Short abstract
Deletion of the second allele of the tumour suppressor gene MEN1 is assoiated with development of duodenal gastrin‐secreting microtumours in MEN1
Zollinger–Ellison syndrome results from hypergastrinaemia and gastric hyperacidity. The disorder is clinically characterised by recurrent peptic ulcer disease, gastroesophageal reflux disease and, often, diarrhoea.1,2 The syndrome is caused by gastrin‐secreting tumours (gastrinomas), which can arise either sporadically (approximately 70–75%) or in association with the autosomal dominant inherited condition, multiple endocrine neoplasia type 1 (MEN1; approximately 25–30%).3 The MEN1 syndrome is also associated with a range of endocrine tumours that predominantly arise in the parathyroid glands, anterior pituitary gland and pancreas.4,5 Sporadic gastrinomas are usually single and are most commonly located in the pancreas, although some are found in the duodenum. By contrast, gastrinomas found in patients with MEN1 are usually multiple and are most often located in the duodenal wall. Although multiple endocrine macrotumours and microtumours are also commonly found in the pancreas of patients with MEN1, these neoplasms usually secrete a variety of other hormones and are rarely gastrinomas.6 These observations on the location and multiplicity of tumours have implications for the surgical resection of gastrinomas, and it is now advised that even when pancreatic tumours are shown by imaging studies, duodenotomy should be performed in all cases that seem to be associated with MEN1.7,8
MEN1 is caused by germline mutations in the tumour suppressor MEN1 gene located on chromosome 11q13.9,10 This gene encodes the protein menin, whose functions have not yet been fully defined, particularly as it has been shown to bind several (mostly nuclear) partner proteins and disrupt several signalling pathways.11 A number of mouse models in which the MEN1 gene is disrupted have been generated, and many of these transgenic animals have developed tumours in the tissues (such as pancreas and parathyroid) that are also affected in the human syndrome. However, gastrinomas are only rarely observed in Men1+/− mice.10,12,13
Recently, there has been considerable interest in the pathogenesis of endocrine tumours in patients with MEN1. It has recently been proposed that tumours may arise from precursor lesions. For example, microadenomas have been identified in the pancreas of patients with MEN1, but currently there is a debate as to whether these arise from islets14 or from ductal epithelium.15 Pancreatic islet hyperplasia has also been identified in some types of Men1+/− mice.13 Recently Anlauf et al16 at the University of Kiel, Kiel, Germany, have investigated the pathogenesis of duodenal gastrinomas arising in patients with MEN1. In a paper published in Gastroenterology16 this group reported distinctive proliferative hyperplastic gastrin cell lesions in the non‐tumorous duodenum of patients with MEN1‐associated multifocal duodenal gastrinomas. The authors proposed that these lesions were preneoplastic, in an analogous fashion to C cell hyperplasia in medullary carcinoma of the thyroid in the setting of MEN217 and enterochromaffin‐like cell hyperplasia in type 1 gastric carcinoids associated with autoimmune chronic atrophic gastritis and hypergastrinaemia.18
In this issue of Gut, Anlauf et al19 (see page 637) have taken their investigations further and have analysed the genetic changes that occur in duodenal gastrinomas and their previously identified precursor lesions. Patients with MEN1 display heterozygous germline mutations in the MEN1 gene. According to Knudson's20 two‐hit hypothesis of tumour development, the formation of MEN1‐associated tumours will involve additional somatic inactivation of the wild‐type MEN1 allele on chromosome 11q13. This may result in loss of a part or all of the chromosome 11. PCR‐based techniques have previously been used to demonstrate loss of heterozygosity (LOH) at 11q13 in approximately half the cases of a variety of MEN1‐associated macrotumours including gastrinomas.21 Other cases may harbour intragenic mutations that inactivate the gene but do not lead to allelic loss, and that would not be detected by this technique.22 PCR‐based methods involving microdissection are not currently precise enough to allow analysis of genetic changes occurring in individual cells. The authors have therefore used a novel combined fluorescence in situ hybridisation and immunofluorescence technique to simultaneously detect allelic deletions and hormone expression in individual duodenal neuroendocrine cells.
