Abstract
Objective
To examine prospectively whether irritable bowel syndrome (IBS) or other variables—that is, psychiatric profiles, health‐related quality of life (HRQoL) and clinical features—are associated with negative appendectomy (NA).
Design
Longitudinal study.
Setting
Inpatient and emergency service in a university‐affiliated teaching hospital.
Patients
430 consecutive patients underwent emergent surgery for suspected appendicitis.
Main outcome measures
Rome‐II IBS questionnaire; the Hospital Anxiety and Depression Scale; the Short‐Form 36 survey; the clinical, pathological and CT findings.
Results
The NA group (n = 68, 15.8%) was younger, with female predominance, higher prevalence of Rome‐II IBS, higher anxiety/depression scores and lower levels of HRQoL than the positive appendectomy group. The patients with NA tended to have atypical presentations (absence of migration pain/fever/muscle guarding), lower white cell count and percentage of polymorphonuclear cells (PMNC) and lower rate of CT scan usage than the positive group. After multiple logistic regression, IBS (OR 2.17; 95% CI 1.14 to 4.24), degree of anxiety (OR 1.12; 95% CI 1.02 to 1.49), absence of migrating pain (OR 3.43; 95% CI 1.90 to 5.95)/muscle guarding (OR 3.72; 95% CI 2.07 to 6.70), a lower PMNC percentage (<75%; OR 3.05; 95% CI 1.69 to 5.51) and no CT scan usage (OR 2.32; 95% CI 1.27 to 4.26) were found to be the independent factors in predicting NA.
Conclusion
Both patient (IBS, anxiety, atypical presentation) and physician (low CT scan usage) factors are the independent determinants predicting NA. Physicians should be cautious before operating on or referring patients with IBS for appendectomy. CT scan should be considered in patients with suspected appendicitis, particularly in those with IBS and atypical clinical presentations.
Acute appendicitis is the most common cause of an acute abdomen with at least 250 000 cases per year in the US.1 The life‐time risk of appendectomy is 12.0% for men and 23.1% for women.2 Although clinical symptoms are often characteristic, a high rate of misdiagnosis, often referred to as negative appendectomy (NA), suggests otherwise. Several studies have shown that over 15% of appendectomies performed revealed no pathological evidence of appendicitis.3,4,5,6 Despite the introduction of modern diagnostic imaging, such as CT/ultrasonography, population‐based rates of NA remain unchanged over time.7 Furthermore, a significant clinical and financial cost incurred by patients undergoing NA exists throughout their presumed course of appendicitis.1
Very few studies have been conducted to evaluate factors predictive of NA. In one study, intense perceived pain and abdominal tenderness, and a white cell count <13×109/l, were independent predictors of NA.8 The authors recommended that laboratory examinations of systemic inflammation (such as white cell count) should be scrutinised closely, and that physical symptoms and signs (such as pain and tenderness) should be interpreted cautiously. This finding implies that abdominal pain and tenderness may be unintentionally augmented in patients with NA themselves, a situation comparable to the so‐called visceral hypersensitivity or hypervigilance for visceral sensation.
Visceral hypersensitivity or hypervigilance for visceral sensation is a crucial factor in the pathogenesis of irritable bowel syndrome (IBS).9,10 Patients with IBS have been reported to undergo disproportionately high rates of appendectomy or other abdominal/pelvic surgeries.11 For example, in a large‐scale retrospective survey, patients with physician‐diagnosed IBS reported a threefold higher incidence of cholecystectomy, twofold higher incidence of appendectomy and hysterectomy, and 50% higher incidence of back surgery than patients without IBS.12 Unfortunately, the true pathological state in resected specimens of the study was unknown. On the other hand, Chaudhary and Truelove13 noted that most of the removed appendices in their patients with IBS were normal, although the exact prevalence of NA in patients with IBS was not addressed. It is likely that the visceral hypersensitivity or hypervigilance for visceral sensation often observed in IBS may mislead surgeons to perform an unnecessary appendectomy. However, this study was a retrospective review of 130 IBS‐like patients without a control group in 1962, when the consensus criteria for the diagnosis of IBS were not established. Furthermore, the modern imaging technology (CT scan and ultrasonography), which facilitates the diagnosis of acute appendicitis, was not available at that time. Therefore, in today's clinical situation, whether the diagnosis of IBS still represents a factor leading to NA requires further evaluation.
