Abstract
Bcl-xL suppresses apoptotic cell death induced by diverse stimuli in cell lines in vitro. To examine the mechanism by which axotomized cholinergic neurons die in vivo, lentiviral vectors expressing Bcl-xL, human nerve growth factor (hNGF), or green fluorescent protein were injected into the septum 3 weeks before transection of the fimbria fornix. Three weeks after transection, Bcl-xL- and hNGF-injected animals showed significantly higher numbers of spared cholinergic neurons compared with control (green fluorescent protein) injected animals. These results provide evidence that adult axotomized cholinergic neurons die of apoptotic death that can be prevented by local delivery of hNGF or intracellular delivery of Bcl-xL.
Cell death plays an important role in the repair, development, and homeostasis of the central nervous system. During development, programmed cell death tightly regulates a variety of cellular responses to extrinsic and intrinsic factors. Neurotrophic factors are responsible not only for the shaping of the developing brain (1) but also for the maintenance of distinct adult neuronal populations. Receptors for neurotrophins are present on specific populations of neurons in the adult brain and these neurons transduce survival signals by neurotrophins (2). Nerve growth factor (NGF) has been shown to promote neuronal survival of septal cholinergic neurons after axotomy when administered exogenously, and its effects are best characterized in the fimbria fornix model (3–5). Neurotrophins may be involved in several neurological degenerative diseases such as Parkinson’s and Alzheimer’s diseases (6).
The discovery of positive and negative cell-death-regulating proteins involved in both traumatic and apoptotic cell death provides in vitro and in vivo tools to explain the causes of cell death. Bcl-xL, isolated from a chicken lymphoid cDNA library, is a member of the growing number of cell death regulators of the Bcl-2 family (7). Similar to Bcl-2, Bcl-xL prevents apoptotic cell death caused by different stimuli in vitro, such as neurotrophin deprivation, glucocorticoids, ionizing radiation, and oxidant stressors (8–11).
Bcl-xL, one of three isoforms of Bcl-x, protects cells from the damaging effect of reactive oxygen species, e.g., lipid peroxidation, which has been shown to induce apoptotic cell death in vitro (12–14). Several studies indicate that Bcl-2/Bcl-xL and interleukin 1β-converting enzyme(-like) proteases modulate apoptotic and some forms of necrotic cell death, suggesting that both cell death pathways involve some common mediators (15). Bcl-xL is expressed in early neurogenesis; however, unlike Bcl-2, which shows a peak of expression during embryonic development and declines postnatally, Bcl-xL expression increases postnatally, reaching a peak in the adult central nervous system (16–19). Recent results have demonstrated that Bcl-xL can protect sympathetic neurons and telencephalic neurons from trophic-factor-withdrawal-induced cell death (20). The bulk of Bcl-xL is localized in the mitochondrial membrane and the nuclear membrane (18). Data suggest that Bcl-xL may maintain cell survival by regulating the permeability of the intracellular membranes to which it is distributed (21). The baseline expression of Bcl-xL may play a role in neuronal maintenance in the adult, and overexpression of Bcl-xL may prevent traumatic-injury-induced cell death by interfering with the apoptotic pathway.
Viral vectors enable the direct transfer of genes encoding for neurotrophic or antiapoptotic factors thereby allowing one to determine the mechanism involved in cell death. We have recently demonstrated that lentiviral vectors injected into the adult rat brain stably transduce terminally differentiated cells in vivo, without a decrease in transgene expression or toxicity for at least 6 months in vivo (22–25). In this study, the injection of highly concentrated lentiviral vector encoding human NGF (hNGF), Bcl-xL, and green fluorescent protein (GFP) prior to lesion was performed to evaluate the ability of these molecules to rescue septal cholinergic neurons after axotomy-induced trophic factor deprivation. Our study demonstrates a role of Bcl-xL in preventing axotomized cell death by way of the apoptotic pathway, in addition to cell savings by intracellular delivery of hNGF.
MATERIALS AND METHODS
Cloning and Construction of Plasmids.
