Abstract
A recent study demonstrated that vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) activate Raf-1 kinase in an experimental neovasculature system. The study showed that bFGF and VEGF activate p21-activated protein kinase-1 (PAK-1) and Src kinase, respectively. PAK-1 and Src kinases phosphorylate specific serine and tyrosine residues within the activation loop of Raf-1 kinase. Their findings further suggest that phosphorylation at these sites protects endothelial cells from apoptosis induced by both intrinsic and extrinsic factors. The tumor neovasculature provides specific molecular markers or "zip codes". This group of investigators has previously shown that nanosphere-aided targeting of the neovasculature with mutant Raf-1 causes regression of the tumor vasculature. Thus, nanoparticles coated with "zip code"-specific homing biomolecules may be useful for delivering anti-angiogenic molecules that can induce tumor regression.
Introduction
For tumors to survive, grow and disseminate, they must be able to secrete critical growth factors and cytokines. It is estimated that 15 to 20 different cytokines are secreted by various tumors. These cytokines determine the characteristics and behaviors of many solid tumors. Some of these cytokines can positively influence neovascularization or angiogenesis, and others negatively regulate this process. It is thought that an "angiogenic switch", or the balance between positive and negative regulators, regulates the process of angiogenesis. The neovascularization process ultimately serves as a conduit to bring in nutrients that promote growth and metastasis [1]. Thus, the angiogenic switch also determines tumor cell growth. Many of these cytokines are also used under normal physiological conditions in various cells and tissues; therefore, direct interference with these cytokines is not a viable option. It has been recently shown that signaling events mediated by bFGF in endothelial cells targets Raf-1 to the mitochondria, which protects these cells from apoptosis [2]. This provides a mechanism that effectively explains why targeting the tumor neovasculature with a mutant Raf-1 gene exerts anti-angiogenic effects [3].
Discussions
Basic FGF and VEGF are survival factors
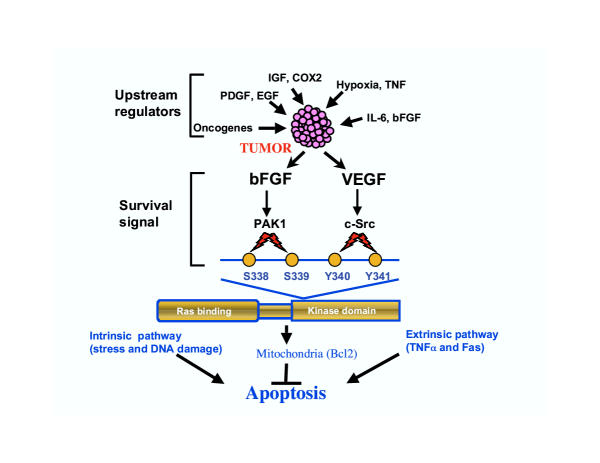
A number of cytokines and growth factor polypeptides have been shown to act as survival factors during angiogenesis, including the acidic and basic fibroblast growth factors (FGFs) and vascular endothelial growth factor (VEGF). Basic FGF (bFGF) and VEGF are two of the cytokines that have been most widely studied, because of their ability to induce many physiological responses, including survival and tumor growth. Both in vitro and in vivo studies suggest that these mediators play a role in angiogenesis. These studies showed that bFGF and VEGF induce mitogenesis and capillary morphogenesis. Furthermore, these factors and their receptors are up-regulated under ischemic conditions in vivo, and administration of these proteins in vivo enhances capillary morphogenesis [1]. The intracellular signaling components regulated by these cytokines have been studied both in cultured cells and in vivo. The production of VEGF by tumors is known occur in response to various upstream factors, including hypoxia, elevated concentrations of bFGF, epidermal growth factor (EGF), insulin-like growth (IGF) and hydrogen peroxide (H2O2) (Figure 1). Several lines of evidence show that VEGF is one of the most important factors in tumor cell survival and neovascularization [4]. For example, deletion of VEGF or its receptor in mice results in the loss of functional blood vessels and early embryonic lethality [4]. Furthermore, blocking VEGF or VEGF receptor functions can induce regression of tumor vasculature in vivo [1,4].
Figure 1.
How do tumors survive, grow and become resistant to drug treatment? The answer may lie in the fact that tumors secrete multiple cytokines and growth factors. A complex interplay between positive and negative factors determines the survival of tumors. The apoptotic signals exerted by "intrinsic" and "extrinsic" pathways could be rescued by bFGF and VEGF. These cytokines activate the PAK-1 and Src kinases, and phosphorylation of specific amino acid residues (indicated by the lightning symbols) within the Raf-1 kinase signals it to be targeted to the mitochondria, which promotes endothelial cell survival [adapted from Alavi, A. et al., Science 301; 94–6 (2003)].
