Abstract
Background
Relatives of BRCA1 and BRCA2 mutation carriers have long been proposed by epidemiological studies to have an increased risk of developing prostate cancer. In the Ashkenazi Jewish (AJ) population, the existence of 3 frequent founder mutations, 185delAG and 5382insC in BRCA1 and 6174delT in BRCA2 greatly facilitates screening for carriers.
Methods
We tested 146 AJ men with confirmed diagnoses of invasive prostate cancer. Thirteen had at least one first degree relative with prostate cancer. The median age at diagnosis of participants was 67.9 years (range 48.6–84.2 years). Subjects were screened for the BRCA1:185delAG, BRCA1:5382insC and BRCA2:6174delT mutations simultaneously using a multiplex sizing assay detecting band shifts in the presence of the variant sequence.
Results
Two out of 146 individuals were found to carry the germline BRCA2 mutation 6174delT (1.4%); the previously reported population frequency for this mutation is ~1% in AJ. We found no BRCA1 mutations. One carrier had 2 uncles affected with prostate cancer, while the other had an uncle and daughter with breast cancer. We combined our results with previously published data examining these 3 founder AJ mutations in men with prostate cancer and in population controls. Including our results, studies to date reported 5/463 (1.1%), 2/293 (0.68%) and 7/461 (1.3%) carriers for the BRCA1:185delAG, BRCA1:5382insC and BRCA2:6174delT mutations in prostate cancer cases, respectively. This compares with combined reported frequencies of 85/9371 (0.91%), 24/8867 (0.27%) and 119/9514 (1.3%) for the same mutations in control individuals. There was no statistically significant excess of mutations in cases compared to controls in either gene.
Conclusions
Our observations remain preliminary. By combining all studies published to date, we have an 80% power to detect ORs of 2.7, 6.6 and 2.5 (185delAG, 5382insC and 6174 delT, respectively) while the values we observed range between 1.0 and 2.5. However, the contribution of rare mutations with such low odds ratios to the population prostate cancer burden is unlikely to be large enough to be clinically useful. Thus, contrary to suggestions from some previous epidemiological data, our observations do not support an important role for AJ founder BRCA1/2 mutations in prostate cancer risk.
Keywords: prostate cancer, BRCA1, BRCA2, founder mutations
Background
Mutations in the BRCA1 and BRCA2 genes are responsible for a large proportion of inherited breast and ovarian cancer cases. Both BRCA1 and BRCA2 comprise many exons and hundreds of specific mutations have been identified to date in these 2 large genes (Breast Cancer Information Core website). This makes screening for mutations in individuals at risk a time consuming task that complicates mutation testing for large numbers of samples.
For over 2000 years, Jews have been a migratory people linked by religion, language, customs and culture, and have been establishing communities throughout the Middle East and the Mediterranean basin (reviewed in [1]). This has resulted in the creation of a Jewish genetic identity which evolved over time, partly through genetic drift and partly due to bottlenecks resulting from wars or epidemics, often followed by rapid population growth thanks to large family sizes. This genetic identity is characterized by the existence of some 40 genetic conditions with Mendelian patterns of transmission with established allele frequencies within distinct Jewish groups. In Ashkenazi Jews, two founder mutations in BRCA1 (185delAG, population frequency ~1%, 5382insC, ~0.13%) and one founder mutation in BRCA2 (6174delT, population frequency ~1%) [2-4], greatly facilitate screening of individuals at risk for breast and ovarian cancer.
Previous studies have suggested an increased risk of prostate cancer in relatives of BRCA1 and BRCA2 mutation carriers or in mutation carriers themselves [5-11]. When comparing BRCA1 and BRCA2 carriers in similarly designed studies, the risk of prostate cancer appears to be lower in BRCA1 carriers [11] than in BRCA2 carriers [10], and mostly confined to early-onset prostate cancer cases. Prostate cancer is a common malignancy, and identifying individuals at risk of developing the disease could be of clinical importance. Therefore, we attempted to determine the contribution of germ-line BRCA1/2 mutations to the risk of prostate cancer by testing a group of unselected AJ prostate cancer cases for the 3 founder mutations.