Abnormal psychosocial profiles and impaired health‐related quality of life (HRQoL) are often associated with IBS.14,15,16 In fact, psychosocial characteristics have also been suggested as the important factors leading to NA. For example, Creed17 demonstrated a significant relationship between depression and NA patients. He also noted that his patients with NA experienced significantly more severe life events than the patients with true appendicitis (59% vs 25%).17 This finding would further suggest that psychological profiles and HRQoL may be impaired in patients with NA.
In this study, we aimed to test the hypothesis that patients with NA will have a higher incidence of Rome‐II‐defined IBS, worse psychological profiles and more impaired HRQoL than patients with an acutely inflamed appendix. We also attempted to identify the factors important in predicting NA.
Patients and methods
Patients
Between April 2002 and April 2006, all patients (⩾18 years old) undergoing emergent appendectomy with the clinical impression of acute appendicitis in the emergency service of Taipei Veterans General Hospital, Taipei, Taiwan, were consecutively enrolled into the study. Taipei Veterans General Hospital is a university‐affiliated and tertiary care teaching hospital with an annual census of approximately 80 000 patient visits in its emergency service, which received patients referred from other hospitals or clinics and self‐referred patients. The clinical impression of acute appendicitis was established according to the overall information obtained from history, physical examination, routine laboratory examination and/or imaging findings, which were recorded on a standardised sheet used in our emergency service. The decision to perform an appendectomy was made by the chief residents and attending surgeons in general surgery, and the experience of those board‐certified surgeons was between 1 and 28 years, with a mean of 13 years. No instructions were given about how to interpret the diagnostic information or how to make the decision to operate. Subjects were excluded if some other cause for their abdominal pain was found at laparotomy. Subjects with current major medical or psychological conditions (medical exclusions are noted in Results) were also excluded.
During the period after appendectomy and before the patients' discharge from the hospital, a trained study nurse met the patients and instructed them on the correct way to answer the questionnaires regarding their abdominal pain and bowel symptoms, healthcare use, psychological profile and HRQoL before the onset of the abdominal pain for the appendectomy. The questionnaires were completed only when the patient was able to sit comfortably in a side room. The study nurse was blinded to both operative findings and pathological results at the time of the interview. The patients would be informed about the operative findings; however, the surgeons would emphasise that the real appendiceal feature can only be determined after pathological examination, especially for those suspected of a normal appendix at surgery. The patients did not know their pathology reports at the time of answering the questionnaire. In addition, their demographic data, associated systemic disease, surgical history, prehospital delay (the duration of symptoms before emergency service visit), in‐hospital delay (the duration between the time of emergency room evaluation and the time sent to the operation room) and hospital days were also recorded.6
All the removed appendices were reviewed by our gastrointestinal pathologist without any knowledge of symptoms and psychological profile of the enrolled subjects. Thereafter, the patients were divided into two groups according to their pathological findings.17 Specimens with normal appendix histology or those with evidence of lymphoid hyperplasia and minimal inflammation were classified in the NA group. On the other hand, subjects placed in the positive appendectomy group were those patients with acute appendicitis, which is defined by the accumulation of leucocytes throughout the appendiceal wall. The study protocol was approved by the institutional review board of Taipei Veterans General Hospital.
Questionnaire
Rome‐II questionnaire for IBS
We used a standardised questionnaire based on a module developed by the Rome‐II working team.18 This Taiwan version has been validated and published elsewhere.19,20
Rome‐II‐defined IBS is based on the presence of abdominal discomfort or pain involving two of the following three characteristics: symptoms relieved with defecation, onset associated with a change in frequency of stools or onset associated with a change in stool form.18 Eleven other IBS‐associated bowel disorders were also evaluated to help diagnosis of IBS: <3 bowel movements/week; >3 bowel movements/day; hard or lumpy stools; loose, mushy stools; straining during a bowel movement; having to rush to the toilet to have a bowel movement; feeling of incomplete emptying after a bowel movement; passing mucus during a bowel movement; abdominal fullness, bloating or swelling; a sensation that the stools cannot be passed when having a bowel movement; and a need to press on or around one's bottom or vagina to try to move the stool to complete the bowel movement.18 In addition, we evaluated the degree of each patient's healthcare usage by asking: “In the past year, how many times have you visited a physician for any illness or bowel movement disorder?”20
