed by their translocation into the nucleus, resulting in suppression of the nuclear accumulation of Smad3 by TGFβ1 stimulation.17
We have hypothesised here that introduction of BMP‐7 by adenoviral infection might suppress the profibrogenic response of the liver. We show that adenoviral gene transfer of BMP‐7 suppresses the expression of type I collagen in cultured HSC and in an in vivo thioacetamide (TAA)‐induced liver fibrosis model via upregulation of an inhibitor of differentiation 2 (Id2), which is a negative regulator for basic helix‐loop‐helix (bHLH) transcription factor. Furthermore, we show that adenoviral gene transfer of Id2 attenuates liver fibrosis in vivo.
Methods
Animals
Pathogen‐free male Wistar rats were obtained from SLC (Shizuoka, Japan). Animals were housed at a constant temperature and had free access to laboratory chow and water. Procedures were performed under the control of the animal care committee of Osaka City University in accordance with the Guideline on Animal Experiments in Osaka City University and the Japanese Government Animal Protection and Management Law.
Construction of recombinant adenovirus vector and virus purification
We used the Adenovirus Cre/loxP Kit (Takara Biomedicals, Tokyo, Japan) and the Adenovirus Expression Vector Kit (Takara Biomedicals) to generate two types of recombinant adenovirus vector. One adenovirus vector expresses the Cre recombinase, AxAw‐CAG‐Cre, under the control of the CAG (a fusion of the cytomegalovirus enhancer and the chicken β‐actin promoter plus part of the 3′ untranslated region of rabbit β‐globin) promoter. The other vector contains a potent expression unit that is activated by Cre recombinase‐mediated removal of an upstream loxP‐flanked ”stuffer” sequence. As a consequence, co‐transfection of these two adenovirus vectors to either cells or animals induces transcription of the down‐stream transgenes of interest (BMP‐7, Id2, Smad6 and Smad1). Cosmid pAxCALNL‐GFP, ‐LacZ, ‐BMP‐7, ‐Id2, ‐Smad6 and ‐Smad1 were constructed by the insertion of green fluorescent protein (GFP) cDNA, LacZ cDNA, mouse BMP‐7 (mBMP‐7) cDNA, mouse Id2 cDNA, mouse Smad1 cDNA and mouse Smad6 cDNA, respectively, into the Swa I cloning site of cosmid pAxCALNLw.18 GFP and LacZ were used to validate this Cre/loxP system in vitro and in vivo. Using the COS‐TPC method,19 recombinant adenoviruses of CAG‐Cre, LNL‐GFP, LNL‐LacZ, LNL‐BMP‐7 and LNL‐Id2 were generated by transfecting the cosmids of individual adenoviruses into HEK‐293 cells as described in the manufacturer's protocol. The titre of the recombinant adenoviruses was measured by the 50% tissue culture infectious dose (TCID50) method.20 Each adenovirus vector was used at the concentration of 1×109 PFU in vivo and the concentration of 50 multiplicity of infection (MOI) PFU/cell number) in vitro.
Preparation of primary cultured HSC and cell lines
HSC were isolated from male Wistar rats and transgenic mice, which expressed a construct that includes the sequences from −17 kb to +54 bp of the mouse pro α2(I) collagen gene which was cloned upstream of the firefly luciferase reporter gene, as previously described.21 Isolated HSC were suspended in Dulbecco's modified Eagle's medium (DMEM; Sigma, St. Louis, MO) supplemented with heat‐inactivated 10% fetal calf serum (FCS; Sigma), 100 IU/ml penicillin and 100 μg/ml streptomycin, and were plated at 5×105 cells/cm3 on non‐coated 35 mm dishes or six‐well plastic plates (Falcon 3001 or Falcon 3046, respectively, Becton Dickinson, Flanklin Lakes, NJ). The human hepatic stellate cell line LX‐222 was cultured in DMEM containing 100 IU/ml penicillin and 100 μg/ml streptomycin and supplemented or not with 10% FCS.
Adenoviral infection of cultured liver cells or cell lines
Primary cultured HSC were co‐infected with 50 MOI of LNL‐GFP, LNL‐LacZ, LNL‐BMP‐7 or LNL‐Id2 together with 50 MOI of CAG‐Cre for 1 h at day 4 of primary culture and incubated in serum‐free DMEM for 48 h. They were then incubated in the presence or absence of TGFβ1 (5 ng/ml) for another 24 or 48 h. Subconfluent LX‐2 cells were infected with 50 MOI of CAG‐Cre and 50 MOI of LNL‐mBMP‐7, LNL‐Smad6, LNL‐Smad1, LNL‐Id2 or LNL‐GFP.
Animal models
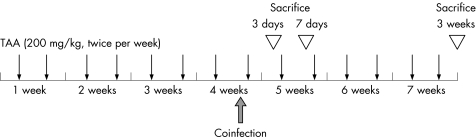
Hepatic fibrosis was induced in rats by intraperitoneal injection of TAA (200 mg/kg body weight; Wako, Osaka, Japan) twice per week for 4 weeks. Rats then each received 2×109 PFU of adenovirus vectors via the tail vein and were repetitively injected with TAA for 3 days, 7 days or 3 weeks as shown in fig 1. Liver tissues were either snap‐frozen in liquid nitrogen or fixed in 4% buffered paraformaldehyde for immunostaining.
