Abstract
The neural cell adhesion molecule (N-CAM) inhibits astrocyte proliferation in vitro and in vivo, and this effect is partially reversed by the glucocorticoid antagonist RU-486. The present studies have tested the hypothesis that N-CAM-mediated inhibition of astrocyte proliferation is caused by homophilic binding and involves the activation of glucocorticoid receptors. It was observed that all N-CAM Ig domains inhibited astrocyte proliferation in parallel with their ability to influence N-CAM binding. The proliferation of other N-CAM-expressing cells also was inhibited by the addition of N-CAM. In contrast, the proliferation of astrocytes from knockout mice lacking N-CAM was not inhibited by added N-CAM. These findings support the hypothesis that it is binding of soluble N-CAM to N-CAM on the astrocyte surface that leads to decreased proliferation. Signaling pathways stimulated by growth factors include activation of mitogen-activated protein (MAP) kinase. Addition of N-CAM inhibited MAP kinase activity induced by basic fibroblast growth factor in astrocytes. In accord with previous findings that RU-486 could partially prevent the proliferative effects of N-CAM, inhibition of MAP kinase activity by N-CAM was reversed by RU-486. The ability of N-CAM to inhibit astrocyte proliferation was unaffected, however, by agents that block the ability of N-CAM to promote neurite outgrowth. Together, these findings indicate that homophilic N-CAM binding leads to inhibition of astrocyte proliferation via a pathway involving the glucocorticoid receptor and that the ability of N-CAM to influence astrocyte proliferation and neurite outgrowth involves different signal pathways.
Cell adhesion molecules mediate cell–cell interactions during development and in the adult central nervous system (1). The neural cell adhesion molecule (N-CAM) is expressed by both neurons and astrocytes and can promote homophilic binding and influence neurite outgrowth (1). N-CAM contains five Ig domains followed by two fibronectin type III repeats in the extracellular portion of the molecule (2). A model for N-CAM homophilic binding has been proposed in which the Ig domains bind in a pairwise antiparallel manner such that Ig I binds Ig V, Ig II binds Ig IV, and Ig III binds Ig III (3).
We recently have shown that exogenously added N-CAM can inhibit the proliferation of cultured neonatal astrocytes and of astrocytes responding to a penetrating lesion in the adult rat brain in vivo (4, 5). We hypothesized that these effects were mediated by homophilic binding to N-CAM on the astrocyte membrane. This hypothesis was based on the observations that purified N-CAM, N-CAM-related recombinant proteins and peptides, and specific anti-N-CAM antibodies all inhibited cell proliferation and that antisense oligonucleotides directed against the N-CAM mRNA also decreased the effectiveness of N-CAM added in solution (4).
To understand the role of N-CAM in inhibiting astrocyte proliferation, it is important to demonstrate definitively that N-CAM homophilic binding is critical in this process. Moreover, the ability of N-CAM binding to inhibit astrocyte proliferation prompts the question of how N-CAM binding relates to other signaling events. Astrocyte proliferation induced by growth factors such as basic fibroblast growth factor (bFGF) is inhibited by N-CAM (4). This inhibition is partially reversed by the glucocorticoid antagonist RU-486, suggesting that N-CAM signaling might activate the glucocorticoid receptor (6). In other studies, signaling after N-CAM binding in neurons has been examined in neurite outgrowth assays. Based on the results of these studies, it has been proposed that N-CAM signaling occurs through the cis interaction of N-CAM with the FGF receptor (reviewed in ref. 7) and intracellular pathways stimulated by the FGF receptor (reviewed in refs. 8 and 9). It therefore is important to assess whether signaling after N-CAM binding involves both the glucocorticoid receptor and FGF receptor pathways or whether there may be different pathways involved depending on cell type or specific cellular events.
In the present studies, we have shown that individual recombinant domains of N-CAM each inhibited astrocyte proliferation in a manner that was correlated with their estimated binding strength. Although N-CAM did not inhibit the proliferation of astrocytes from knockout mice lacking N-CAM, it was able to inhibit proliferation of a variety of N-CAM-expressing cells in addition to astrocytes. In examining signal pathways activated by N-CAM binding, we observed that stimulation of mitogen-activated protein (MAP) kinase by bFGF was reduced by N-CAM and that this effect was completely reversed in the presence of the glucocorticoid antagonist RU-486. In contrast, perturbation of signaling pathways reported by others to be involved in N-CAM-mediated neurite outgrowth (7) did not reverse the effect of N-CAM on astrocyte proliferation. These results reinforce the hypothesis that N-CAM-mediated inhibition of astrocyte proliferation is caused by homophilic binding and that it involves the activation of glucocorticoid receptors. Furthermore, they suggest that the pathways by which N-CAM binding signals the inhibition of cell proliferation differ from pathways signaling neurite extension.