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression Scale (HADS) is a self‐completed questionnaire specifically developed for use in the hospital outpatient setting, which yields two subscales: anxiety and depression.21,22 Each subscale includes seven questions rated with a score of 0–3 depending on the severity of the problem described in each question. The two subscales can also be aggregated to provide an overall anxiety and depression score. A patient with a higher score had a poorer mental health status. The HADS does not consider the somatic domains of anxiety and depression, and thereby excludes the influence of confounding factors such as physical signs and symptoms. The Chinese version of the HADS is in agreement with the English origin.22
Short Form‐36 Health Survey
Short Form‐36 (SF‐36) is a generic, subjective measure of HRQoL. This assessment is extensively documented as being reliable and valid. The SF‐36 consists of 36 items that measure eight multi‐item scales and aspects of health status from physical health to mental health: physical function; role limitations due to physical problems; bodily pain (BP); general health perceptions (GH); vitality (VT); social functions (SF); role limitations due to emotional problems; and mental health (MH). These eight domains are grouped into two health dimension scales—physical (physical function; role limitations due to physical problems; BP; GH) and mental (VT; SF; role limitations due to emotional problems; MH). After summing the Likert‐scaled items in the SF‐36, each scale was standardised so that it ranged from 0 (lowest level of functioning) to 100 (highest level).23 The translation of the Taiwanese version of the SF‐36 was developed using a standard methodology followed by the International Quality of Life Assessment Projects.24 The SF‐36 Taiwan version showed good reliability and validity in a healthy adult sample and varying disease status.25,26
Power calculation
Our primary end point was the difference in prevalence of IBS between the patients with negative and positive appendectomy. A sample size of 430 patients receiving emergent appendectomy provides adequate statistical power (0.80) to detect a 19% difference in the prevalence of IBS between the patients with NA and the patients with positive appendectomy with an α of 0.01 (two‐sided).
Statistical analysis
All eligible questionnaires were coded and entered into a computer. The diagnosis of IBS was obtained via a computer‐generated algorithm. Response to the items on the SF‐36 Health Survey were compiled using standard procedures to obtain overall scores for each of the eight subscales.23
Data are expressed as mean (SD). SPSS V.10.0 was used for statistical analysis. The statistics used included Student's t test and χ2 test. A multiple logistic regression analysis was designed to identify factors predictive of NA. The strategy of analysis was as follows. Three models were first developed according to the groups of the potential risk factors—that is, chronic bowel symptoms, psychosocial profiles and clinical features in emergency service. Each of these included variables that were statistically significant in the univariate analysis; the selection was done on the basis of clinical and scientific knowledge. This strategy was necessary because of the correlation between many factors. The variables tested in the chronic bowel symptom group model were: Rome‐II‐defined IBS, having to rush to the toilet to have a bowel movement, feeling of incomplete emptying after a bowel movement and total numbers of presenting bowel symptoms. The psychological profile model included mean anxiety and depression score, BP, VT, GH, SF and MH. The emergency service model included migrating pain, fever, muscle guarding, rebounding pain, white cell counts (<11×109/l and ⩾11×109/l), percentage of polymorphonuclear cells (PMNC; <75% and ⩾75%) and the use of CT scan. In the second step of the multivariate analysis, each factor that made an independent contribution in the three models specified previously was included in a single final model along with age (<40 years and ⩾40 years) and gender. A value of p<0.05 was considered to be significant.
Results
Demographics of the subjects receiving emergent appendectomy with suspected appendicitis
During the study period, 455 patients received an emergent appendectomy as a result of suspected appendicitis. Ten patients were excluded because other causes for their abdominal pain were discovered at laparotomy (four, gynaecological conditions; three, appendix tumour; two, colon cancer; and one, perforated diverticulitis). Another seven patients were excluded due to their major medical (one, tongue cancer; two, chronic HIV infection; one, recent cerebral vascular accident) or psychiatric (two, bipolar disorder; one, schizophrenia) diseases. Eight patients refused to answer the questionnaire and were also excluded from the survey. A total of 430 patients were enrolled in this study. According to our definition, 362 (84.2%) had inflamed appendix (positive appendectomy group), whereas 68 (15.8%) were classified as not acutely inflamed (NA group). No surgical mortality was noted in the cohort.
Comparison of the demographics, Rome‐II IBS prevalence and bowel symptoms of the study groups
Table 1 compares the demographic characters and bowel symptoms of both study groups. The NA group was younger and female‐predominant relative to the positive group. A greater number of patients with NA fulfilled the Rome‐II‐defined IBS criteria compared with the positive group (p = 0.001). Although the NA group tended to have a higher proportion of various bowel symptoms suggestive of IBS, only the symptom of having to rush to the toilet to have a bowel movement reached a significant level. Nevertheless, the total symptom score was significantly higher in the NA group.