Figure 1 Experimental hepatic fibrosis model in rats. Hepatic fibrosis was induced by intraperitoneal injection of thioacetamide (TAA; 200 mg/kg body weight) twice per week for 4 weeks. Rats then each received 2×109 plaque forming units (PFU) of adenovirus vectors via the tail vein and were repetitively injected with TAA for 3 days, 7 days or 3 weeks.
Analyses of hepatic fibrosis and immunohistochemistry
Fixed liver tissues were dehydrated and then embedded in Polybed. Sections were cut at 4 μm thickness. Following deparaffinisation and hydration, the sections were stained for 1 h in 0.1% (w/v) Sirius red (Direct Red 80, Aldrich, Milwaukee, WI) in a saturated aqueous solution (about 1.2% w/v) of picric acid (Wako, Osaka, Japan). After staining, the slides were rinsed for 30 min in 0.01 N HCl to remove unbound dye. Following dehydration by an alcohol series, the slides were mounted and observed under a light microscope (HC 2500, Fuji Photo Film, Tokyo, Japan). For semiquantitative analysis of liver fibrosis, ten fields from each slide were randomly selected, recorded and the red stained area per total area (mm2/mm2) was measured using Macscope Analyzer (Mitani, Fukui, Japan).
A microwave‐based antigen retrieval method was used to enhance antigenic capture. Fixed liver samples from rats infected with both LNL‐mBMP‐7 and CAG‐Cre were embedded in paraffin. Antigen unmasking of deparaffinised sections (4 μm thickness) was accomplished by heating in phosphate‐buffered saline buffer in a microwave oven for 15 min. The sections were then allowed to cool for 20 min. Immunohistochemistry for anti‐BMP‐7 was performed according to the method previously described.23 The sections were blocked against endogenous peroxidase using 3% H2O2. The sections were treated first with a 1:1000‐diluted goat polyclonal anti‐BMP‐7 (Sigma) and then with horseradish peroxidase‐conjugated anti‐immunoglobulins. Immunocomplexes were visualised by 3,3′‐diaminobenzidine tetrahydrochloride (DAB; Wako, Osaka, Japan). The tissue sections were counterstained with haematoxylin for 10 s. BMP‐7 expression was documented using an HC 2500 camera (Fuji Photo Film).
Assay of hydroxyproline content
Wet liver samples (100 mg) were subjected to acid hydrolysis to determine the amount of hydroxyproline as previously described.24 The data were expressed as hydroxyproline (mg)/wet liver weight (g).
Western blot analysis
Samples were homogenised in a lysis buffer (50 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, 5 mM EDTA, 10% glycerol, 1% Triton X‐100, 0.5% Nonidet P‐40, 10 mM NaF, 1 mM Na3VO4, pH 7.5) supplemented with a protease inhibitor cocktail from Roche (Summerville, NJ). To determine nuclear protein, cells were fractionated into cytoplasm and nuclei by using a ProteoExtract Subcellular Proteome Extraction kit (Merck Biosciences) according to the manufacturer's protocol. After heat denaturation at 95°C for 5 min, the samples (10 or 20 μg protein) were subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane (Bio‐Rad, Richmond, CA). The membranes were subsequently treated with 5% skim milk and then with primary antibodies overnight at 4°C. The following antibodies were used: anti‐phosphorylated Smad 1/5/8 antibody (1:1000 dilution; Cell Signaling Technology, Beverly, MA), anti‐collagen α2 (I) antibody (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), anti‐αSMA antibody (1:1000 dilution; Sigma), anti‐Smad3 antibody (1:1000 dilution; Zymed, San Francisco, CA), anti‐histone H1 antibody (1:1000 dilution; MBL, Nagoya, Japan), anti‐GAPDH antibody (1:25 000 dilution; Chemicon, Temecula, CA). After vigorous washing, the membranes were incubated with secondary horseradish peroxidase‐conjugated anti‐immunoglobulins for 2 h at room temperature. After washing, immunoreactive bands were visualised by using an enzyme‐linked chemiluminescence detection reagent (Amersham, Buckinghamshire, UK) and documented using an LAS 1000 analyser (Fuji Photo Film).
Luciferase assay
Cell extracts were prepared using Cell Culture Lysis Reagent (Promega, Madison, WI). The luciferase activity was measured according to the manufacturer's protocol (Promega) using a luminometer (Wallac 1420 Victor 2 multilabel counter system; PerkinElmer, Waltham, MA) and normalised for protein concentrations measured by DC Protein Assay (Bio‐Rad, Hercules, CA). Values are the means±SD of five independent experiments.
Quantitative real time‐polymerase chain reaction analysis of mRNA expression
Expression of collagen 1A2 (COL1A2), BMP‐7 and Id2 mRNAs was measured by a reverse transcription polymerase chain reaction (RT‐PCR) method using TaqMan One‐Step RT‐PCR Master Mix Reagents and the Applied Biosystems Prism 7700 system (PE Applied Biosystems, Foster City, CA) according to the previously reported procedure.25 Total RNA was isolated from HSC or whole liver tissues using ISOGEN (Nippon Gene, Tokyo, Japan). Primers and the oligonucleotide probe used are shown in table 1. Individual gene expression was normalised by GAPDH mRNA expression. As a standard reaction, cDNA corresponding to 200, 40, 8, and 1.6 ng of total RNA from one sample was examined and used as a reference value. We analysed each sample by using 100 ng of total RNA. The conditions of RT‐PCR were as follows: 20 min at 50°C (stage 1, reverse transcription), 10 min at 95°C (stage 2, reverse transcription inactivation and AmpliTaq Gold activation), and then 40 cycles of amplification for 15 s at 94°C and 1 min at 60°C (stage 3, PCR).