EXPERIMENTAL PROCEDURES
Reagents and Cell Lines.
Drugs were purchased from Calbiochem, except bFGF (Upstate Biotechnology), and RU-486 (Biomol, Plymouth Meeting, PA). Cell culture reagents were obtained from GIBCO. Cell lines were obtained from the American Type Culture Collection. C6 and N2A cells were maintained in 10% fetal bovine serum (GIBCO) and DMEM. NG-108 cells were maintained in 5% calf serum/DMEM supplemented with hypoxanthine, aminopterin, and thymine (HAT).
Astrocyte and Fibroblast Cultures.
Primary cultures of astrocytes were prepared from the forebrains of 4- to 5-day-old neonatal rats as previously described (4, 6). Cell preparations were checked regularly for the expression of GFAP, a marker for astrocytes, and other marker antibodies (6). Cultures used routinely contained more than 98% astrocytes. Fibroblasts were obtained from embryonic day 18 mice as previously described (10).
Purification of N-CAM and Recombinant Domains.
N-CAM was purified from early postnatal rat brains by affinity chromatography as described (4). Recombinant N-CAM fragments were prepared and characterized as previously described (3).
Proliferation Assay.
Primary astrocyte cultures or other cell types were trypsinized and transferred into 96-well plates at a density of 2 × 105 cells/ml (equivalent to 7 × 104 cells/cm2 or 2 × 104 cells/well). After cell attachment for 24 hr, the culture medium was exchanged for serum-free media. Growth factors, purified N-CAM, recombinant N-CAM domains, and pharmacological agents were added after the cells had remained in serum-free media for 48 hr (T = 48). After 12 hr (at T = 60), [3H]thymidine (New England Nuclear, 20 Ci/mmol) was added (0.38 μM, 10 μCi/ml) and the incubation proceeded for an additional 12 hr (T = 72). Incorporation of [3H]thymidine was measured as described (6). The time of exposure to [3H]thymidine was altered for the different cell lines to reflect their doubling times, C6 cells (6 hr), N2A (12 hr), and NG-108 cells and fibroblasts (24 hr).
Peptide Synthesis.
Peptides were synthesized and purified as described previously (4).
MAP Kinase Assay.
The PathDetect system (Stratagene) was used to measure MAP kinase activity. In brief, astrocytes were electroporated with 10 μg of pFR-luciferase vector, 2 μg of pFA-ELK vector, and 5 μg of the CMV-β vector (Promega) (6). The cells were plated in 24-well dishes in serum containing media for 24 hr and then switched to serum-free media for an additional 48 hr. The cells were treated with the reagents for 6 hr, after which they were harvested and assayed for luciferase and β-galactosidase activity. The luciferase activity of each sample was normalized to an internal reference standard of β-galactosidase activity.
RESULTS
Effects of Recombinant N-CAM Domains on Astrocyte Proliferation.