Table 1 Demographic data and bowel disorders in patients with negative and positive appendectomy.
| Negative appendectomy (n = 68) | Positive appendectomy (n = 362) | p Value | |
|---|---|---|---|
| Mean (SD) age (years) | 39.9 (18.5) | 47.5 (18.6) | 0.002 |
| Female, n (%) | 42 (61.8) | 144 (39.8) | 0.001 |
| Smoking, n (%) | 18 (26.5) | 80 (22.1) | NS |
| Alcohol consumption, n (%) | 6 (8.8) | 26 (7.2) | NS |
| Higher education (⩾ senior high), n (%) | 51 (75) | 269 (74.3) | NS |
| History, n (%) | |||
| Diabetes mellitus | 3 (4.4) | 24 (6.6) | NS |
| Hypertension | 9 (13.2) | 20 (16.6) | NS |
| Cholecystectomy | 0 (0) | 7 (1.9) | NS |
| Hysterectomy (in females) | 1 (2.4) | 9 (6.3) | NS |
| Rome‐II IBS, n (%) | 22 (32.4) | 50 (13.8) | 0.001 |
| Bowel symptoms, n (%) | |||
| <3 bowel movements a week | 4 (5.9) | 24 (6.6) | NS |
| >3 bowel movements a day | 6 (8.8) | 25 (6.9) | NS |
| Hard or lumpy stools | 7 (10.3) | 36 (9.9) | NS |
| Loose, mushy stools | 14 (20.6) | 49 (13.5) | NS |
| Straining during a bowel movement | 13 (19.1) | 56 (15.5) | NS |
| Having to rush to the toilet to have a bowel movement | 20 (29.4) | 68 (18.8) | <0.05 |
| Feeling of incomplete emptying after a bowel movement | 22 (32.4) | 80 (22.1) | 0.08 |
| Passing mucus during a bowel movement | 7 (10.3) | 24 (6.6) | NS |
| Abdominal fullness, bloating or swelling | 25 (36.8) | 102 (28.2) | NS |
| A sensation that the stools cannot be passed when having a bowel movement | 6 (8.8) | 45 (12.4) | NS |
| A need to press on or around one's bottom or vagina to try to move the stools in order to complete the bowel movement | 2 (2.9) | 11 (3.0) | NS |
| Total number of presenting bowel symptoms | 1.96 (1.65) | 1.31 (1.56) | 0.03 |
IBS, irritable bowel syndrome.
Comparison of the HADS scores, healthcare use and SF‐36 psychometric results of the study groups
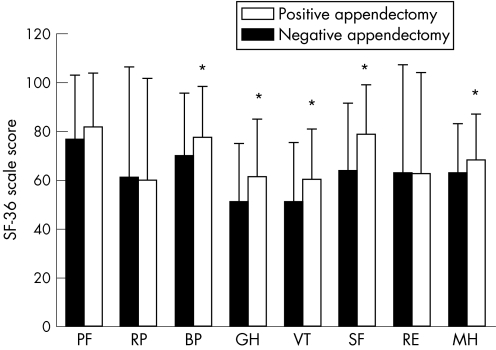
Table 2 shows that patients with NA have higher HADS scores (both anxiety and depression) than those of the positive group (p<0.01). In the previous year, patients with NA consulted physicians more for their general and gastrointestinal (GI)‐related symptoms (p<0.01). Figure 1 shows the SF‐36 scores of the study groups; patients with NA have significantly lower levels in both physical (BP, GH) and mental domains (VT, SF, MH; p<0.01).
Table 2 Hospital Anxiety and Depression Scale scores and health‐seeking behaviour in patients with negative and positive appendectomy.
| Negative appendectomy (n = 68) | Positive appendectomy (n = 362) | p Value | |
|---|---|---|---|
| HADS scores | 15.7 (8.2) | 11.9 (6.6) | 0.002 |
| Mean anxiety score | 8.30 (4.33) | 6.17 (3.59) | 0.001 |
| Mean depression score | 7.43 (4.49) | 5.77 (3.78) | <0.01 |
| Total number of visits to doctor during the previous year | |||
| Any illness | 4.9 (7.0) | 3.6 (7.1) | <0.01 |
| GI symptoms | 2.1 (4.7) | 1.0 (2.2) | <0.01 |
GI, gastrointestinal; HADS, Hospital Anxiety and Depression Scale.
Data are presented as mean (SD).
Figure 1 Health‐related quality of life is significantly worse in patients with negative appendectomy (n = 68) than in those with positive appendectomy (n = 362). Short Form‐36 (SF‐36) maximum score, 100. *p<0.01. BP, bodily pain; GH, general health perception; MH, mental health; PF, physical function; RE, role limitation–emotional; RP, role limitation–physical; SF, social functioning; VT, vitality.