Table 1 Sequences of primers and probes for quantitative RT‐PCR.
| Sequences | |
|---|---|
| Rat collagen | F: 5′‐AAG GGT CCT TCT GGA GAA CC‐3′ |
| 1A2 | R: 5′‐TGG AGA GCC AGG GAG ACC CA‐3′ |
| P: 5′‐CAG GGT CTT CTT GGT GCT CCC GGT AT‐3′ | |
| Human | F: 5′‐AAG GGT CCC TCT GGA GAG‐3′ |
| collagen 1A2 | R: 5′‐TCT CGA GCC AGG GAG ACC CA‐3′ |
| P: 5′‐CAG GGT CTT CTT GGT GCT CCT GGT AT‐3′ | |
| Mouse | F: 5′‐GGC TGG CAG GAC TGG ATC AT‐3′ |
| BMP‐7 | R: 5′‐ACC AGT GTC TGG ACG ATG GC‐3′ |
| P: 5′‐CCT TCC CTC TGA ACT CCT ACA TGA ACG CC ‐3′ | |
| Rat | F: 5′‐GGC TGG CAG GAC TGG ATC AT‐3′ |
| BMP‐7 | R: 5′‐ACC AGT GTC TGG ACG ATA GC‐3′ |
| P: 5′‐CCT TCC CTC TGA ACT CCT ACA TGA ACG CC ‐3′ | |
| Human | F: 5′‐GGC TGG CAG GAC TGG ATC AT‐3′ |
| BMP‐7 | R: 5′‐ACC AGC GTC TGC ACG ATG GC‐3′ |
| P: 5′‐CCT TCC CTC TGA ACT CCT ACA TGA ACG CC ‐3′ | |
| Rodent | F: 5′‐AAA ACA GCC TGT CGG ACC AC‐3′ |
| Id2 | R: 5′‐CTG GGC ACC AGT TCC TTG AG‐3′ |
| P: 5′‐CCG GTG GAC GAC CCG ATG AGT CT‐3′ | |
| Human | F: 5′‐CAG CAT CCC CCA GAA CAA GA‐3′ |
| Id2 | R: 5′‐TAG TGG GAT GCG AGT CCA GG‐3′ |
| P: 5′‐TGA GCA AGA TGG AAA TCC TGC AGC AC‐3′ | |
| Rodent | F: 5′‐TGC ACC ACC AAC TGC TTA G‐3′ |
| GAPDH | R: 5′‐GGA TGC AGG GAT GAT GTT C‐3′ |
| P: 5′‐CAG AAG ACT GTG GAT GGC CCC TC‐3′ | |
| Human | F: 5′‐TGC ACC ACC AAC TGC TTA G‐3′ |
| GAPDH | R: 5′‐AGA GGC AGG GAT GAT GTT C‐3′ |
| P: 5′‐CAG AAG ACT GTG GAT GGC CCC TC‐3′ |
F, forward primer; P, probe; R, reverse primer.
Statistical analysis
Values reported in the figures are the means±SD of three or more independent samples. The results were analysed by unpaired Student's t test. Statistical significance was set at p<0.05.
Results
Adenoviral gene transfer of BMP‐7 increased the expression of BMP‐7 mRNA in primary cultured rat HSC
We first observed that endogenous BMP‐7 gene expression was hardly detectable in primary cultured HSC. As shown in fig 2A, adenoviral gene transfer of BMP‐7 (Ad‐BMP‐7) augmented BMP‐7 mRNA expression, which was much greater than in Ad‐LacZ treated HSC. As shown in fig 2B, BMP‐7 overexpression decreased COL1A2 mRNA in the presence or absence of 5 ng/ml of TGFβ.
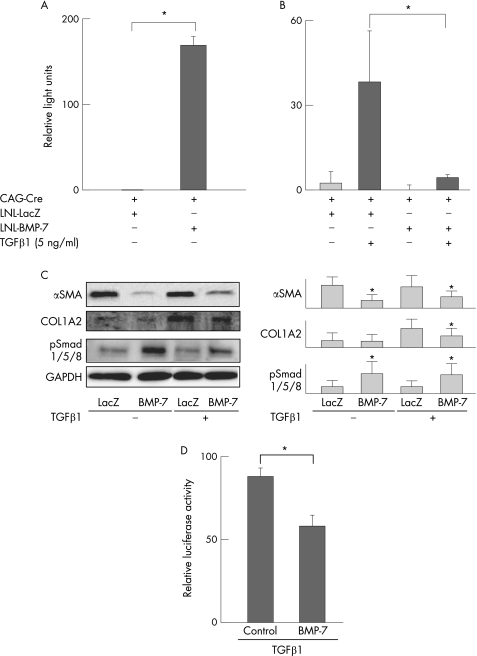
Figure 2 Effect of BMP‐7 on αSMA and COL1A2 expression in primary cultured rodent HSC. Primary cultured rat HSC were co‐infected with CAG‐Cre and either LNL‐LacZ or LNL‐BMP‐7 for 1 h and then incubated in serum‐free DMEM for 48 h. They were next incubated in the presence or absence of TGFβ1 (5 ng/ml) for another 24 h. (A) BMP‐7 and (B) COL1A2 mRNA were analysed using real‐time RT‐PCR as described in the Methods section. The relative levels of mRNA were normalised by GAPDH. Data are the means±SD of at least five independent experiments. *p<0.05. (C) Western blot analyses of αSMA, COL1A2 and phospho‐Smad 1/5/8 were performed as described in the Methods section. *Significant difference compared with control, p<0.05. (D) Primary cultured mouse HSC isolated from transgenic mice harbouring the COL1A2 upstream sequence fused to luciferase were stimulated with TGFβ (5 ng/ml) in the presence or absence of recombinant human BMP‐7 (250 ng/ml). Analysis of COL1A2 promoter activity was performed by luciferase assay, as described in the Methods section. *p<0.05.