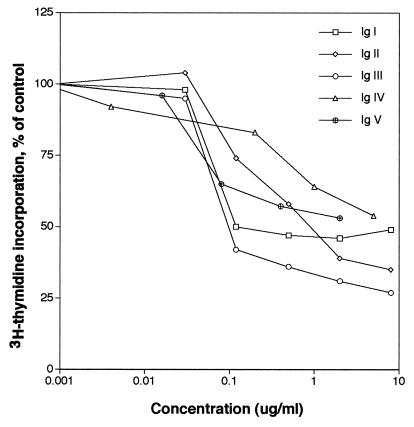
Astrocyte proliferation was analyzed in the presence of N-CAM recombinant proteins corresponding to each of its five individual Ig domains. Recombinant Ig domains were added at concentrations ranging from 5 ng/ml to 10 μg/ml. Each Ig domain inhibited astrocyte proliferation in a dose-dependent manner (Fig. 1). An N-CAM mAb that recognizes the cytoplasmic domain (11) and normal rabbit Ig Fab′ fragments were added to astrocyte cultures and were found not to affect astrocyte proliferation (data not shown). This result suggests that the inhibition of astrocyte proliferation was specific to the N-CAM Ig domains and not to Ig proteins in general. Addition of Ig III at higher concentrations produced a larger inhibition in [3H]thymidine incorporation than did the other domains. At an intermediate concentration of the Ig domains (≈100 ng/ml; 9 nM for each of the domains) there was a distinct difference in the ability of each domain to inhibit proliferation Ig III > Ig I > Ig V > Ig II > Ig IV. The Ig IV domain used in the above experiment did not contain the VASE sequence (12) but addition of the Ig IV domain containing the VASE sequence to astrocyte cultures in a separate experiment produced similar results (data not shown). The concentration of Ig III necessary to achieve 50% of maximal activity in decreasing [3H]thymidine incorporation (ID50) by astrocytes was 6.5 nM. The ID50 of intact N-CAM (0.8 nM) was substantially less than that for Ig III despite the fact that addition of Ig III resulted in the largest decrease in proliferation of any of the Ig domains. This finding is consistent with the observation that all five N-CAM Ig domains were effective at inhibiting proliferation.
Figure 1.
Effects of recombinant N-CAM Ig domains on astrocyte proliferation. Astrocyte proliferation assays were performed as described in Experimental Procedures in the presence of each of the five N-CAM Ig domains.
Effect of N-CAM on N-CAM-Deficient Astrocytes.
To confirm that N-CAM homophilic binding was indeed required for the inhibition of astrocyte proliferation, astrocytes were prepared from mice that were heterozygous or homozygous for a disrupted N-CAM allele (13). Astrocytes from the homozygous N-CAM knockout mice did not express N-CAM protein, whereas astrocytes derived from heterozygous mice expressed approximately half the amount of N-CAM found on wild-type astrocytes (unpublished observations). Addition of N-CAM or N-CAM Ig III inhibited proliferation of astrocytes from heterozygous, but not homozygous, N-CAM knockout mice (Table 1). This finding and those described above on the individual domains provide strong support for the hypothesis (4–6) that inhibition of astrocyte proliferation by N-CAM reagents requires interaction with N-CAM on the astrocyte surface.
Table 1.
Proliferation assays with heterozygous and homozygous N-CAM knockout astrocytes
| Reagent | Concentration | Heterozygous N-CAM knockout cells (−/+)
|
Homozygous N-CAM knockout cells (−/−)
|
|---|---|---|---|
| [3H]thymidine incorporation, % of control | [3H]thymidine incorporation, % of control | ||
| N-CAM | 5 μg/ml | 30 ± 1 | 101 ± 8 |
| N-CAM | 2 μg/ml | 57 ± 7 | 115 ± 2 |
| Ig III | 3 μg/ml | 29 ± 4 | 94 ± 5 |
| Ig III | 1 μg/ml | 33 ± 2 | 106 ± 3 |
Astrocytes were obtained from transgenic mice that were heterozygous or homozygous for the disrupted N-CAM allele. N-CAM mRNA and protein was not detected in homozygous astrocytes (13). The amount of [3H]thymidine incorporation is presented as the percent incorporated compared to astrocytes treated with PBS alone. Addition of N-CAM or Ig III recombinant protein decreased the amount of [3H]thymidine incorporation in heterozygous but not homozygous astrocytes. Data are presented as mean ± SD (n = 4 for each condition).
Inhibition of Proliferation of Other Cell Types by N-CAM.
To examine whether soluble N-CAM could inhibit proliferation of other N-CAM-expressing cell types, N-CAM or Ig III was added to mitotically active cells that expressed N-CAM, including C6 glioma, NG-108 neuroblastoma cells, and N2A neuroblastoma cells (Table 2). N-CAM and Ig III were able to inhibit proliferation of these cells in a dose-dependent manner. The proliferation of NG-108 and N2A neuroblastoma cells was inhibited by N-CAM or Ig III but the level of inhibition was less than that observed with the C6 glioma cells. Embryonic mouse fibroblasts, which express N-CAM, also were examined to determine whether N-CAM could inhibit the proliferation of non-neural cells. Both N-CAM and Ig III showed robust inhibition of fibroblast proliferation.
