Abstract
Objective: Assessments of endothelial cell function with acetylcholine have typically used systemic, regional intra-arterial, or iontophoretic delivery of drug. Each of these techniques induces systemic and/or local changes that compromise their safety or effectiveness. Using translucent drug preparations applied under laser Doppler flowmetry (LDF) probes, we tested whether local vasodilation can be induced with non-iontophoretic transdermal delivery of acetylcholine and how such dilation would compare to the dilation achieved with topical nitroglycerin in healthy volunteers. Methods: Ten subjects without known vascular disease were recruited for LDF monitoring at sites of drug application for this preliminary investigation. Topical acetylcholine chloride, nitroglycerin, and placebo were applied via translucent patches to the forehead directly below LDF probes. Results: LDF readings increased by 406 percent (245 percent to 566 percent) and 36 percent (26 percent to 46 percent), respectively, at the acetylcholine and placebo sites (p = .005 by Wilcoxon Signed Rank Test (WSRT) for acetylcholine vs. placebo); and they increased by 365 percent (179 percent to 550 percent) at the nitroglycerin site (p = .005 by WSRT for nitroglycerin vs. placebo; p = .6 vs. acetylcholine). Conclusion: Transdermal delivery of acetylcholine can induce significant local vasodilatory responses comparable to those achieved with nitroglycerin without requiring iontophoresis. The means of transdermal delivery and monitoring described herein may constitute a new minimally invasive way to interrogate the microvasculature and thereby assess the microcirculatory changes induced by various disorders and therapeutic interventions.
Introduction
Since the discovery that it acts as an endothelium-dependent vasodilator [1], acetylcholine has been used to document the presence and degree of endothelial cell dysfunction in a variety of disease states. In the context of atherosclerotic disease, intracoronary injection of acetylcholine is associated with impaired vasodilation or frank vasoconstriction, leading to the suggestion that it serve as a test for early detection of coronary artery disease [2–4]. Likewise, the vasodilatory response to brachial or femoral artery injection of acetylcholine is compromised in the presence of coronary artery disease [5] and hypertension [6]. These approaches have been used to track attempts to improve endothelial function in patients with coronary disease [7] and peripheral-arterial disease [8–12]. However, the need for an intra-arterial injection has led such tests to be primarily confined to research settings.
In an effort to avoid the invasive nature of intra-coronary, intra-femoral, or intra-brachial injection, investigators have sought other means of acetylcholine delivery. Laser Doppler flowmetry (LDF) of the microvasculature has identified vasodilation in response to iontophoretic application of acetylcholine in healthy subjects and documented that this response is compromised in patients with diabetes [13]. While iontophoresis offers a less invasive method of drug delivery than intra-arterial injections, it, too, is far from ideal. In addition to the slight risk of electrical burn from the technique [14], electric current itself has been shown to be a potent vasodilator [14], whose effects are impaired in some vasculopathic subjects [15]. Even when data are modified to account for a potential current-induced effect, the current remains a potential confounder in such studies [16]; its effect varies among specific vehicles and drugs as well as spatially over the area of drug delivery [17,18].
In order to avoid these limitations, we prepared and tested a means for LDF monitoring of acetylcholine responses via a translucent, non-iontophoretic, transdermal delivery system. In addition, a commercially available translucent patch for delivery of nitroglycerin (Minitran™ Patch, 3M, Minnesota) was adapted for LDF monitoring of local changes in microvascular perfusion without remote or systemic effects. The primary endpoint of the present investigation was whether non-iontophoretic topical application of acetylcholine would induce local vasodilation greater than placebo. We also sought a preliminary understanding of whether the acetylcholine-induced vasodilation was comparable to that achieved with nitroglycerin.
To our knowledge, this is both the first report of the use of non-iontophoretic translucent patches for continuous LDF monitoring of drug effects during topical application and the first published report of the non-iontophoretic, transdermal effects of acetylcholine on the microcirculation.