BMP‐7 utilises Smad 1/5/8 as signalling intermediates and decreases the expression of type I collagen and αSMA in HSC
To investigate whether BMP‐7 activates a Smad 1/5/8 signal, Western blot analysis for phospho‐Smad 1/5/8 was performed. The phosphorylation of Smad 1/5/8 was upregulated by exogenously added BMP‐7 in the presence or absence of TGFβ1 stimulation (fig 2C). BMP‐7 decreased the protein level of type I collagen and αSMA in primary cultured HSC (fig 2C). To investigate the effect of BMP‐7 on collagen promoter activity, HSC were isolated from transgenic mice harbouring the COL1A2 upstream sequence fused to luciferase, and luciferase activity was assayed. As shown in fig 2D, COL1A2 promoter activity was inhibited by BMP‐7.
Effect of BMP‐7 on LX‐2, a human HSC cell line
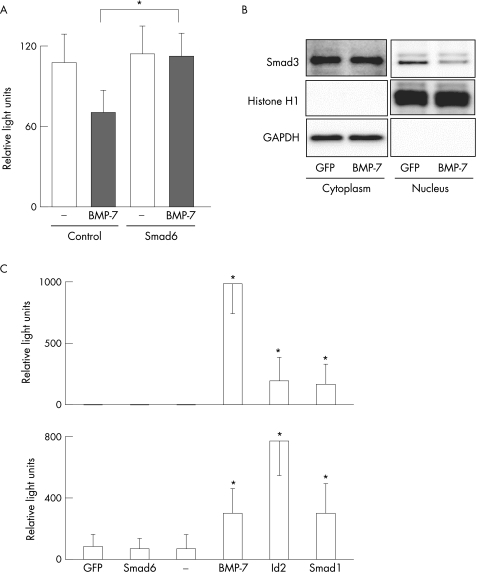
We further examined the effect of BMP‐7 using LX‐2, a human HSC cell line. As shown in fig 3A, BMP‐7 decreased the expression of COL1A2 mRNA in LX‐2, which is consistent with the data obtained from primary cultured rat HSC (fig 2B). The inhibitory effect of BMP‐7 on COL1A2 expression was blocked by Smad6 overexpression (fig 3A). These data clearly demonstrate that BMP‐7 decreased the expression of COL1A2 via Smad 1/5/8 phosphorylation. BMP‐7 also inhibited nuclear localisation of Smad3 in LX‐2 cells (fig 3B).
Figure 3 Effect of BMP‐7 on the function of LX‐2 cells. LX‐2 cells were co‐infected with CAG‐Cre and either LNL‐GFP (B, C), LNL‐BMP‐7 (B, C), Smad6 (A, C), Smad1 (C) or Id2 (C) for 1 h and incubated in serum‐free DMEM for 48 h. (A) LX‐2 cells were then incubated in the presence or absence of recombinant human BMP‐7 (250 ng/ml) for another 24 h. Expression of type I α2 collagen (COL1A2) mRNA was analysed using real‐time RT‐PCR as described in the Methods section. The relative levels of mRNA were normalised by GAPDH. Data are the means±SD of at least five independent experiments. *p<0.05. (B) Cytoplasmic and nuclear fractions were prepared and subjected to western blot with antibody against Smad3. Antibodies against GAPDH and Histone H1 were used to validate the fraction procedure. (C) Expression of BMP‐7 upper graph and Id2 lower graph mRNA was analysed using real‐time RT‐PCR as described in the Methods section. The relative levels of mRNA were normalised by GAPDH. Data are the means±SD of at least five independent experiments. *Significant difference compared with control, p<0.05.
Exogenous BMP‐7 increases the expression of Id2 in LX‐2
Id2 is known to be upregulated by BMP‐7 and to antagonise Smad 2/3 signalling. We utilised LX‐2 cells to confirm this effect in HSC. As shown in fig 3C, overexpression of BMP‐7 increased Id2 expression in LX‐2 cells. Smad1 overexpression also increased Id2 expression. Expression of BMP‐7 was barely detectable in untreated LX‐2 cells. However, both Smad1 and Id2 overexpression increased BMP‐7 expression in LX‐2 cells (fig 3C). Id2 overexpression decreased the expression of COL1A2 mRNA in LX‐2 cells (data not shown).