Table 2.
N-CAM inhibits proliferation of glioma and neuroblastoma cells and fibroblasts
| Cell type | Media | Reagent | Concentration | [3H]thymidine incorporation, % of control |
|---|---|---|---|---|
| C6 glioma | Serum-free | N-CAM | 5 μg/ml | 62 ± 5 |
| 2 μg/ml | 87 ± 6 | |||
| 1 μg/ml | 102 ± 4 | |||
| bFGF | N-CAM | 5 μg/ml | 52 ± 7 | |
| 2 μg/ml | 80 ± 6 | |||
| 1 μg/ml | 97 ± 9 | |||
| NG-108 neuroblastoma | 1% FBS | N-CAM | 5 μg/ml | 68 ± 4 |
| 2 μg/ml | 84 ± 3 | |||
| 1 μg/ml | 94 ± 4 | |||
| 1% FBS | Ig III | 10 μg/ml | 70 ± 8 | |
| 3 μg/ml | 79 ± 4 | |||
| 1 μg/ml | 93 ± 7 | |||
| N2A neuroblastoma | Serum-free | N-CAM | 5 μg/ml | 80 ± 2 |
| 2 μg/ml | 92 ± 5 | |||
| 1 μg/ml | 100 ± 2 | |||
| Serum-free | Ig III | 10 μg/ml | 89 ± 4 | |
| 3 μg/ml | 91 ± 2 | |||
| 1 μg/ml | 95 ± 3 | |||
| Fibroblasts | Serum-free | N-CAM | 5 μg/ml | 20 ± 12 |
| 2 μg/ml | 74 ± 11 | |||
| 1 μg/ml | 93 ± 7 | |||
| Serum-free | Ig III | 10 μg/ml | 60 ± 5 | |
| 3 μg/ml | 65 ± 9 | |||
| 1 μg/ml | 79 ± 10 |
Proliferation assays, as described in Experimental Procedures, were performed on C6 glioma, NG-108 neuroblastoma, N2A neuroblastoma, and primary fibroblasts. Concentrations of N-CAM or Ig III were added to the cells that were in serum-free media or in the presence of 1% fetal bovine serum (FBS) or bFGF (20 ng/ml). The amount of [3H]thymidine incorporation by cells in each condition was compared to cells treated with PBS alone. Data are presented as mean ± SD (n = 4 for each condition).
N-CAM Inhibition of MAP Kinase Activation Is Dependent on the Glucocorticoid Receptor.
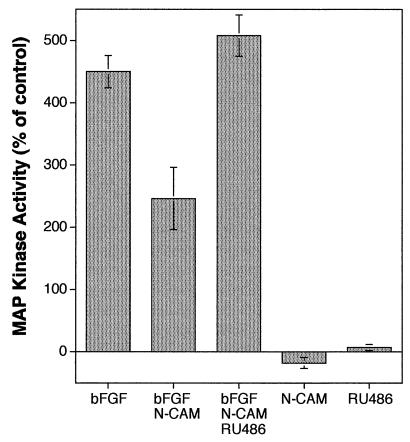
One of the convergence points in growth factor receptor-activated pathways is the phosphorylation and activation of MAP kinase (reviewed in ref. 14). We observed that MAP kinase activity in astrocytes was increased over 4-fold after bFGF treatment (Fig. 2). When N-CAM was added simultaneously with bFGF, however, MAP kinase activity was reduced to 46% of the value stimulated by bFGF alone. This inhibitory effect on MAP kinase activity was completely reversed if the glucocorticoid antagonist RU486 was included with bFGF and N-CAM. The addition of N-CAM alone produced a small, but reproducible, decrease in basal MAP kinase activity whereas the addition of RU486 alone had little or no effect. These results suggest that soluble N-CAM inhibits growth factor-induced MAP kinase activity and that this inhibition requires activation of the glucocorticoid receptor.
Figure 2.
Quantitation of MAP kinase activity in astrocytes. The addition of bFGF (20 ng/ml) stimulated MAP kinase activity 450% of untreated control values. The addition of Ig III (10 μg/ml) prevented stimulation of MAP kinase activity by 46%. Inclusion of RU-486 (100 nM) with Ig III completely blocked its ability to inhibit MAP kinase activation. Added individually, Ig III and RU-486 had only minor effects on basal MAP kinase activity. Data are presented as mean ± SD (n = 4 for each condition).