Comparison of clinical features in the emergency service of the study groups
According to the standardised recording in our emergency service, patients with NA experienced significantly less migration pain, fever and muscle guarding than those in the positive group at the initial presentation (p<0.01, table 3). The negative group also had a lower white cell count and a lower percentage of PMNC than the positive group. The patients in the negative group received less CT scan examinations compared with those in the positive group. No difference could be identified in the prehospital delay, in‐hospital delay and hospital days between the two groups.
Table 3 Clinical presentations, laboratory findings, use of CT scan, patient and hospital delays, and hospitalisation days in patients with negative and positive appendectomy.
| Negative appendectomy (n = 68) | Positive appendectomy (n = 362) | p Value | |
|---|---|---|---|
| Clinical presentation, n (%) | |||
| Anorexia/nausea | 32 (47.1) | 191 (52.8) | NS |
| Vomiting | 15 (22.1) | 97 (26.8) | NS |
| Migrating pain | 30 (44.1) | 228 (63.0) | 0.004 |
| Fever | 14 (20.6) | 143 (39.5) | 0.003 |
| Physical findings, n (%) | |||
| Tenderness | 100 (100) | 100 (100) | NS |
| Localisation | 60 (88.2) | 335 (92.5) | NS |
| Muscle guarding | 34 (50) | 255 (70.4) | 0.002 |
| Rebounding pain | 46 (67.6) | 251 (69.3) | NS |
| Laboratory findings | |||
| WBC count (×109/l) | 11.6 (3.9) | 13.1 (4.1) | 0.007 |
| PMNC (%) | 76.3 (10.2) | 80.7 (9.9) | 0.001 |
| C reactive protein (mg/dl) | 3.90 (5.87) | 4.79 (6.20) | NS |
| Use of CT scan, n (%) | 21(30.9) | 183 (50.6) | 0.003 |
| Pre‐hospital delay (days) | 1.89 (2.08) | 1.35 (1.31) | NS |
| In‐hospital delay (days) | 0.30 (0.22) | 0.37 (0.37) | NS |
| Hospital day (days) | 3.46 (3.18) | 4.17 (3.91) | NS |
PMNC, polymorphonuclear cells; WBC, white blood cell.
Data are presented as mean (SD) unless otherwise mentioned.
Multivariate analysis to identify the risk factors for predicting NA
In the first step of multivariate analysis of the three models, the following variables were independently predictive of NA: Rome‐II‐defined IBS (odd ratio (OR) 2.65; 95% CI 1.34 to 5.23) in chronic bowel symptom model; degree of anxiety (OR 1.16; 95% CI 1.05 to 1.29) and SF impairment (OR 1.35; 95% CI 1.12 to 1.63) in psychosocial profile model; absence of migrating pain (OR 2.95; 95% CI 1.62 to 5.36) and muscle guarding (OR 3.96; 95% CI 2.00 to 7.83); lower percentage of PMNC (<75%) (OR 2.34; 95% CI 1.15 to 4.75); and lack of CT imaging (OR 2.98; 95% CI 1.59 to 5.60) in the emergency service model. The above‐listed variables were combined in a final model with age and gender for prediction of NA. The variables predictive of NA in the final model includes Rome‐II‐defined IBS, higher degree of anxiety, absence of migrating pain and muscle guarding, lower percentage of PMNC (<75%) and lack of CT imaging (table 4).
Table 4 Predictive variables in patients with negative appendectomy based on the multiple logistic regression analysis.
| Adjusted OR (95% CI) | p Value | |
|---|---|---|
| Rome‐II IBS | 2.17 (1.14 to 4.24) | 0.02 |
| Degree of anxiety | 1.12 (1.02 to 1.49) | 0.04 |
| Absence of migrating pain | 3.43 (1.90 to 5.95) | <0.001 |
| Absence of muscle guarding | 3.72 (2.07 to 6.70) | <0.001 |
| PMNC (<75%) | 3.05 (1.69 to 5.51) | <0.001 |
| No use of CT scan | 2.32 (1.27 to 4.26) | <0.01 |
IBS, irritable bowel syndrome; PMNC, polymophonuclear cells.