Methods
Study Population
Following IRB approval, we used hospital-based registries to identify 435 self-reported AJ men with prevalent prostate cancer, diagnosed between 1991 and 2002, who were known to be alive in 2002. Patients were considered Ashkenazi Jewish if both parents were reported as Ashkenazi Jewish, with no Sephardic heritage. Individuals not fulfilling these criteria were excluded. All were diagnosed and/or treated in one of three large McGill University affiliated hospitals in metropolitan Montreal, Canada. The diagnosis of invasive prostate cancer was confirmed by examining pathology reports from patients' medical charts. At the time of writing, 250 patients had been contacted (reasons for lack of contact: physician approval pending, returned unopened letters, address unknown, left town). Of these, 205 responded to our letter; 48 refused and 157 agreed to participate in the study. One-hundred-and-forty-six had provided a blood sample for this study at the time of analysis and were genotyped for two BRCA1 and one BRCA2 AJ founder mutations, making this the largest series of AJ prostate cancer cases studied thus far. All participants were given the option of receiving genetic counseling.
Thirteen cases had a family history (at least one first degree relative with prostate cancer) (8.9%); a single proband was included in the study from each family with multiple prostate cancer cases. The median age at diagnosis of participants was 67.9 years (range 48.6–84.2 years) and the participants were tested at a median of 5.7 years since diagnosis (range 0.3–23.7 years, 5 cases had missing information). Overall, 75/146 participants (51.4%) were found to have a Gleason score of 6/10 or greater (median number of months since diagnosis = 55) and 53/146 (36.35%) were found to have a Gleason score of 5 or less (median number of months since diagnosis = 78). This difference in the time interval between diagnosis and blood collection, dichotomized at a Gleason score of 6, is statistically significant (P = 0.042, Mann-Whitney U-test, 2-sided). However, when we regressed the Gleason score against the time interval in months between the date of diagnosis and blood draw, there was no evidence for an effect across all scores. The correlation coefficient (r) was -0.037 (r2 = 0.0013, P = 0.68). We do not have a Gleason score for 18 men (12.3%, median number of months since diagnosis = 96).
Molecular Analysis
We screened for founder BRCA1/2 mutations using a multiplex sizing assay in which all 3 PCR products can be visualized on a single polyacrylamide gel as described previously by Kuperstein et al [12]. Samples demonstrating a band shift were re-amplified and run again for confirmation. All tests included a positive control previously confirmed by sequencing.
Results and Discussion
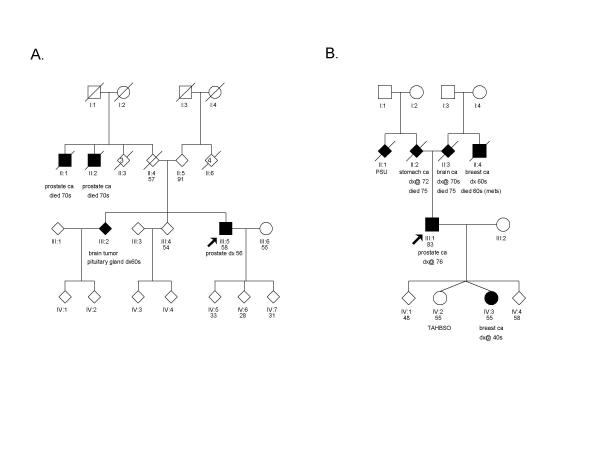
We observed 2 BRCA2 6174delT mutations and no BRCA1 mutations (Table 1). One carrier, diagnosed at age 56 with a Gleason score of 7/10, had two uncles with prostate cancer; the second carrier, diagnosed at age 76 with a Gleason score of 9/10, had no relatives with prostate cancer but had an uncle and a daughter with breast cancer (Figure 1).
Table 1.