Relation of Signaling Pathways Involved in Inhibition of Astrocyte Proliferation to Those Involved in N-CAM-Dependent Neurite Outgrowth.
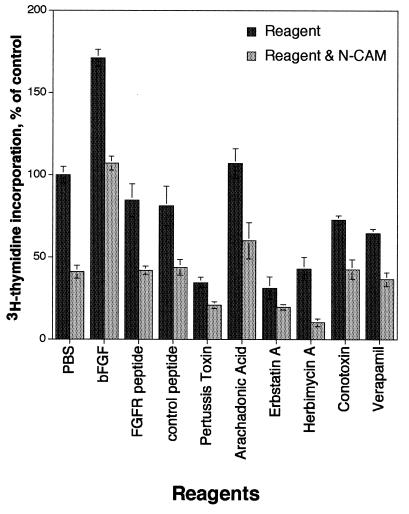
The ability of N-CAM to promote neurite outgrowth in neurons has been postulated to occur through cis binding of N-CAM to a segment of the FGF receptor called the CAM homology domain (15). This conclusion was based on the observation that the presence of a 20-aa synthetic peptide corresponding to this domain could inhibit N-CAM-dependent neurite outgrowth (16). We found that proliferation stimulated by bFGF in astrocytes was reduced in the presence of N-CAM and we tested the effects of the FGF receptor peptide on the ability of N-CAM to affect astrocyte proliferation (Fig. 3). Inclusion of N-CAM with either the FGF receptor peptide or a control peptide with the same amino acids in a random order (16) reduced the amount of [3H]thymidine incorporation to levels equivalent to that of N-CAM alone, although addition of either peptide alone resulted in a slight decrease in proliferation. These results suggest that the ability of N-CAM to inhibit astrocyte proliferation is not likely to occur through direct interactions of N-CAM with the FGF receptor.
Figure 3.
Astrocyte proliferation assays were performed in the presence of PBS, N-CAM, and reagents that act on the FGF receptor or modulate intracellular signaling pathways. bFGF (20 ng/ml), a peptide corresponding to the CAM homology domain in the FGF receptor (10 μg/ml), a scrambled peptide (10 μg/ml) containing the same amino acids as the CAM homology peptide, mastoparan (500 nM), pertussis toxin (200 ng/ml), arachidonic acid (500 nM), erbstatin A (1 μg/ml), herbimycin A (100 nM), conotoxin (50 nM), and verapamil (500 nM) were added individually to astrocytes in the presence or absence of N-CAM (5 μg/ml). Data are presented as mean ± SD (n = 4 for each condition).
Pharmacological reagents that modulate pathways activated by bFGF binding to the FGF receptor (reviewed in ref. 17) have pointed to a role for G proteins, lipid hydrolysis, and calcium channels as key intermediates in the ability of N-CAM to promote neurite outgrowth. Accordingly, we used these reagents to determine whether perturbation of the bFGF-activated pathways also could reverse the effect of N-CAM on astrocyte proliferation. These reagents included pertussis toxin, which is an inhibitor of G proteins, arachidonic acid, which is a product of lipid hydrolysis, and conotoxin and verapamil, which are inhibitors of N- and L-type calcium channels, respectively. Although the individual reagents alone each had different effects on proliferation (Fig. 3), none of these reagents blocked the ability of N-CAM to inhibit proliferation.
DISCUSSION
The present studies show that binding of each of the five N-CAM Ig domains can lead to inhibition of astrocyte proliferation, an effect that previously was reported for intact N-CAM (4–6). The proliferation of astrocytes from knockout mice lacking N-CAM was not affected by the addition of N-CAM. In the presence of N-CAM, proliferation and activation of MAP kinase in response to bFGF in astrocytes both were inhibited. The inhibition of MAP kinase activity was completely reversed in the presence of the glucocorticoid antagonist RU-486, consistent with our previous finding (6) that RU486 reduced the ability of N-CAM to inhibit astrocyte proliferation. However, perturbation of intracellular pathways reported to influence N-CAM-dependent neurite outgrowth (17) did not alter the ability of N-CAM to inhibit astrocyte proliferation. Together, these findings strengthen the hypothesis that N-CAM homophilic binding inhibits astrocyte proliferation by a mechanism involving the glucocorticoid receptor. They also suggest that at least some of the signal pathways involved in the inhibition of cell proliferation in astrocytes are different from those involved in neurite outgrowth by neurons.