Discussion
Our prospective study indicated that Rome‐II‐defined IBS is an independent determinant in predicting NA. IBS is a common functional GI disorder manifesting as recurrent abdominal pain with an alteration of bowel function, but without organic pathology.9 Patients with IBS received disproportionately high rates of appendectomy or other abdominal/pelvic surgery.11 In a retrospective study, patients with IBS showed a fourfold increase in the rates of appendectomy relative to patients with ulcerative colitis (34.5% vs 7.9%).27 Other retrospective studies reported that 25–46% of patients with IBS underwent appendectomy, rates that are considerably greater than those of the general population.13,28,29 In a recent review of a health maintenance organisation dataset, the physician‐labelled patients with IBS (not criteria‐defined IBS, but patients recalling this event) reported that the prevalence of appendectomy was twofold higher than that in the examinees without IBS (21.1% vs 11.7%).12 Despite increased appendectomy rates often observed in patients with IBS, the aforementioned studies did not examine whether the rate of NA was also increased in patients with IBS. Only one report has shown that IBS might contribute to NA.13 In this retrospective study of 130 patients with IBS conducted 40 years ago, before the era of criteria‐defined IBS and the application of modern imaging (CT/ultrasonography), one‐third of the patients with IBS underwent an appendectomy and most of the removed appendices were normal. In the current prospective study, we further showed that the prevalence of Rome‐II‐defined IBS is higher in patients with NA than in those with true acute appendicitis.
The reasons why IBS predisposes to NA cannot be resolved in this study. However, we speculate that the visceral hypersensitivity or hypervigilance for visceral sensation often observed in patients with IBS is one of the potential mechanisms leading to NA. Disturbed visceral sensation is one of the pathogenic factors contributing to the IBS symptoms. It is postulated that IBS results from sensitisation of afferent pathways such that normal physiological gut stimuli not perceived by healthy individuals induce pain in patients with IBS.30 For example, postprandial pain has been temporally related to the entry of a food bolus into the caecum in 74% of patients with IBS.31 Exaggerated symptoms can also be induced by rectal balloon distension in patients with IBS.32 The patients with IBS tend to label a wide range of visceral stimuli in negatively affective terms.10 Andersson noted that patients with NA experienced more erroneous diagnoses due to the great intensity of their pain and tenderness, which results from an unintentional augmentation of the patient's perceived pain.8 Thus, some patients with IBS, especially those with visceral hypersensitivity or hypervigilance for visceral sensation, may unintentionally augment their abdominal pain and thus confuse the surgeons, resulting in an incorrect diagnosis. Another possible factor underlying the higher rate of normal appendices removed from patients with IBS is that these patients have a tendency towards healthcare‐seeking, which is supported by our finding of increased physician visits during the preceding year for GI and non‐GI symptoms. Thus, patients with IBS report pain as great in intensity and are also predisposed to seek care for it (and other problems), which could contribute to the removal of normal appendices. Accordingly, we suggest that surgeons should know the history of IBS in patients suspected of acute appendicitis before performing emergent appendectomy. In addition, other physicians should also be cautious in referring patients with IBS for an appendectomy.
With multivariate analysis, the degree of anxiety was proved to be an independent factor predictive of NA, which supports our hypothesis that mental stress can lead to NA. In two earlier studies, patients with NA showed increased anxiety, emotional instability and increased likelihood of childhood parental separations compared with those with true appendicitis.33,34 Depression from lasting threat or unpleasantness (such as separation of a spouse) is also associated with NA.35,36 Furthermore, patients with NA experienced more life events and psychiatric symptoms.17 The clinical significance of the psychological abnormalities may be two‐pronged. Firstly, the higher anxiety may play a role in the development of symptom (pain) and enhanced health‐seeking behaviour, a phenomenon also observed in patients with IBS.14 We also confirmed that the patients in the NA group would consult physicians more for their general or GI symptoms before the onset of acute pain. Secondly, these psychiatric symptoms may contribute to the continued abdominal pain after an NA.37
The diagnosis of acute appendicitis is based primarily on the history of pain, abdominal tenderness, and the clinical and laboratory signs of inflammation. Only one study tried to identify the factors influencing the decision to operate from these three sources of information.8 After multivariate analysis, intense perceived pain and abdominal tenderness, and a lower white cell count (<13×109/l), were the independent predictors of negative exploration in patients with suspected appendicitis. The authors concluded that NA is associated with patients placing too much emphasis on pain and tenderness. We also found that the absence of migrating pain/muscle guarding and low PMNC percentage would better predict NA. All the data suggested that patients with NA tended to have an atypical clinical presentation for acute appendicitis. Negative explorations were more likely in patients who presented with unintentional augmentation of their pain and tenderness, but without symptoms/signs of systemic infection.