Materials and Methods
With Institutional Review Board (IRB) approval, 10 healthy volunteers were recruited for LDF measurements of forehead perfusion at sites of transdermal application of acetycholine, nitroglycerin, and placebo. For each session, after informed consent was obtained, subjects lay on a bed in a semi-recumbent position in a temperature-controlled room (22 ± 1°C). A three-lead electrocardiogram and a non-invasive brachial artery blood pressure cuff were applied. Local forehead skin oils were decreased by wiping lightly with an alcohol swab, followed by wet and dry gauze.
Per agreement with our IRB, the application of acetylcholine to the highly vascular forehead in this preliminary investigation was limited to doses of acetylcholine that did not exceed 10 percent of that used safely in other clinical settings. We initially tested 10 percent of the standard 20 mg dose of an acetylchloline-containing preparation (Miochol-E™, Norvartis Ophthalmics, East Hanover, New Jersey) that is used to induce pupillary constriction during cataract surgery. While initial (unpublished) trials by our investigative team found that a 5 percent solution (20 mg/0.4 ml) of Miochol-E™ induced a 100 percent to 300 percent increase in local blood flow after topical application to the forehead under an LDF probe, the presence of mannitol in the Miochol-E™ preparation may have affected local vascular changes. This prompted our decision to dissolve pure acetylcholine chloride powder in water.
To achieve a concentrated solution for delivery of a dose ≤2 mg (≤10 percent of that in the Miochol-E™), 100-mg acetylcholine chloride (Spectrum Chemical, New Brunswick, New Jersey) powder was freshly mixed with 0.6 ml high pressure liquid chromatography (HPLC) grade water to a final volume of approximately 0.7 ml. Then 0.02 ml of this 143 mg/ml acetylcholine solution (containing 2.86 mg of drug) was spread on a double stick clear disk (diameter: 12.6 mm, area: 124.7 mm2). The study sites were delineated by placing double-stick discs (3M Health Care, Neuss, Germany) with an overall diameter of 38.1 mm and a central hole diameter of 8.7 mm on the forehead. These discs served as an adherent base onto which laser Doppler probes were placed, as well as to define the area (59.4 mm2) for drug application. Drug delivery was limited to the area over the central hole of the double-stick disc (59.4 mm2), or approximately one-half of the total drug in solution. The anticipated dose of 1.43 mg was ≤10 percent of the 20 mg dose used for intraocular injection and allowed for safe, unanticipated delivery of a slightly greater quantity of drug. The dry side of the acetylcholine patch was adhered to the end of an LDF probe (PF 5010 with Probe Model 407, Perimed, Järfälla, Sweden). A sham drug patch was made in the same way with 0.02 ml of HPLC-grade water instead of acetylcholine solution.
For transdermal delivery of nitroglycerin, we used a portion of a commercially available translucent nitroglycerin patch (Minitran™ nitroglycerin patch, 3M, St. Paul, Minnesota) which delivers at a homogenous rate of 0.03 mg/hr/100 mm2. A section of a standard 20 cm2 patch (designed to deliver a clinical dose at a rate of 0.6 mg/hr) was obtained by using a standard hole-punch to cut a 6 mm diameter (28.3 mm2 area). The delivery of 0.008 mg/hr from this reduced area was well within the 10 percent safety limit. The patch then was placed onto a double-stick disc and placed over an LDF probe in a similar manner to the acetylcholine patch.
The three LDF probes with patches attached were placed on a double-stick disc at three forehead sites so as to enable undisturbed monitoring and drug delivery. LDF monitoring was performed continuously at each site for a maximum of 20 minutes or until a vasodilatory plateau was maintained for ≥3 minutes. Data were collected using Chart for Windows (ADInstruments, Colorado Springs, Colorado). All LDF sensors were calibrated using a motility standard (Perimed, Sweden) twice during the three-month study period.