BMP‐7 inhibits fibrosis in TAA‐treated rat liver
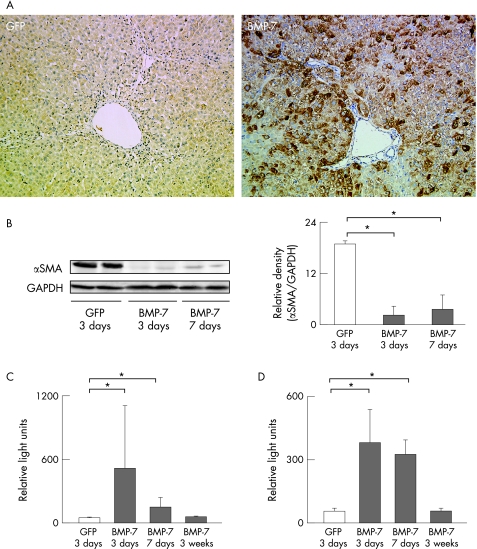
Based on these in vitro data, we hypothesised that BMP‐7 inhibits the development of fibrosis in TAA‐treated rat liver. Liver fibrosis was induced by intraperitoneal injection of TAA (200 mg/kg body weight) twice per week for 4 weeks. Rats then each received 2×109 PFU of CAG‐Cre and LNL‐BMP‐7 via the tail vein and were repetitively injected with TAA until sacrifice. Expression of BMP‐7 was evaluated by immunohistochemistry of TAA‐treated rat livers that were co‐infected with Ad‐CAG‐Cre and Ad‐LNL‐BMP‐7. As shown in fig 4A, BMP‐7 was expressed mainly in hepatocytes, sinusoidal cells and fibroblasts of periportal regions 3 days after adenovirus injection (fig 4A). In these livers, expression of αSMA was already suppressed as early as 3 days after Ad‐BMP‐7 infection (fig 4B). We confirmed that expression of BMP‐7 mRNA peaked at 3 days after injection, as shown in fig 4C, and returned to basal levels by 3 weeks after injection. Ad‐BMP‐7 induced Id2 mRNA expression that peaked at 3 days and returned to basal levels 3 weeks after injection (fig 4D).
Figure 4 Effect of BMP‐7 on the expression of Id2 and αSMA in TAA‐treated rat liver. Rats were treated with 200 mg/kg body weight of TAA twice per week for 4 weeks. They were then injected with 2.0×109 PFU of CAG‐Cre and LNL‐BMP‐7 via the tail vein. Rats co‐infected with LNL‐GFP were used as controls. Animals were sacrificed at 3 or 7 days after injection. (A) Immunohistochemistry for BMP‐7. Note that BMP‐7 was positive mainly in hepatocytes, sinusoidal cells and fibroblasts of periportal regions at 3 days after adenovirus injection. (B) Western blot analysis. Expression of αSMA and GAPDH was determined by immunoblotting. Densitometric measurement of αSMA was normalised by GAPDH. (C, D) Expression of BMP‐7 mRNA (C) and Id2 mRNA (D) was analysed using real‐time RT‐PCR. Relative levels of mRNA were normalised against that of GAPDH. Data are the means±SD of at least five independent experiments. *p<0.05.
Repetitive injection of TAA for 7 weeks induced prominent hepatic fibrosis. A single administration of Ad‐CAG‐NCre and Ad‐LNL‐BMP‐7 via the tail vein 4 weeks after the initiation of TAA injection considerably reduced the extent of fibrosis, as confirmed by morphometric analysis of Sirius red‐stained liver sections (fig 5A,B) and quantitative estimation of hydroxyproline content in the liver (fig 5C). At this time point, αSMA and collagen expression in the liver was persistently decreased (fig 5D,E).
Figure 5 Preventive effect of BMP‐7 on the progression of TAA‐induced hepatic fibrosis. Rats were treated with 200 mg/kg body weight of TAA twice per week for 4 weeks and were then injected with 2.0×109 PFU of CAG‐Cre and LNL‐BMP‐7 via the tail vein. TAA injection was continued for the next 3 weeks and animals were then sacrificed. (A) Sirius red staining. Note that fibrotic septa caused by TAA treatment (control) were dramatically suppressed by BMP‐7 expression induced by CAG‐Cre and LNL‐BMP‐7 injection. Rats co‐infected with LNL‐GFP were used as controls. (B, C) Estimation of liver fibrosis. The degree of hepatic fibrosis was quantified by measuring the area positive for Sirius red staining (B) and hydroxyproline content (C). Data obtained from eight rats in each group are given as means±SD. *p<0.05. (D) COL1A2 mRNA was analysed using real‐time RT‐PCR as described in the Methods section. Relative expression of COL1A2 mRNA was normalised against that of GAPDH mRNA. Data obtained from six rats in each group are given as means±SD. *p<0.05. (E) Western blot analysis. Expression of αSMA and GAPDH was determined by immunoblotting.
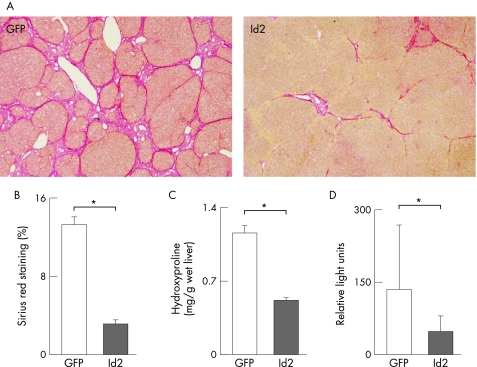
Id2 inhibits fibrosis in TAA‐treated rat liver
Finally, we examined whether Id2 suppressed fibrosis in TAA‐treated rat liver. Liver fibrosis was induced by repetitive injection of TAA for 7 weeks. A single administration of Ad‐CAG‐NCre and Ad‐LNL‐Id2 via the tail vein 4 weeks after the initiation of TAA injection reduced the extent of fibrosis, as confirmed by morphometric analysis of Sirius red‐stained liver sections (fig 6A,B) and quantitative estimation of hydroxyproline content in the liver (fig 6C) similarly to the effects of Ad‐BMP‐7. Expression of COL1A2 mRNA was also reduced by Ad‐Id2 (fig 6D).