To reduce the incidence of perforation, the surgical community traditionally accepts that approximately 15% of appendectomies will yield a non‐inflamed appendix.3,38 Many investigators have demonstrated that, in a research environment, advanced diagnostic testing with CT imaging decreases the frequency of misdiagnosis.39,40 Thus, CT imaging is suggested in patients suspected of having appendicitis in whom the diagnosis is unclear. In our study, no use of CT imaging is an independent predictive factor for NA. The high intensity of the abdominal symptoms/signs may mislead the surgeon to perform appendectomy without appropriate CT imaging. Together with higher chances of atypical clinical presentation in patients with NA, we suggest that a CT scan be considered in these patients to decrease the rate of NA.
In conclusion, patients with NA have a higher prevalence of IBS than those in the positive group. Both the patient (IBS, anxiety, atypical presentation) and the physician factor (low CT scan usage) are the independent factors predicting NA. Physicians should be cautious before operating on or referring patients with IBS for emergent appendectomy. CT scan should be considered in patients suspected of having appendicitis, particularly in those with IBS and atypical clinical presentations.
Abbreviations
BP - bodily pain
GH - general health perceptions
GI - gastrointestinal
HADS - Hospital Anxiety and Depression Scale
HRQoL - health‐related quality of life
IBS - irritable bowel syndrome
MH - mental health
NA - negative appendectomy
PMNC - polymorphonuclear cells
SF - social functions
SF‐36 - Short Form‐36
VT - vitality
Footnotes
Funding: This study was sponsored by a grant of Taipei Veterans General Hospital (No. 94‐230).
Competing interests: None.
References
- 1.Flum D R, Koepsell T. The clinical and economic correlates of misdiagnosed appendicitis: nationwide analysis. Arch Surg 2002137799–804. [DOI] [PubMed] [Google Scholar]
- 2.Addiss D G, Shaffer N, Fowler B S.et al The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol 1990132910–925. [DOI] [PubMed] [Google Scholar]
- 3.Korner H, Sondenaa K, Soreide J A.et al Incidence of acute nonperforated and perforated appendicitis: age‐specific and sex‐specific analysis. World J Surg 199721313–317. [DOI] [PubMed] [Google Scholar]
- 4.Pittman‐Waller V A, Myers J G, Stewart R M.et al Appendicitis: why so complicated? Analysis of 5755 consecutive appendectomies. Am Surg 200066548–554. [PubMed] [Google Scholar]
- 5.Flum D R, Morris A, Koepsell T.et al Has misdiagnosis of appendicitis decreased over time? A population‐based analysis. JAMA 20012861748–1753. [DOI] [PubMed] [Google Scholar]
- 6.Liu C C, Lu C L, Yen D H.et al Diagnosis of appendicitis in the ED: comparison of surgical and nonsurgical residents. Am J Emerg Med 200119109–112. [DOI] [PubMed] [Google Scholar]
- 7.Flum D R, McClure T D, Morris A.et al Misdiagnosis of appendicitis and the use of diagnostic imaging. J Am Coll Surg 2005201933–939. [DOI] [PubMed] [Google Scholar]
- 8.Andersson R E, Hugander A P, Ghazi S H.et al Why does the clinical diagnosis fail in suspected appendicitis? Eur J Surg 2000166796–802. [DOI] [PubMed] [Google Scholar]
- 9.Drossman D A, Camilleri M, Mayer E A.et al AGA technical review on irritable bowel syndrome. Gastroenterology 20021232108–2131. [DOI] [PubMed] [Google Scholar]
- 10.Naliboff B D, Munakata J, Fullerton S.et al Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut 199741505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasler W L, Schoenfeld P. Systematic review: abdominal and pelvic surgery in patients with irritable bowel syndrome. Aliment Pharmacol Ther 200317997–1005. [DOI] [PubMed] [Google Scholar]
- 12.Longstreth G F, Yao J F. Irritable bowel syndrome and surgery: a multivariable analysis. Gastroenterology 20041261665–1673. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhary N A, Truelove S C. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med 196231307–322. [PubMed] [Google Scholar]
- 14.Drossman D A, McKee D C, Sandler R S.et al Psychosocial factors in the irritable bowel syndrome. A multivariate study of patients and non patients with irritable bowel syndrome. Gastroenterology 198895701–708. [DOI] [PubMed] [Google Scholar]
- 15.Whitehead W E, Bosmajian L, Zonderman A B.et al Symptoms of psychologic distress associated with irritable bowel syndrome. Comparison of community and medical clinic samples. Gastroenterology 198895709–714. [DOI] [PubMed] [Google Scholar]