The local microvascular effects of transdermal acetylcholine and nitroglycerin were tested in 10 healthy volunteers (seven males, three females; mean age: 36.1; range: 19 to 56 years) without known vascular disease. An investigator blinded to the status of the subject and to the nature of the study site assessed the laser Doppler tracing at each study site. Our trials demonstrated that within 10 seconds of probe application, a steady baseline interval was consistently obtainable and that a progressive drug-induced rise in flow with both acetylcholine and nitroglycerin was noted to begin no sooner than 30 seconds after probe application. This enabled a 20-second baseline period to be obtained, without the need to remove the probe for subsequent drug application. The drug-induced increase in flow in the present study was determined by comparing the mean during the lowest 10-second interval during the baseline period with the mean during the maximum 10-second interval after a plateau was reached. If no distinct rise in blood flow was evident, then the highest 10-second interval that occurred beyond the first two minutes of readings was used.
All data are presented as the mean with 95 percent confidence intervals (CI). Since the primary goal was to test whether the means of delivery and testing introduced herein generate and delineate a significant vasodilatory response compared to placebo, the primary endpoint was the percent change in LDF voltage after transdermal application of each active drug vs. placebo in the study population. Data were analyzed with the Wilcoxon Signed Rank Test (WSRT), using SPSS for Macintosh (SPSS Inc., Chicago, Illinois). Power analysis was based on reports that iontophoretic delivery of acetylcholine generated increases in skin blood flow ranging from 160 percent to 710 percent, depending on concentration of drug and iontophoretic current [19]. Given these data and our preliminary trials, we anticipated at least a 100 percent increase in blood flow (with a standard deviation of 50 percent) at the sites of active patches vs. a 20 percent difference (with a standard deviation of 10 percent) at sites of placebo application. In order to identify the difference between the active and placebo patches with an alpha of 0.025 (simple Bonferroni correction of p = .05 given two comparisons) and a beta of 0.8, we calculated that a sample size of seven subjects would be required. For values < 0.05, the actual value is reported; p values greater than 0.025 were considered not significant. With the realization that our study design was not powered to assess inter-drug comparisons, a secondary endpoint was assessed. The effects of the single dose of acetylcholine were compared to the single dose of nitroglycerin in the healthy volunteers, with the differences analyzed using WSRT.
Results
Acetylcholine and nitroglycerin both induced a marked rise in LDF voltage within two minutes of drug application in each subject. This was evident not only with respect to mean flow, but also with respect to the amplitude of the pulsation coincident with each heart beat.
Mean blood flow readings increased by 406 percent (245 percent to 566 percent) and 36 percent (26 percent to 46 percent), respectively, at the acetylcholine and placebo sites (p = .005 by WSRT for acetylcholine vs. placebo); and they increased by 365 percent (179 percent to 550 percent) at the nitroglycerin site (p = .005 by WSRT nitroglyerin vs. placebo; p = NS nitroglycerin vs. acetylcholine) [Figure 1].
Figure 1.
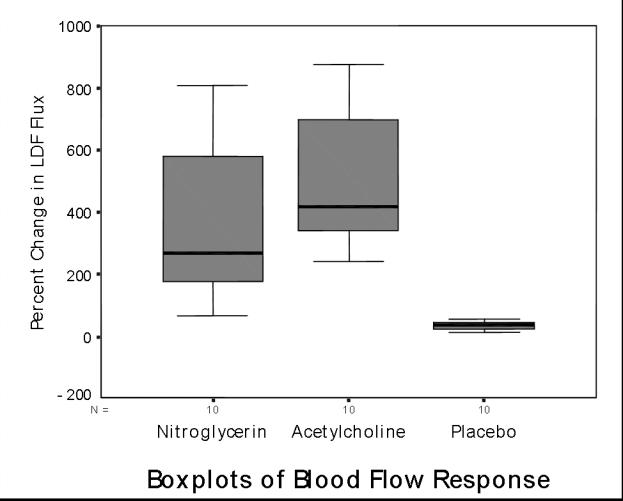
Boxplots of percent change in blood flow at sites of nitroglycerin, acetylcholine, and placebo patches. The boxes incorporate the data between the 25th and 75th percentiles; the line within the boxes is the median; the whiskers on either side show the full range of data values to within 1.5 times the interquartile range. Beyond that range, any outliers would have been shown as individual data points. For both drugs vs. placebo: p = .005).