Figure 6 Preventive effect of Id2 expression on the progression of TAA‐induced hepatic fibrosis. Rats were treated with 200 mg/kg body weight of TAA twice a week for 4 weeks and then injected with 2.0×109 PFU of CAG‐Cre and LNL‐Id2 via the tail vein. TAA injection was continued for the next 3 weeks and the animals were then sacrificed. (A) Sirius red staining. Note that fibrotic septa caused by TAA treatment (control) were dramatically suppressed by Id2 expression induced by CAG‐Cre and LNL‐Id2 injection. Rats co‐infected with LNL‐GFP were used as controls. (B, C) Estimation of liver fibrosis. The degree of hepatic fibrosis was quantified by measuring the area positive for Sirius red staining (B) and hydroxyproline content (C). Data obtained from eight rats in each group are given as means±SD. *p<0.05. (D) COL1A2 mRNA was analysed using real‐time RT‐PCR as described in the Methods section. Relative expression of COL1A2 mRNA was normalised against that of GAPDH mRNA. Data obtained from six rats in each group are given as means±SD. *p<0.05.
Discussion
Liver fibrosis is largely dependent on local TGFβ activity, since TAA and carbon tetrachloride‐induced hepatic fibrosis is attenuated by the administration of Smad7‐expressing adenovirus26 and soluble decoy proteins for TGFβ receptor, respectively.27 We had therefore predicted that BMP‐7, which reportedly antagonises the effects of TGFβ in renal fibrosis,8 might have a similar effect against hepatic fibrosis. Indeed, the present study clearly demonstrated that BMP‐7 overexpression by Ad‐BMP‐7 attenuated the expression of COL1A2 mRNA, collagen protein and αSMA in primary cultured HSC and suppressed liver fibrosis induced in rat liver by TAA treatment.
Activation of BMP receptor phosphorylates Smad1, Smad5 and Smad8, as shown in fig 2C, whereas Smad2 and Smad3 are phosphorylated by TGFβ receptor‐dependent signalling. One of the mechanisms by which BMP‐7 antagonises TGFβ signalling is by interference of phospho‐Smad 1/5/8 by Smad3 for specific DNA binding and/or recruitment of co‐factors.17 Another mechanism is by enhancing Smad7 expression via Smad1 signalling.28 In addition, independently of Smad, BMP‐7 can initiate intracellular pathways which can activate Jun N‐terminal kinase, p38 kinase and PI3 kinase/Akt pathways, which can protect hepatocytes.29,30
Id proteins, which were upregulated in HSC by BMP‐7 stimulation (figs 3 and 4), play important roles in the biological function of BMP‐7.31 Id proteins were identified as negative regulators of bHLH transcription factors. Subsequently, retinoblastoma (Rb) and Ets family members were also shown to interact with Id proteins.32,33 They act as positive regulators for cell proliferation and negative regulators for cell differentiation.34 They lack a basic DNA binding region but possess an HLH dimerisation motif, allowing them to interact with the ubiquitous bHLH transcription factor.32 As a result, these heterodimers containing Id proteins are unable to bind DNA. COL1A2 promoter activity is regulated by Smad3, Sp1 and Ets transcription factors.35 As mentioned above, Id proteins associate with Ets transcription factors and shut down their activity.34 Therefore, Id proteins might regulate COL1A2 promoter activity by inhibiting Ets. Alternatively, although Ids are not oncogenic proteins, the potential of Ids for epithelial cell (hepatocyte) proliferation may also contribute to ameliorating the fibrotic change in a manner similar to the effect of hepatocyte growth factor (HGF).36
Collagen synthesis in HSC and fibroblasts in the liver is regulated at the transcriptional and post‐transcriptional levels.37 In the present study, we demonstrated that BMP‐7 directly affected the COL1A2 promoter activity of HSC (fig 2D). Our recent study using pulmonary myofibroblasts also demonstrated that BMP‐7 decreased COL1A2 promoter activity to ∼70% of normal, whereas BMP‐7 lowered COL1A2 mRNA expression to 25–50% of normal.38 BMP‐7 is thus presumed to influence the stability and degradation of COL1A2 mRNA. This issue may require further analysis in future studies.
BMP‐7 is also reported to antagonise EMT in tissue repair, a hallmark of which is the reduction of E‐cadherin expression.39 E‐cadherin is the central component of cell‐cell adhesion junctions and is required for the formation of epithelium. On the other hand, the expression of αSMA is also a marker of mesenchymal myofibroblasts. TGFβ can induce EMT, which can be suppressed by BMP‐7 in renal tubular epithelial cells.8 In the liver, fetal hepatocytes are reported to undergo EMT,40,41 but there is no report describing EMT in adult hepatocytes. As shown in fig 2C, expression of αSMA was actually suppressed by the overexpression of BMP‐7 in primary cultured rat HSC both in the presence and absence of TGFβ, suggesting that BMP‐7 might be involved in the reversion of myofibroblast‐like cells to HSC. To further examine whether adult hepatocytes have a potential for undergoing EMT, we stimulated primary cultured rat hepatocytes with BMP‐7. However, there was no significant impact on the expression of E‐cadherin by BMP‐7 stimulation (data not shown).