The authors initially analysed 28 duodenal gastrin‐producing neuroendocrine tumours from six patients with Zollinger–Ellison syndrome and MEN1, and found LOH for 11q13 in 46% of patients.19 Different tumours from the same patient often showed different patterns of allelic loss (allelic loss of 11q13 only, loss of the whole chromosome or no LOH), suggesting that each gastrinoma arising in an individual patient developed as a result of an independent second hit. In addition to the tumours studied, all six patients showed several foci of hyperplastic gastrin cells.16 However, none of these lesions showed LOH at the tested loci. The authors therefore conclude that the hyperplastic lesions (which are proposed to be preneoplastic) retain both MEN1 alleles and that allelic loss (or alternatively MEN1 gene mutation, which may be present in 54% of tumours not demonstrating LOH) is responsible for the initial development of neoplastic lesions. Similar findings were made in five somatostatin‐secreting tumours from the same patients, two of which showed LOH at the 11q13 locus, whereas there was no LOH in hyperplastic somatostatin cell lesions.19
Several questions still need to be answered. Perhaps one of the more intriguing questions is the cause of hyperplasia of gastrin‐secreting and somatostatin‐secreting cells in the duodenums of patients with MEN1, especially as these lesions do not show LOH of the MEN1 gene. Could the hyperplasia of these cells be hormonally regulated as is observed in the enterochromaffin‐like cell hyperplasia associated with hypergastrinaemia, or is it simply the result of a germline mutation in a single MEN1 gene? If a germline mutation in a single MEN1 gene is the case, why are the lesions predominantly composed of gastrin‐secreting or somatostatin‐secreting cells rather than other endocrine cell types? Also, as analysis of the hyperplastic lesions showed no LOH at 11q13, it remains to be answered whether these lesions are truly preneoplastic. Is it possible that these lesions harbour inactivating intragenic mutations that do not lead to LOH? This remains to be tested. Do gastrinomas arising in MEN1 arise within foci of gastrin‐cell hyperplasia or can they arise from any cell that acquires a second‐hit mutation? Moreover, do any external or luminal factors cause the second hit to develop? Even if the hyperplastic lesions are not truly preneoplastic, they may be clinically useful as their detection by biopsy in a patient with Zollinger–Ellison syndrome suggests that the condition is likely to be MEN1‐associated rather than being the sporadic form.
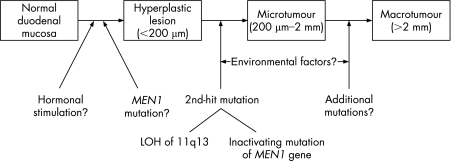
In conclusion, the study by Anlauf et al19 has enhanced our knowledge of the pathogenesis of MEN1‐associated gastrinomas. Multiple tumours seem to arise in susceptible patients as a result of multiple independent second‐hit mutations in the MEN1 gene (fig 1). The described combined fluorescence in situ hybridisation/immunofluorescence technique is a very powerful approach for analysing genetic changes in hyperplastic lesions and microtumours involving a few cells and avoids the potential errors that may result from microdissection. Further investigations of the significance of the duodenal gastrin and somatostatin hyperplastic lesions found in patients with MEN1 are warranted. From a clinical perspective, it is now crucial at the time of diagnosis to determine, if possible, whether a patient with Zollinger–Ellison syndrome has a sporadic or MEN1‐associated gastrinoma, because the different locations and numbers of tumours in these two contexts now suggest different therapeutic approaches, particularly with regard to surgery.
Figure 1 Proposed mechanism for pathogenesis of duodenal gastrinomas in multiple endocrine neoplasia type 1 (MEN1). LOH. Loss of heterozygosity.
Footnotes
Competing interests: None.