The ability of the various individual Ig domains of N-CAM to inhibit proliferation was correlated with their estimated strength of binding. Previous studies have shown that Ig III has a dominant role in N-CAM homophilic binding (18, 19) and have suggested a model in which Ig III binds itself, Ig I binds Ig V, and Ig II binds IV (3, 20). In the present study, addition of Ig III to astrocyte cultures produced the largest decrease in [3H]thymidine incorporation of any of the recombinant domains. It is of interest that these individual monovalent domains are capable of transducing signals presumably by binding to N-CAM on the cell surface. This monovalent binding may cause a change in protein conformation, leading to an activated signaling state. Although each individual Ig domain was able to inhibit astrocyte proliferation, purified N-CAM was the most efficacious on a molar basis. In accord with previous studies (3, 20), it is likely that all five Ig domains are not only required for optimal binding but also for subsequent signal transducing activity.
The ability of N-CAM to inhibit proliferation was not limited to astrocytes. The proliferation of glioma cells, neuroblastoma cells, and fibroblasts was reduced in the presence of soluble N-CAM. Indeed, the inhibition of fibroblast proliferation by cell–cell contact in vitro previously has been attributed to N-CAM-N-CAM binding (21). This finding suggests that the proliferation of many, if not all, N-CAM-expressing cells is sensitive to N-CAM binding and that this binding therefore may play an important role in contact inhibition (22). The failure of N-CAM reagents to inhibit the proliferation of astrocytes from knockout mice lacking N-CAM suggests that expression of N-CAM on the cell surface is required for N-CAM and related reagents to block cell proliferation.
N-CAM binding in neurons has been proposed to involve interactions with the FGF receptor and signaling pathways associated with receptor activation (17). The ability of N-CAM to inhibit astrocyte proliferation was not affected, however, by the addition of a peptide from the FGF receptor that has been reported to block the ability of N-CAM to support neurite outgrowth (16). Activation of phospholipase C gamma that results in lipid hydrolysis and calcium influx has been proposed to be necessary (17) for N-CAM induced neurite outgrowth. In the present studies, however, pharmacological manipulation of this pathway did not reverse the effect of N-CAM on astrocyte proliferation. The combined data suggest that homophilic N-CAM binding may activate multiple signaling pathways and that these can differ in different cell types.
The signal pathways activated by N-CAM binding in astrocytes appear to involve glucocorticoid receptors. In previous studies, glucocorticoids were shown to inhibit astrocyte proliferation. The ability of N-CAM binding to influence proliferation and gene expression was reversed by the glucocorticoid receptor antagonist RU-486 (6). These findings prompted the hypothesis that N-CAM binding leads to activation of the glucocorticoid receptor. The present results showing that RU-486 blocked the ability of N-CAM to inhibit MAP kinase activity are in line with this hypothesis.
Together these experimental results suggest that N-CAM and glucocorticoids share certain signaling pathways. In other systems, glucocorticoid receptor activation has been shown to inhibit MAP kinase activity. For example, in 3T3 fibroblasts and mast cells, the synthetic glucocorticoid, dexamethasone, inhibited MAP kinase activity induced by growth factors and antigenic stimulation, respectively (23, 24). In addition, it has been observed that MAP kinase can phosphorylate the glucocorticoid receptor and affect its ability to alter transcription (25). Determining the causal connections between N-CAM binding, glucocorticoid receptor activation, and growth factor-stimulated MAP kinase activity is a challenging task for further investigation.
Acknowledgments
We thank Dr. John Hemperly for the N-CAM mAb; Nguyen Tran, Lisa Remedios, Shaun Hammer, Catherine Cowley, and Adrian Badillo for excellent technical assistance; Dr. Todd Ranheim for help in the preparation of the N-CAM Ig domains; and Drs. Joseph Gally, George Miklos, and Ralph Greenspan for their critical reading of the manuscript. This work was supported by U.S. Public Health Service Grants NS/OD34874 (to K.L.C.), HD09635 (to G.M.E.), and HD16550 to (B.A.C.) and a grant from the G. Harold and Leila Y. Mathers Foundation (to G.M.E.). G.M.E., B.A.C., and K.L.C. are consultants to Becton Dickinson and Company.