Mean baseline pulse amplitude at the acetylcholine site was 0.13 V (0.06 to 0.19) at baseline vs. 0.43 V (0.25 to 0.61) during drug effect (p = .005 by WSRT). For nitroglycerin, baseline pulse amplitude was 0.09 V (0.05 to 0.13) vs. 0.25 V (0.13 to 0.36) during drug effect (p = .005 by WSRT, p = NS for nitroglycerin vs. acetylcholine). Mean placebo pulse amplitude did not change significantly, going from 0.17 V (0.09 to 0.24) to 0.16 V (0.06 to 0.26) during drug effect (p = NS by WSRT) [Figure 2].
Figure 2.
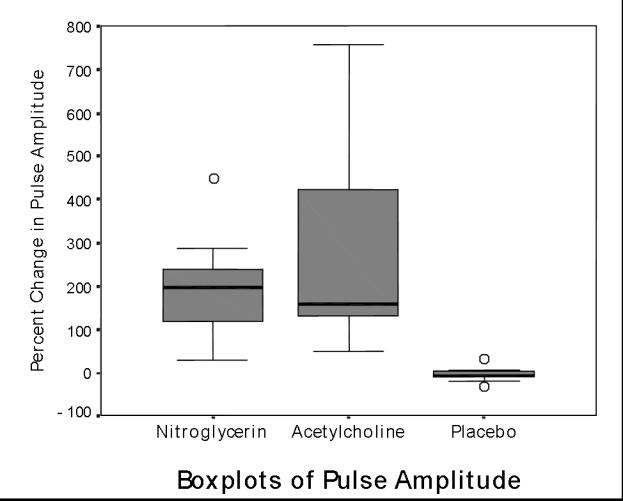
Boxplots of percent change in pulse amplitude at sites of nitroglycerin, acetylcholine, and placebo patches. (See Figure 1 legend). Both drugs vs. placebo: p = .005.
The lack of significant systemic effect was suggested by the lack of vasodilation at the placebo site; mean post-drug and baseline flow and amplitude values did not change significantly. The slight increase in flow at the placebo site seen in Figure 2 is attributable to our method of comparing the highest 10 second interval after drug application with the lowest 10 second interval at baseline. LDF readings have some temporal variation even under baseline conditions, a feature that would be emphasized by looking at maximal differences. A clinically trivial drop in mean blood pressure was recorded from 81 mmHg (75 to 86) at baseline to 78 mmHg (73 to 83) after drug absorption (p = .03 by WSRT). There was no significant change in heart rate: 1.00 Hz (0.90 to 1.11) at baseline vs. 1.04 Hz (0.94 to 1.15) after drug absorption (p = NS).
Discussion
We have demonstrated that microvascular dilation comparable to that achieved with iontophoretic delivery [13,20] can be induced via topical application of acetylcholine and nitroglycerin and that such effects easily can be monitored via LDF directly over a translucent delivery vehicle. The local vasodilatory effect of transdermal nitroglycerin has long been appreciated; to the best of our knowledge, a comparable response to non-iontophoretic transdermal acetylcholine has not been reported. Its rapid breakdown by plasma cholinesterase makes it well-suited to transdermal application for local testing of the microvasculature as well as possible vasodilatory therapy without significant systemic effect.