Adenovirus vector systems are useful to transfer and express genes in hepatocytes, even in liver fibrosis models.42 A single injection of adenoviral vectors in the tail vein attenuated fibrogenesis as shown in figs 5 and 6. One disadvantage of this system is the inability to repeat administration due to acquisition of the immune response against the virus. This problem may be overcome by several new methodologies. First, new adenoviral vectors that express the transgene of interest together with either the dimeric human monoclonal antibodies against CD40 and CD8043 or CTLA4Ig,44 which mediate immunogenic responses to the virus, have been developed. Second, repeated retrograde administration of recombinant adenoviral vector into the common bile duct may be useful to successfully re‐express the transgene in the liver despite the existence of neutralising antibodies in the serum.45 Third, adenoviral vectors are being developed that do not express any viral protein.46,47 These experiments could eventually contribute to the development of gene therapy for hepatic fibrosis using either adenoviral vectors or other viral transduction systems.
Acknowledgements
The authors thank Ms M Yamauchi and Ms M Nakazawa for excellent technical assistance. This work was supported in part by a grant from the Ministry of Education, Science, Sports and Culture of Japan (C16590150 to KI) and a grant from the NIH (DK56621 to SLF) as well as the Feld Fibrosis Center.
Abbreviations
αSMA - smooth muscle α‐actin
bHLH - basic helix‐loop‐helix
BMP‐7 - bone morphogenetic protein‐7
COL1A2 - type I α2 collagen
DMEM - Dulbecco's modified Eagle's medium
FCS - fetal calf serum
GFP - green fluorescent protein
HSC - hepatic stellate cells
Id2 - inhibitors of differentiation 2
MOI - multiplicity of infection
PFU - plaque forming units
TAA - thioacetamide
TGFβ - transforming growth factor‐β
Footnotes
Competing interests: None declared.
References
- 1.Wake K. Perisinusoidal stellate cells (fat‐storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A‐storing cells in extrahepatic organs. Int Rev Cytol 198066303–353. [DOI] [PubMed] [Google Scholar]
- 2.Friedman S L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 20002752247–2250. [DOI] [PubMed] [Google Scholar]
- 3.Friedman S L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 19933281828–1835. [DOI] [PubMed] [Google Scholar]
- 4.Pinzani M. Novel insights into the biology and physiology of the Ito cell. Pharmacol Ther 199566387–412. [DOI] [PubMed] [Google Scholar]
- 5.Hui A Y, Friedman S L. Molecular basis of hepatic fibrosis. Expert Rev Mol Med 200320031–23. [DOI] [PubMed] [Google Scholar]
- 6.Knittel T, Mehde M, Kobold D.et al Expression patterns of matrix metalloproteinases and their inhibitors in parenchymal and non‐parenchymal cells of rat liver: regulation by TNF‐alpha and TGF‐beta1. J Hepatol 19993048–60. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H Y, Phan S H. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am J Respir Cell Mol Biol 199921658–665. [DOI] [PubMed] [Google Scholar]
- 8.Zeisberg M, Hanai J, Sugimoto H.et al BMP‐7 counteracts TGF‐beta1‐induced epithelial‐to‐mesenchymal transition and reverses chronic renal injury. Nat Med 20039964–968. [DOI] [PubMed] [Google Scholar]
- 9.Wozney J M, Rosen V, Celeste A J.et al Novel regulators of bone formation: molecular clones and activities. Science 19882421528–1534. [DOI] [PubMed] [Google Scholar]
- 10.Wall N A, Hogan B L. TGF‐beta related genes in development. Curr Opin Genet Dev 19944517–522. [DOI] [PubMed] [Google Scholar]
- 11.Kingsley D M, Bland A E, Grubber J M.et al The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell 199271399–410. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 19961222977–2986. [DOI] [PubMed] [Google Scholar]
- 13.Jena N, Martin‐Seisdedos C, McCue P.et al BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp Cell Res 199723028–37. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Hirschberg R. BMP7 antagonizes TGF‐beta‐dependent fibrogenesis in mesangial cells. Am J Physiol Renal Physiol 2003284F1006–F1013. [DOI] [PubMed] [Google Scholar]
- 15.Botchkarev V A. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. J Invest Dermatol 200312036–47. [DOI] [PubMed] [Google Scholar]
- 16.Franzen A, Heldin N E. BMP‐7‐induced cell cycle arrest of anaplastic thyroid carcinoma cells via p21(CIP1) and p27(KIP1). Biochem Biophys Res Commun 2001285773–781. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Hirschberg R. Bone morphogenetic protein‐7 signals opposing transforming growth factor beta in mesangial cells. J Biol Chem 200427923200–23206. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Tanaka K, Lee G.et al Enhanced and specific gene expression via tissue‐specific production of Cre recombinase using adenovirus vector. Biochem Biophys Res Commun 1998244455–462. [DOI] [PubMed] [Google Scholar]
- 19.Miyake K, Tohyama T, Shimada T. Two‐step gene transfer using an adenoviral vector carrying the CD4 gene and human immunodeficiency viral vectors. Hum Gene Ther 199672281–2286. [DOI] [PubMed] [Google Scholar]
- 20.Kanegae Y, Miyake S, Sato Y.et al Adenovirus vector technology: an efficient method for constructing recombinant adenovirus and on/off switching of gene expression. Acta Paediatr Jpn 199638182–188. [DOI] [PubMed] [Google Scholar]