BRCA1/2 founder mutation frequencies in AJ unselected prostate cancer cases and controls
| Ref # | 185delAG (%) | 5382insC (%) | 6174delT (%) | |
| Cases | ||||
| Lehrer et al (1998) | 13 | 0/60 | n.t. | 0/60 |
| Hubert et al (1999) | 14 | 2/87 (2.3%) | 0/87 | 1/87 (1.1%) |
| Nastiuk et al (1999) | 15 | 1/83 (1.2%) | n.t. | 2/82 (2.4%) |
| Vazina et al (2000) | 16 | 2/87 (2.3%) | 2/60 (3.3%)* | 1/86 (1.1%) |
| Hamel et al (this study) | 0/146 | 0/146 | 2/146 (1.4%) | |
| Total | 5/463 (1.1%) | 2/293 (0.68%) | 6/461 (1.3%) | |
| Controls | ||||
| Struewing et al (1995) | 2 | 8/858 (0.93%) | 0/433 | n.t. |
| Roa et al (1996) | 3 | 34/3108 (1.1%) | 4/3116 (0.13%) | 47/3085 (1.5%) |
| Oddoux et al (1996) | 4 | n.t. | n.t. | 12/1255 (0.96%) |
| Struewing et al (1997)† | 8 | 41/5318 (0.77%) | 20/5318 (0.38%) | 59/5087 (1.2%) |
| Hubert et al (1999) | 14 | 2/87 (2.3%) | 0/87 | 1/87 (1.1%) |
| Total | 85/9371 (0.91%) | 24/8867 (0.27%) | 119/9514 (1.3%) | |
| Overall OR (95%CI) | 1.2 (0.38–2.9) | 2.5 (0.29–10) | 1.0 (0.37–2.4) | |
| P Value (2-sided) | 0.62 | 0.20 | 0.83 |
n.t. Not tested Odds ratios were calculated according to the method of Gart. 95% confidence intervals were calculated using the exact Clopper-Pearson method and P values are two-sided Fisher exact tests. *This frequency was reported as significantly different from observations in a control population in the original publication. †Data from this publication were used as control by Vazina et al, 2000.
Figure 1.
Pedigrees of the 2 BRCA2:6174delT mutation carriers. Probands are identified with an arrow. PSU = primary site unkown; TAHBSO = total abdominal hysterectomy with bilateral salpingo-oophorectomy.
Recent studies reporting BRCA1/2 founder mutation frequencies in unselected AJ men with prostate cancer have also failed to find an excess of carriers among affected individuals [13-15], with the exception of one study [16] where a slight but significant excess of BRCA1:5382insC carriers was observed. However, all studies were small, and lack of power may have prevented some differences from being detected. We therefore combined our results with observations in cases and, when available, controls from these studies. In addition, we compiled data from various reports which previously examined mutation frequencies in unselected AJ population controls [2-4] and AJ volunteers [8]. There were no statistically significant differences between the frequencies of the founder mutations in individuals affected with prostate cancer compared with their frequencies in AJ population controls (Table 1).
Different recruitment methods were used by the various groups cited in Table 1 as sources of controls. Hubert et al [14] selected a group of 87 healthy elderly Israeli men (median age 71 years) with no history of cancer as controls, thereby attempting to compensate for possible age-related variations in mutation frequencies. The larger populations studies [2-4] performed mutation testing on AJ individuals from either the US or Israel (or both) who were referred for unrelated genetic testing (e.g. Tay Sachs, Cystic Fibrosis, Fanconi Anemia, etc.), with no information available on gender, age or cancer history for these participants. Since genetic testing for the recessive conditions listed above would usually be undertaken prior to making the decision of having children, this larger group is more likely to have a lower median age than that observed in prostate cancer cases. This group should, however, otherwise provide representative population frequencies for the mutations. Finally, controls from Struewing et al [8] were AJ volunteers from the Washington D.C. area who wished to participate in a study on breast and ovarian cancer. The authors acknowledge that such a recruitment scheme led to a higher than expected proportion of participants reporting a personal or family history of breast and ovarian cancer. This may conceivably result in an exaggerated mutation frequency in this control group compared to frequencies in the general population. In addition, participants in this control group included a number of siblings and relatives, including some who were mutation carriers, potentially further increasing the apparent frequency of BRCA1 and BRCA2 mutations in this cohort. We therefore compared the mutation frequencies in volunteers from the Washington D.C. area [8] to frequencies obtained from combining results from our other sources of controls [2-4,14] to determine if frequencies from the volunteer group were different from those from the general population. Frequencies for BRCA1:185delAG and BRCA2:6174delT were slightly lower in the volunteer group, although the difference was not statistically significant. In contrast, the BRCA1:5382insC mutation was significantly over-represented in volunteer controls compared to random population controls (OR = 3.3, 95%CI: 1.1–13; P = 0.02). In consequence, we compared our combined cases to controls once again, this time excluding control data from the Washington D.C. study. While there was no change for BRCA1:185delAG and BRCA2:6174delT, we now observed a stronger association between the low frequency BRCA1:5382insC mutation and prostate cancer (OR = 6.1, 95%CI: 0.54–42; P = 0.07). However, as indicated by the wide confidence interval, this effect is driven entirely by 2 carriers observed by Vazina et al [16], the only report of BRCA1:5382insC mutations among prostate cancer cases across 3 studies. According to the authors, neither carrier has a family history of prostate cancer.