References
- 1.Jensen R T. Gastrinomas: advances in diagnosis and management. Neuroendocrinology 200480(Suppl 1)23–27. [DOI] [PubMed] [Google Scholar]
- 2.Campana D, Piscitelli L, Mazzotta E.et al Zollinger–Ellison syndrome. Diagnosis and therapy. Minerva Med 200596187–206. [PubMed] [Google Scholar]
- 3.Gibril F, Jensen R T. Zollinger–Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep 20046454–463. [DOI] [PubMed] [Google Scholar]
- 4.Brandi M L, Gagel R F, Angeli A.et al Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 2001865658–5671. [DOI] [PubMed] [Google Scholar]
- 5.Doherty G M. Multiple endocrine neoplasia type 1. J Surg Oncol 200589143–150. [DOI] [PubMed] [Google Scholar]
- 6.Anlauf M, Garbrecht N, Henopp T.et al Sporadic versus hereditary gastrinomas of the duodenum and pancreas: distinct clinico‐pathological and epidemiological features. World J Gastroenterol 2006125440–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton J A. Surgical treatment and prognosis of gastrinoma. Best Pract Res Clin Gastroenterol 200519799–805. [DOI] [PubMed] [Google Scholar]
- 8.Norton J A, Alexander H R, Fraker D L.et al Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases, or survival in patients with Zollinger–Ellison syndrome? Ann Surg 2004239617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandrasekharappa S C, Guru S C, Manickam P.et al Positional cloning of the gene for multiple endocrine neoplasia‐type 1. Science 1997276404–407. [DOI] [PubMed] [Google Scholar]
- 10.Marx S J. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer 20055367–375. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal S K, Kennedy P A, Scacheri P C.et al Menin molecular interactions: insights into normal functions and tumorigenesis. Horm Metab Res 200537369–374. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree J S, Scacheri P C, Ward J M.et al A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci USA 2001981118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loffler K A, Biondi C A, Gartside M.et al Broad tumor spectrum in a mouse model of multiple endocrine neoplasia type 1. Int J Cancer 2007120259–267. [DOI] [PubMed] [Google Scholar]
- 14.Anlauf M, Schlenger R, Perren A.et al Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol 200630560–574. [DOI] [PubMed] [Google Scholar]
- 15.Vortmeyer A O, Huang S, Lubensky I.et al Non‐islet origin of pancreatic islet cell tumors. J Clin Endocrinol Metab 2004891934–1938. [DOI] [PubMed] [Google Scholar]
- 16.Anlauf M, Perren A, Meyer C L.et al Precursor lesions in patients with multiple endocrine neoplasia type 1‐associated duodenal gastrinomas. Gastroenterology 20051281187–1198. [DOI] [PubMed] [Google Scholar]
- 17.Guyetant S, Blechet C, Saint‐Andre J P. C‐cell hyperplasia. Ann Endocrinol (Paris) 200667190–197. [DOI] [PubMed] [Google Scholar]
- 18.Burkitt M D, Pritchard D M. Review article: pathogenesis and management of gastric carcinoid tumours. Aliment Pharmacol Ther 2006241305–1320. [DOI] [PubMed] [Google Scholar]
- 19.Anlauf M, Peren A, Henopp T.et al Allelic deletion of the MEN1 gene in duodenal gastrin and somatostatin cell neoplasms and their precursor lesions. Gut 200756637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knudson A G. Two genetic hits (more or less) to cancer. Nat Rev Cancer 20011157–162. [DOI] [PubMed] [Google Scholar]
- 21.Debelenko L V, Zhuang Z, Emmert‐Buck M R.et al Allelic deletions on chromosome 11q13 in multiple endocrine neoplasia type 1‐associated and sporadic gastrinomas and pancreatic endocrine tumors. Cancer Res 1997572238–2243. [PubMed] [Google Scholar]
- 22.Pannett A A, Thakker R V. Somatic mutations in MEN type 1 tumors, consistent with the Knudson “two‐hit” hypothesis. J Clin Endocrinol Metab 2001864371–4374. [DOI] [PubMed] [Google Scholar]