The robust response to non-iontophoretic acetylcholine delivery in the present study may be attributable, in part, to our choice of the forehead. Parasympathetic innervation is more prominent in this central region than in the vasculature of the periphery [14–18], and the vasodilatory response to iontophoretic delivery of acetylcholine has been shown to decrease in the distal periphery as compared to more proximal sites [21]. Prior research by our team [22,23] and others [24] noted that perfusion in central skin such as that of the forehead tends to be maintained in the context of vasoconstrictive challenges that cause decreased perfusion in the periphery. It has been suggested that this may be a consequence of homeostatic cholinergic mechanisms designed to preserve organ blood flow [25,26].
It should be noted that the patch technique described herein may be prone to the spatial and temporal variation that characterizes LDF readings in general. The translucent delivery systems enabled us to obtain a short baseline reading in the initial period of drug application, thereby avoiding an impact of spatial variation on baseline-to-drug comparisons. However, the technique would be improved by a delayed-release patch that would allow for a more prolonged baseline measurement. With the LDF probes employed in this study, it has been determined that one to three arteriolar-capillary networks are typically interrogated in the approximate 1 mm3 volume of tissue that is monitored [27]. Spatial variability may be reduced with a larger LDF probe and local mapping with a moving probe [28] or a laser Doppler scanner.
Whether the test described will be useful for microvascular assessments of the endothelium or for therapeutic induction of local vasodilation remains to be determined. With further elaboration, this technique may constitute a useful and minimally invasive way to interrogate the microvasculature in general, including its responses in various disorders and the microcirculatory changes induced by therapeutic interventions.
Abbreviations
- CI
confidence interval
- IRB
Institutional Review Board
- HPLC
high pressure liquid chromatography
- LDF
laser Doppler flowmetry
- WSRT
Wilcoxon Signed Rank Test
References
- Furchgott RF , Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- Drexler H, Zeiher AM. Progression of coronary endothelial dysfunction in man and its potential clinical significance. Basic Res Cardiol. 1991;2:223–232. doi: 10.1007/978-3-642-72461-9_22. [DOI] [PubMed] [Google Scholar]
- Bossaller C, Habib GB, Yamamoto H, Williams C, Wells S, Henry PD. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987;79:170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanyasiri P, Celermajer DS, Adams MR. Endothelial dysfunction occurs in peripheral circulation patients with acute and stable coronary artery disease. Am J Physiol Heart Circ Physiol. 2005;289:H513–H517. doi: 10.1152/ajpheart.01086.2004. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Imaizumi T, Ando S, Hirooka Y, Harada S, Takeshita A. Impaired forearm vasodilatation by acetylcholine in patients with hypertension. Heart Vessels. 1991;6:218–223. doi: 10.1007/BF02125100. [DOI] [PubMed] [Google Scholar]
- Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing Endothelial Dysfunction) Study. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in forearm resistance vessels of humans with hypercholesterolemia. Circulation. 1997;95:2617–2622. doi: 10.1161/01.cir.95.12.2617. [DOI] [PubMed] [Google Scholar]
- Prasad A, Tupas-Habib T, Schenke WH, et al. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101:2349–2354. doi: 10.1161/01.cir.101.20.2349. [DOI] [PubMed] [Google Scholar]
- Husain S, Andrews NP, Mulcahy D, Panza JA, Quyyumi AA. Aspirin improves endothelial dysfunction in atherosclerosis. Circulation. 1998;97:716–720. doi: 10.1161/01.cir.97.8.716. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within one month. Circulation. 1997;95:1126–1131. doi: 10.1161/01.cir.95.5.1126. [DOI] [PubMed] [Google Scholar]
- Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Diabetologia. 1995;38:137–144. doi: 10.1007/BF00401767. [DOI] [PubMed] [Google Scholar]
- Ledger P. Skin biological issues in electrically enhanced transdermal delivery. Adv Drug Deliv Rev. 1991;9:289–307. [Google Scholar]
- Peters EJ, Armstrong DG, Wunderlich RP, Bosma J, Stacpoole-Shea S, Lavery LA. The benefit of electrical stimulation to enhance perfusion in persons with diabetes mellitus. J Foot Ankle Surg. 1998;37:396–400. doi: 10.1016/s1067-2516(98)80048-3. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Ramsay JE, Brooks N, et al. Elimination of electrically induced iontophoretic artefacts: implications for non-invasive assessment of peripheral microvascular function. J Vasc Res. 2002;39:447–455. doi: 10.1159/000064515. [DOI] [PubMed] [Google Scholar]
- Droog EJ, Sjoberg F. Nonspecific vasodilatation during transdermal iontophoresis: the effect of voltage over the skin. Microvasc Res. 2003;65:172–178. doi: 10.1016/s0026-2862(03)00002-5. [DOI] [PubMed] [Google Scholar]
- Khan F, Newton DJ, Smyth EC, Belch JJ. Influence of vehicle resistance on transdermal iontophoretic delivery of acetylcholine and sodium nitroprusside in humans. J Applied Physiol. 2004;97:883–887. doi: 10.1152/japplphysiol.00373.2004. [DOI] [PubMed] [Google Scholar]
- Christen S, Delachaux A, Dischl B, et al. Dose-dependent vasodilatory effects of acetylcholine and local warming on skin microcirculation. J Cardiovasc Pharmacol. 2004;44:659–664. doi: 10.1097/00005344-200412000-00006. [DOI] [PubMed] [Google Scholar]
- Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol. 1996;496 (Pt 2):531–542. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli S, Waeber B, Dalle-Ave A, Feihl F. Reproducibility of laser Doppler imaging of skin blood flow as a tool to assess endothelial function. J Cardiovasc Pharmacol. 2000;36:640–648. doi: 10.1097/00005344-200011000-00014. [DOI] [PubMed] [Google Scholar]
- Silverman DG, Stout RG, Lee FA, Ferneini EM. Detection and characterization of cholinergic oscillatory control in the forehead microvasculature in response to systemic alpha-agonist infusion in healthy volunteers. Microvasc Res. 2001;61:144–147. doi: 10.1006/mvre.2000.2283. [DOI] [PubMed] [Google Scholar]
- Silverman DG, Jotkowitz AB, Gutter V, Braverman IM, O'Connor TZ. Regional vs systemic responses to mental stress: a potential mechanism for non-demand-related ischemia. Microvasc Res. 1996;51:396–399. doi: 10.1006/mvre.1996.0036. [DOI] [PubMed] [Google Scholar]
- Hertzman AB, Roth LW. The absence of vasoconstrictor reflexes in the forehead circulation: effects of cold. Am J Physiol. 1942;136:692–697. [Google Scholar]
- Silverman DG, Jotkowitz AB, Freemer M, Gutter V, O'Connor TZ, Braverman IM. Peripheral assessment of phenylephrine-induced vasoconstriction by laser Doppler flowmetry and its potential relevance to homeostatic mechanisms. Circulation. 1994;90:23–26. doi: 10.1161/01.cir.90.1.23. [DOI] [PubMed] [Google Scholar]
- Silverman DG, Stout RG. Distinction between atropine-sensitive control of microvascular and cardiac oscillatory activity. Microvasc Res. 2002;63:196–208. doi: 10.1006/mvre.2001.2385. [DOI] [PubMed] [Google Scholar]
- Braverman IM, Schechner JS, Silverman DG, Keh-Yen A. Topographic mapping of the cutaneous microcirculation using two outputs of laser-Doppler flowmetry: flux and the concentration of moving blood cells. Microvasc Res. 1992;44:33–48. doi: 10.1016/0026-2862(92)90100-4. [DOI] [PubMed] [Google Scholar]
- Nissen A, Rose M, Schonberger RB, Silverman TJ, Silverman DG. Consistency of laser Doppler Assessments of vasoreactivity; Abstracts of the Annual Meeting of the American Society of Anesthesiologists; 2006. p. A244. [Google Scholar]