It is difficult to reconcile our observations with results from previous epidemiological studies suggesting an increased prostate cancer risk in relatives of mutation carriers [5-11]. One possible explanation may be that a diagnostic bias exists in families where hereditary cancer cases are found. Specifically, having a relative affected with BRCA1/2-related breast or ovarian cancer may encourage relatives to undergo testing and reveal the existence of prostate tumors which may otherwise have remained asymptomatic and undetected, thereby artificially increasing the incidence of prostate cancer cases in families bearing BRCA1/2 mutations.
If there were a significant decline in the mutation frequency of founder BRCA1 and/or BRCA2 mutations in older Jewish males, then it is possible that using frequencies of 0.91% for BRCA1:185delAG, 0.27% for BRCA1:5382insC and 1.3% for BRCA2: 6174delT (Table 1) from a potentially younger population will result in an underestimation of the odds ratios observed. There are no data published that address this possibility, but if we assume that age-matched controls would have overall allele frequencies of 0.64%, 0.19% and 0.91% (observed frequencies decreased by 30%) for the 185delAG, 5382insC and 6174delT alleles, respectively, then using the cases and controls in Table 1 we still do not reach statistical significance for an association between founder mutations and prostate cancer: BRCA1:185delAG, OR = 1.7 (P = 0.23); BRCA1:5382insC, OR = 3.6 (P = 0.12); BRCA2:6174delT, OR = 1.4 (P = 0.33). Thirty percent is a generous reduction; these results suggest that a bias due to an age-related decline in mutation frequency in the control population is unlikely to be a major explanatory factor.
A third possibility is that only certain BRCA1/2 mutations are associated with an increased prostate cancer risk. In the initial report from the Breast Cancer Linkage Consortium [10], there was a significant excess risk of prostate cancer for male BRCA2 mutation carriers (RR: 4.65, 95% CI: 3.48–6.22). In a further analysis, Thompson et al [17] found that the risk of prostate cancer was lower in carriers of BRCA2 mutations located in the ovarian cancer cluster region (OCCR; nucleotides 3035–6629, including the AJ founder 6174delT) than in carriers of mutations clustering elsewhere in the gene (RR = 0.52; 95%CI = 0.24–1.00; P = 0.05). This observation was recently indirectly supported by a large study examining 263 men with early-onset prostate cancer (55 years and less) where the authors sequenced the entire BRCA2 coding region and found 6 truncating mutations, all located outside the OCCR [18]. However, this hypothesis cannot explain discrepancies in findings between epidemiological and direct mutation detection studies where an increased prostate cancer risk was also observed in relatives of AJ founder mutation carriers, including BRCA2:6174delT [9]. It is notable, however, that in the study of Warner et al. [9], a significant difference in cumulative incidence of prostate cancer to age 85 (33.6% vs. 12.6%, P = 0.049) was observed when comparing relatives of women with and without founder BRCA1/2 mutations, who were themselves affected by breast cancer. Thus other factors may account for the differences in prostate cancer incidence observed.