- 21.Kawada N, Seki S, Inoue M.et al Effect of antioxidants, resveratrol, quercetin, and N‐acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology 1998271265–1274. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Hui A Y, Albanis E.et al Human hepatic stellate cell lines, LX‐1 and LX‐2: new tools for analysis of hepatic fibrosis. Gut 200554142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ikeda K, Kawada N, Wang Y Q.et al Expression of cellular prion protein in activated hepatic stellate cells. Am J Pathol 19981531695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuno M, Akita K, Moriwaki H.et al Prevention of rat hepatic fibrosis by the protease inhibitor, camostat mesilate, via reduced generation of active TGF‐beta. Gastroenterology 20011201784–1800. [DOI] [PubMed] [Google Scholar]
- 25.Uyama N, Shimahara Y, Okuyama H.et al Carbenoxolone inhibits DNA synthesis and collagen gene expression in rat hepatic stellate cells in culture. J Hepatol 200339749–755. [DOI] [PubMed] [Google Scholar]
- 26.Dooley S, Hamzavi J, Breitkopf K.et al Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 2003125178–191. [DOI] [PubMed] [Google Scholar]
- 27.Yata Y, Gotwals P, Koteliansky V.et al Dose‐dependent inhibition of hepatic fibrosis in mice by a TGF‐beta soluble receptor: implications for antifibrotic therapy. Hepatology 2002351022–1030. [DOI] [PubMed] [Google Scholar]
- 28.Benchabane H, Wrana J L. GATA‐ and Smad1‐dependent enhancers in the Smad7 gene differentially interpret bone morphogenetic protein concentrations. Mol Cell Biol 2003236646–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada N, Hatano E, Koizumi N.et al Akt activation protects rat liver from ischemia/reperfusion injury. J Surg Res 2004121159–170. [DOI] [PubMed] [Google Scholar]
- 30.Carini R, Grazia De Cesaris M, Splendore R.et al Role of phosphatidylinositol 3‐kinase in the development of hepatocyte preconditioning. Gastroenterology 2004127914–923. [DOI] [PubMed] [Google Scholar]
- 31.Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE 20022002PE40. [DOI] [PubMed] [Google Scholar]
- 32.Norton J D, Deed R W, Craggs G.et al Id helix‐loop‐helix proteins in cell growth and differentiation. Trends Cell Biol 1998858–65. [PubMed] [Google Scholar]
- 33.Yokota Y, Mori S. Role of Id family proteins in growth control. J Cell Physiol 200219021–28. [DOI] [PubMed] [Google Scholar]
- 34.Ruzinova M B, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol 200313410–418. [DOI] [PubMed] [Google Scholar]
- 35.Jinnin M, Ihn H, Yamane K.et al Alpha2(I) collagen gene regulation by protein kinase C signaling in human dermal fibroblasts. Nucleic Acids Res 2005331337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueki T, Kaneda Y, Tsutsui H.et al Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med 19995226–230. [DOI] [PubMed] [Google Scholar]
- 37.Lindquist J N, Marzluff W F, Stefanovic B. Fibrogenesis. III. Posttranscriptional regulation of type I collagen. Am J Physiol Gastrointest Liver Physiol 2000279G471–G476. [DOI] [PubMed] [Google Scholar]
- 38.Izumi N, Mizuguchi S, Inagaki Y.et al BMP‐7 opposes TGF‐beta1‐mediated collagen induction in mouse pulmonary myofibroblasts through Id2. Am J Physiol Lung Cell Mol Physiol 2006290L120–L126. [DOI] [PubMed] [Google Scholar]
- 39.Kowanetz M, Valcourt U, Bergstrom R.et al Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor beta and bone morphogenetic protein. Mol Cell Biol 2004244241–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chagraoui J, Lepage‐Noll A, Anjo A.et al Fetal liver stroma consists of cells in epithelial‐to‐mesenchymal transition. Blood 20031012973–2982. [DOI] [PubMed] [Google Scholar]
- 41.Valdes F, Alvarez A M, Locascio A.et al The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes. Mol Cancer Res 2002168–78. [PubMed] [Google Scholar]
- 42.Inagaki Y, Kushida M, Higashi K.et al Cell type‐specific intervention of transforming growth factor beta/Smad signaling suppresses collagen gene expression and hepatic fibrosis in mice. Gastroenterology 2005129259–268. [DOI] [PubMed] [Google Scholar]
- 43.Haegel‐Kronenberger H, Haanstra K, Ziller‐Remy C.et al Inhibition of costimulation allows for repeated systemic administration of adenoviral vector in rhesus monkeys. Gene Ther 200411241–252. [DOI] [PubMed] [Google Scholar]
- 44.Thummala N R, Ghosh S S, Lee S W.et al A non‐immunogenic adenoviral vector, coexpressing CTLA4Ig and bilirubin‐uridine‐diphosphoglucuronate‐glucuronosyltransferase permits long‐term, repeatable transgene expression in the Gunn rat model of Crigler‐Najjar syndrome. Gene Ther 20029981–990. [DOI] [PubMed] [Google Scholar]
- 45.Tominaga K, Kuriyama S, Yoshiji H.et al Repeated adenoviral administration into the biliary tract can induce repeated expression of the original gene construct in rat livers without immunosuppressive strategies. Gut 2004531167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim I H, Jozkowicz A, Piedra P A.et al Lifetime correction of genetic deficiency in mice with a single injection of helper‐dependent adenoviral vector. Proc Natl Acad Sci U S A 20019813282–13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morsy M A, Gu M, Motzel S.et al An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc Natl Acad Sci U S A 1998957866–7871. [DOI] [PMC free article] [PubMed] [Google Scholar]