Signaling through Raf-1 kinase
VEGF and bFGF act through specific cell surface receptor tyrosine kinases, which both utilize the canonical Ras/Raf/Mitogen-activated protein kinase (MAP) / extracellular-signal-regulated kinase (ERK) signaling events that link growth factor receptors to nuclear events. The Raf signaling pathway has been highly conserved throughout evolution, and activation of the Raf protein kinase is considered to be a primary event in the Ras signaling pathway [5]. Depending on the specific stimulus and cell type involved, this signaling pathway can promote cell survival, proliferation, or apoptosis. The Raf genes encode cytoplasmic protein serine/threonine kinases that play a critical role in cell growth and differentiation [6]. There are three Raf genes, c-Raf (Raf-1), A-Raf and B-Raf. The expression of A-Raf and B-Raf are known to be somewhat restricted. Structural and functional studies have shown that Raf is composed of two distinct domains, an N-terminal Ras interacting domain and a C-terminal serine/threonine kinase domain. The GTP-bound form of Ras directly interacts with N-terminal region of Raf-1 (Figure 1). This binding localizes Raf to the plasma membrane. Targeting of Raf-l to mitochondria links Ras to a cell survival pathway [7,8]. The binding of Raf-1 to Ras is not sufficient to promote its activation. Raf-1 has been shown to be phosphorylated on tyrosines 340 and 341, as well as on serines 43, 259, 499, and 621 and threonine 269. Intracellular protein tyrosine kinase Src has been implicated in the tyrosine phosphorylation of Raf-1 [8,9]. Phorbol ester is also known to induce phosphorylation of serine 259 and 499. Another recent study suggested that phosphorylation of threonine 491 and serine 494, two phosphorylation sites within the catalytic domain of Raf-1, may be required for its activation, but not inhibition [10]. While these phosphorylation events positively regulate Raf-1 activity, phosphorylation induced by protein kinase A and possibly ERK may negatively impact Raf-1 functions [8].
Targeting of Raf-1 to mitochondria protects endothelial cells from apoptosis
Recently, Alavi et al. demonstrated that bFGF and VEGF utilize the same target, i.e. Raf-1, kinase with distinct specificity [2]. In this elegant study, Alavi et al. examined the role of bFGF-and VEGF-induced activation of p21-activated protein kinase-1 (PAK-1) and Src kinase in the activation of Raf-1 kinase. Both cytokines induced activation of Focal adhesion kinase (FAK) and ERK, but only bFGF-induced phosphorylation of serines 338 and 339 promoted endothelial cell survival. Serum starvation or DNA damage (intrinsic pathway) induced apoptosis. This pathway requires the action of bFGF through PAK-1 kinase, which directly phosphorylates the serine 338 and 339 residues of Raf-1 and targets it to the mitochondria (Figure 1). Once in the mitochondria, Bcl-2 mediated anti-apoptotic mechanisms promote cell survival. In contrast to the effects of bFGF, VEGF induced Src-mediated phosphorylation of tyrosines 340 and 341. This required activation of MEK-1 and ERK1/2, and conferred protection of endothelial cell against apoptosis induced by exposure to tumor necrosis factor (extrinsic pathway) [2]. It appears that a loss-of-function mutant form of Raf-1 i.e., Raf-1 SS338/339AA+YY340/341FF blocks both bFGF- and VEGF-mediated protection of endothelial cells against 'intrinsic' and 'extrinsic' apoptotic events. Based on this study, it will be of considerable interest to investigate the regulation of Raf-1 kinase in response to EGF, IGF and platelet-derived growth factor (PDGF), and to examine how these growth factors affect endothelial cell survival. Furthermore, it will also be important to evaluate the role of adhesion receptors αvβ3 and α5β1 integrins in the regulation of Raf-1 kinase activity.
Conclusions
Previously Hood et al. showed that the nanocrystal-aided targeting of the neovasculature with mutant Raf-1 exerts anti-angiogenic effects [3,11]. Taken together with the recent work by Alavi et al. [2], these studies suggest new possibilities for targeting the tumor neovasculature with small molecule drugs directed against Raf-1 that could promote the apoptosis of endothelial cells and cause regression of tumor vasculature. Endothelial cell death also plays a key role in myocardial infarction and heart failure. These studies also suggest opportunities for inducing therapeutic angiogenesis, in tissues where unwanted apoptosis could be prevented by promoting the translocation of activated Raf-1 kinase into the mitochondria. Taken together, these studies clearly bring nanotechnology-aided anti-angiogenic molecular therapeutics a step closer to reality.
Authors contribution
KKW prepared and approved the manuscript.
Acknowledgments
Acknowledgements
The author (K.K.W) acknowledges research support obtained from American Heart Association, National Council. K.K.W is a member of Mission Connect (TIRR foundation); Cardiovascular Research Institute (CVRI), Texas A & M University System Health Science Center; and U.T. & TAMU-IBT Graduate School of Biomedical Sciences (GSBS), Houston.
References
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–96. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Kolch W, Heidecker G, Lloyd P, Rapp UR. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991;349:426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Magnuson NS, Beck T, Vahidi H, Hahn H, Smola U, Rapp UR. The Raf-1 Serine/threonine protein kinase. Semin Cancer Biol. 1994;5:247–253. [PubMed] [Google Scholar]
- Wang HG, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Kolch W. Untying the regulation of the Raf-1 kinase. Arch Biochem Biophys. 2002;404:3–9. doi: 10.1016/S0003-9861(02)00244-8. [DOI] [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- Tran NH, Frost JA. Phosphorylation of Raf-1 by p21-activated kinase 1 and Src regulates Raf-1 autoinhibition. J Biol Chem. 2003;278:11221–11226. doi: 10.1074/jbc.M210318200. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Antiangiogenics meet nanotechnology. Cancer Cell. 2002;2:97–98. doi: 10.1016/S1535-6108(02)00100-9. [DOI] [PubMed] [Google Scholar]