With the combined results from nine studies, we have an 80% power to detect ORs of 2.7, 6.6 and 2.5 (185delAG, 5382insC and 6174 delT, respectively), while the values we observed range between 1.0 and 2.5. Therefore, we do not have the power to rule out small effects even with our combined sample size. For a mutation with a population frequency near 1%, we would need more than 3,200 cases and 3,200 controls to rule out an OR of 2.0 or greater; over 10,000 cases and 10,000 controls would be needed to exclude an OR of 1.5 or greater. These numbers indicate that larger studies will be needed to rule out a small effect by BRCA1/2 founder mutations on prostate cancer risk. However, using the observed mutation frequency in controls, we can estimate the population attributable risk per cent (PAR%) of these mutations as follows:
PAR% = Pe (RR-1)/ [1 + Pe(RR-1)] × 100
where Pe = proportion of exposure in controls and RR = observed relative risk between cases and controls. PAR% values will reflect the proportion of prostate cancer cases attributable to these AJ founder mutations based on their frequency in the population; from the combined data in Table 1, these values are 0.18% (BRCA1:185delAG), 0.40% (BRCA1:5382insC) and 0% (BRCA2:6174delT). In comparison, based on previously published data on cases diagnosed after 55 years of age [19,20], the PAR% of AJ BRCA1 (185delAG and 5382insC combined) and BRCA2 (6174delT) founder mutations are 3.8% and 2.5 %, respectively, in the case of breast cancer, and 23.8% and 16.7%, respectively, in the case of ovarian cancer.
Conclusions
We screened 146 AJ men with prostate cancer for germline AJ BRCA1/2 founder mutations, and found only two carriers of the BRCA2:6174delT mutation. As was the case in previous smaller studies, our observations failed to support previous data suggesting that AJ founder BRCA1/2 mutations might contribute significantly to prostate cancer risk. While even the combined results of publications to date do not have the power to rule out a small effect, PAR percentages above do not support a major role for these founder mutations in prostate cancer susceptibility. Any modest but statistically significant contribution of these three mutations to prostate cancer risk that may be uncovered using larger studies is unlikely to be of clinical significance.
Electronic Database
Breast Cancer Information Core: http://research.nhgri.nih.gov/bic/
Authors' Contribution
NH carried out the molecular genetic studies, the statistical analyses and drafted the manuscript. KK recruited the participants and collected all epidemiological information in Montreal. WDF designed and coordinated the study and contributed to drafting the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
Funded by the Department of Defense (DAMD17-00-1-0033) the Canadian Genetic Diseases Network and the National Institutes of Health. We thank McGill University urologists- Drs. A Aprikian, J Corcos, M. Elhilali, Y Taguchi and S Tanguay for help with recruitment.
Contributor Information
Nancy Hamel, Email: nancy.hamel@mail.mcgill.ca.
Kimberley Kotar, Email: dolphins@colba.net.
William D Foulkes, Email: william.foulkes@mcgill.ca.
References
- Ostrer H. A genetic profile of contemporary Jewish populations. Nat Rev Genet. 2001;2:891–898. doi: 10.1038/35098506. [DOI] [PubMed] [Google Scholar]
- Struewing JP, Abeliovich D, Peretz T, Avishai N, Kaback MM, Collins FS, Brody LC. The carrier frequency of the BRCA1 185delAG mutation is approximately 1 percent in Ashkenazi Jewish individuals. Nat Genet. 1995;11:198–200. doi: 10.1038/ng1095-198. [DOI] [PubMed] [Google Scholar]
- Roa BB, Boyd AA, Volcik K, Richards CS. Ashkenazi Jewish population frequencies for common mutations in BRCA1 and BRCA2. Nat Genet. 1996;14:185–187. doi: 10.1038/ng1096-185. [DOI] [PubMed] [Google Scholar]
- Oddoux C, Struewing JP, Clayton CM, Neuhausen S, Brody LC, Kaback M, Haas B, Norton L, Borgen P, Jhanwar S, Goldgar D, Ostrer H, Offit K. The carrier frequency of the BRCA2 6174delT mutation among Ashkenazi individuals is approxiamtely 1-percent. Nat Genet. 1996;14:188–190. doi: 10.1038/ng1096-188. [DOI] [PubMed] [Google Scholar]
- Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692–695. doi: 10.1016/S0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Easton DF, Steele L, Fields P, Ormiston W, Averill D, Daly PA, McManus R, Neuhausen SL, Ford D, Wooster R, Cannon-Albright LA, Stratton MR, Goldgar DE. Cancer risks in two large breast cancer families linked to BRCA2 on chromosome 13q12-13. Am J Hum Genet. 1997;61:120–128. doi: 10.1086/513891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson S, Thorlacius S, Tomasson J, Tryggvadottir L, Benediktsdottir K, Eyfjord JE, Jonsson E. BRCA2 mutation in Icelandic prostate cancer patients. J Mol Med. 1997;75:758–761. doi: 10.1007/s001090050162. [DOI] [PubMed] [Google Scholar]
- Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Timmerman MM, Brody LC, Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N Engl J Med. 1997;336:1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, Paterson C, Ozcelik H, Goss P, Allingham-Hawkins D, Hamel N, Di Prospero L, Contiga V, Serruya C, Klein M, Moslehi R, Honeyford J, Liede A, Glendon G, Brunet JS, Narod S. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst. 1999;91:1241–1247. doi: 10.1093/jnci/91.14.1241. [DOI] [PubMed] [Google Scholar]
- The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- Thompson D, Easton DF, The Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–1365. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- Kuperstein G, Foulkes WD, Ghadirian P, Hakimi J, Narod SA. A rapid fluorescent multiplexed-PCR analysis (FMPA) for founder mutations in the BRCA1 and BRCA2 genes. Clin Genet. 2000;57:213–220. doi: 10.1034/j.1399-0004.2000.570307.x. [DOI] [PubMed] [Google Scholar]
- Lehrer S, Fodor F, Stock RG, Stone NN, Eng C, Song HK, McGovern M. Absence of 185delAG mutation of the BRCA1 gene and 6174delT mutation of the BRCA2 gene in Ashkenazi Jewish men with prostate cancer. Br J Cancer. 1998;78:771–773. doi: 10.1038/bjc.1998.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert A, Peretz T, Manor O, Kaduri L, Wienberg N, Lerer I, Sagi M, Abeliovich D. The Jewish Ashkenazi founder mutations in the BRCA1/BRCA2 genes are not found at an increased frequency in Ashkenazi patients with prostate cancer. Am J Hum Genet. 1999;65:921–924. doi: 10.1086/302525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastiuk KL, Mansukhani M, Terry MB, Kularatne P, Rubin MA, Melamed J, Gammon MD, Ittmann M, Krolewski JJ. Common mutations in BRCA1 and BRCA2 do not contribute to early prostate cancer in Jewish men. Prostate. 1999;40:172–177. doi: 10.1002/(SICI)1097-0045(19990801)40:3<172::AID-PROS5>3.3.CO;2-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazina A, Baniel J, Yaacobi Y, Shtriker A, Engelstein D, Leibovitz I, Zehavi M, Sidi AA, Ramon Y, Tischler T, Livne PM, Friedman E. The rate of the founder Jewish mutations in BRCA1 and BRCA2 in prostate cancer patients in Israel. Br J Cancer. 2000;83:463–466. doi: 10.1054/bjoc.2000.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Easton D. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68:410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SM, Kote-Jarai Z, Meitz J, Hamoudi R, Hope Q, Osin P, Jackson R, Southgate C, Singh R, Falconer A, Dearnaley DP, Ardern-Jones A, Murkin A, Dowe A, Kelly J, Williams S, Oram R, Stevens M, Teare DM, Ponder BA, Gayther SA, Easton DF, Eeles RA. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am J Hum Genet. 2003;72:1–12. doi: 10.1086/345310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satagopan JM, Offit K, Foulkes W, Robson ME, Wacholder S, Eng CM, Karp SE, Begg CB. The lifetime risks of breast cancer in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2001;10:467–473. [PubMed] [Google Scholar]
- Satagopan JM, Boyd J, Kauff ND, Robson M, Scheuer L, Narod S, Offit K. Ovarian cancer risk in Ashkenazi Jewish carriers of BRCA1 and BRCA2 mutations. Clin Cancer Res. 2002;8:3776–3781. [PubMed] [Google Scholar]