ABBREVIATIONS
- N-CAM
neural cell adhesion molecule
- FGF
fibroblast growth factor
- bFGF
basic FGF
- MAP kinase
mitogen-activated protein kinase
References
- 1.Edelman G M, Crossin K L. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham B A, Hemperly J J, Murray B A, Prediger E A, Brackenbury R, Edelman G M. Science. 1987;236:799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- 3.Ranheim T S, Edelman G M, Cunningham B A. Proc Natl Acad Sci USA. 1996;93:4071–4075. doi: 10.1073/pnas.93.9.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sporns O, Edelman G M, Crossin K L. Proc Natl Acad Sci USA. 1995;92:542–546. doi: 10.1073/pnas.92.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krushel L A, Sporns O, Cunningham B A, Crossin K L, Edelman G M. Proc Natl Acad Sci USA. 1995;92:4323–4327. doi: 10.1073/pnas.92.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crossin K L, Tai M-H, Krushel L A, Mauro V P, Edelman G M. Proc Natl Acad Sci USA. 1997;94:2687–2692. doi: 10.1073/pnas.94.6.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty P, Smith P, Walsh F S. Perspect Dev Neurobiol. 1996;4:157–168. [PubMed] [Google Scholar]
- 8.Jaye M, Schlessinger J, Dionne C A. Biochim Biophys Acta. 1992;1135:185–199. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- 9.Denhardt D T. Biochem J. 1996;318:729–747. doi: 10.1042/bj3180729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crossin K L, Carney D H. Cell. 1981;23:61–71. doi: 10.1016/0092-8674(81)90270-1. [DOI] [PubMed] [Google Scholar]
- 11.Warren H S, Kinnear B F. In: Leucocyte Typing V. Schlossman S F, Boumsell L, Gilks W, Harlan J M, Kishimoto T, et al., editors. Cambridge: Oxford Univ. Press; 1995. pp. 1404–1406. [Google Scholar]
- 12.Small S J, Haines S L, Akeson R A. Neuron. 1988;1:1007–1017. doi: 10.1016/0896-6273(88)90158-4. [DOI] [PubMed] [Google Scholar]
- 13.Holst B D, Vanderklish P, Krushel L A, Zhou W, Langdon R A, McWhirter J R, Edelman G M, Crossin K L. Proc Natl Acad Sci USA. 1998;95:2597–2602. doi: 10.1073/pnas.95.5.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelech S L, Sanghera J S. Trends Biochem Sci. 1992;17:233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- 15.Doherty P, Walsh F S. Mol Cell Neurosci. 1996;8:99–111. doi: 10.1006/mcne.1996.0049. [DOI] [PubMed] [Google Scholar]
- 16.Williams E J, Furness J, Walsh F S, Doherty P. Neuron. 1994;13:583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 17.Baldwin T J, Fazeli M S, Doherty P, Walsh F S. J Cell Biochem. 1996;61:502–513. doi: 10.1002/(sici)1097-4644(19960616)61:4<502::aid-jcb3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 18.Rao Y, Wu X-F, Gariepy J, Rutishauser U, Siu C-H. J Cell Biol. 1992;118:937–949. doi: 10.1083/jcb.118.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao Y, Wu X-F, Yip P, Gariepy J, Siu C-H. J Biol Chem. 1993;268:20630–20638. [PubMed] [Google Scholar]
- 20.Zhou H, Fuks A, Alcaraz G, Bolling T J, Stanners C P. J Cell Biol. 1993;122:951–960. doi: 10.1083/jcb.122.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki J, Umeda M, Takio K, Titani K, Utsumi H, Sasaki M, Inoue K. J Cell Biol. 1991;115:1751–1761. doi: 10.1083/jcb.115.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todaro G J, Green H, Goldberg B D. Proc Natl Acad Sci USA. 1964;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansson A, Hehenberger K, Thoren M. Cell Biochem Funct. 1996;14:121–129. doi: 10.1002/cbf.656. [DOI] [PubMed] [Google Scholar]
- 24.Rider L G, Hirasawa N, Santini F, Beaven M A. J Immunol. 1996;157:2374–2380. [PubMed] [Google Scholar]
- 25.Krstic M D, Rogatsky I, Yamamoto K R, Garabedian M J. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]