- 16.Gralnek I M, Hays R D, Kilbourne A.et al The impact of irritable bowel syndrome on health‐related quality of life. Gastroenterology 2000119654–660. [DOI] [PubMed] [Google Scholar]
- 17.Creed F. Life events and appendicectomy. Lancet 198111381–1385. [DOI] [PubMed] [Google Scholar]
- 18.Drossman D A C, E, Talley N J.ROME II. The functional gastrointestinal disorders. 2nd edn. Lawrence: Allen Press, 2000
- 19.Lu C L, Lang H C, Chang F Y.et al Social and medical impact, sleep quality and the pharmaceutical costs of heartburn in Taiwan. Aliment Pharmacol Ther 200522739–747. [DOI] [PubMed] [Google Scholar]
- 20.Lu C L, Chang F Y, Lang H C.et al Gender difference on the symptoms, health‐seeking behaviour, social impact and sleep quality in irritable bowel syndrome: a Rome II‐based survey in an apparent healthy adult Chinese population in Taiwan. Aliment Pharmacol Ther 2005211497–1505. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond A S, Snaith R P. The hospital anxiety and depression scale. Acta Psychiatr Scand 198367361–370. [DOI] [PubMed] [Google Scholar]
- 22.Leung C M, Ho S, Kan C S.et al Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale. A cross‐cultural perspective. Int J Psychosom 19934029–34. [PubMed] [Google Scholar]
- 23.Ware J E S, K K, Kosinski M.et alSF‐36 Health Survey‐manual and interpretation guide. Boston: New England Medical Center, The Health Institute, 1993
- 24.Ware J E, Jr, Keller S D, Gandek B.et al Evaluating translations of health status questionnaires. Methods from the IQOLA project. International Quality of Life Assessment. Int J Technol Assess Health Care 199511525–551. [DOI] [PubMed] [Google Scholar]
- 25.Wang S J, Fuh J L, Lu S R.et al Quality of life differs among headache diagnoses: analysis of SF‐36 survey in 901 headache patients. Pain 200189285–292. [DOI] [PubMed] [Google Scholar]
- 26.Fuh J L, Wang S J, Lu S R.et al Psychometric evaluation of a Chinese (Taiwanese) version of the SF‐36 health survey amongst middle‐aged women from a rural community. Qual Life Res 20009675–683. [DOI] [PubMed] [Google Scholar]
- 27.Burns D G. The risk of abdominal surgery in irritable bowel syndrome. S Afr Med J 19867091. [PubMed] [Google Scholar]
- 28.Havia T, Manner R. The irritable colon syndrome. A follow‐up study with special reference to the development of diverticula. Acta Chir Scand 1971137569–572. [PubMed] [Google Scholar]
- 29.Fielding J F, Bianchi P, Brown J M.et al The O.M.G.E. Irritable Bowel Syndrome Survey. Scand J Gastroenterol Suppl 19849570–72. [PubMed] [Google Scholar]
- 30.Hasler W L, Owyang C.Irritable bowel syndrome. 3rd edn. Philadelphia: Lippincott Williams & Wilkins, 1999
- 31.Cann P A, Read N W, Brown C.et al Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut 198324405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitehead W E, Engel B T, Schuster M M. Irritable bowel syndrome: physiological and psychological differences between diarrhea‐predominant and constipation‐predominant patients. Dig Dis Sci 198025404–413. [DOI] [PubMed] [Google Scholar]
- 33.Blanton S, Kirk V. A psychiatric study of sixty‐one appendicectomy cases. Ann Surg 1947126305–314. [PMC free article] [PubMed] [Google Scholar]
- 34.Barraclough B M. Appendicectomy in men. J Psychosom Res 196711203–206. [DOI] [PubMed] [Google Scholar]
- 35.Paulley J W. Psychosomatic factors in the aetiology of acute appendicitis. Arch Middlesex Hosp 1955535–44. [PubMed] [Google Scholar]
- 36.Harding H E. A notable source of error in the diagnosis of appendicitis. BMJ 196253111028–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker A, Mayou R. Psychological factors in patients with non‐specific abdominal pain acutely admitted to a general surgical ward. J Psychosom Res 199236715–722. [DOI] [PubMed] [Google Scholar]
- 38.Detmer D E, Nevers L E, Sikes E D., Jr Regional results of acute appendicitis care. JAMA 19812461318–1320. [PubMed] [Google Scholar]
- 39.Rao P M, Rhea J T, Novelline R A.et al Effect of computed tomography of the appendix on treatment of patients and use of hospital resources. N Engl J Med 1998338141–146. [DOI] [PubMed] [Google Scholar]
- 40.Balthazar E J, Rofsky N M, Zucker R. Appendicitis: the impact of computed tomography imaging on negative appendectomy and perforation rates. Am J Gastroenterol 199893768–771. [DOI] [PubMed] [Google Scholar]