The genes encoding the hNGF and Bcl-xL were amplified by PCR using primers with BamHI and XhoI sites for subsequent cloning into the lentiviral vector backbone (phRCMV). The GFP insert of pHR′CMVGFP was cloned from the pEGFP-C1 plasmid (a gift from Hiroyuki Miyoshi). In all constructs, the transgene was driven by the human cytomegalovirus immediate early promoter (hCMV). Lentiviral vector was produced by cotransfection with three plasmids into human kidney 293T cells by the method of Pear (26, 27). In the pHR′CMV plasmid, large portions of the envelope coding sequence were deleted. The packaging plasmid (pCMVΔR8.2) provides all vector proteins driven by the hCMV but the envelope, which is encoded by the third plasmid (pMD.G) that provides the heterologous vesiculo stomatitis virus envelope protein. The hCMV promoter drives the VSV.G reading frame. The viral vector was obtained by calcium phosphate precipitation by cotransfecting 293T cells on 10-cm plates with 15 μg of pCMVΔR8.2, 20 μg of pHR′CMVhNGF, pHR′Bcl-xL, or pHR′CMVGFP and 5 μg of pMD.G. After 62 hr the conditioned medium was harvested, centrifuged at low speed, and filtered through a 0.45-μm (pore size) filter. p24 antigen was detected by ELISA (Dupont). Further vector concentration was achieved by ultracentrifugation at 50,000 × g for 90 min, resuspension in TBS containing 10 mM MgCl2, pooling, and incubation with all four dNTPs (each at 0.1 mM)/3 mM spermine/0.3 mM spermidine for 2 hr at 37°C. After the second ultracentrifugation, the pellet was resuspended in sterile saline with Polybrene (2 μg/ml). The vector concentrate was stored at −80°C until thawed for in vitro use or in vivo injection.
Immunohistochemistry.
293T cells were transiently transfected by using Dotap (Boehringer) with the pHR′CMVhNGF, pHR′CMVBcl-xL, or pHR′CMVGFP plasmid. Forty-eight hours after transfection, cells were passaged onto polyornithine/laminin-coated chamber slides and, 24 hr later, fixed with 4% paraformaldehyde in TBS for 20 min. Undifferentiated and differentiated PC12 cells infected with Bcl-xL vector were also fixed and stained. All staining procedures were carried out at room temperature. Cells were washed three times with TBS and preincubated with 5% donkey serum/0.3% Triton X-100 for 1 hr in TBS (TBS++), followed by incubation with primary antibodies in TBS++ [rabbit anti-hNGF (Santa Cruz Biotechnology), 1:500 dilution; rabbit anti Bcl-x (PharMingen), 1:500 dilution; mouse anti-tyrosine hydroxylase (Boehringer-Mannheim), 1:500 dilution] for 2 hr. The cells were washed three times with TBS and preincubated again with TBS++ for 30 min, followed by incubation in secondary antibody [donkey anti-rabbit Biotin (Jackson ImmunoResearch), 1:80 dilution; donkey anti-mouse (Jackson ImmunoResearch), 1:250 dilution] for 2 hr. After three washes in TBS, cells were incubated for 1 hr in streptavidin Cy3 (Jackson ImmunoResearch; 1:250 dilution), Cy5 (Jackson ImmunoResearch; 1:250 dilution), and 4,6-diamidino-2-phenylindole (Sigma; 10 ng/ml) as a fluorescent counterstain for cell nuclei. The slides were cover-slipped in 100 mM Tris (pH 8.5), containing 25% glycerol, 10% polyvinyl alcohol (Air Products and Chemicals, Allentown, PA), and 2.5% 1,4-diazobicycla-(2,2,2)-octane (Sigma). Labeled cells were visualized by using confocal scanning microscopy (Zeiss Axiovert and Bio-Rad MRC1000).
NGF Immunoassay.
NGF was detected in the culture medium of transiently transfected 293T cells, and stably infected 3T3 fibroblasts were measured with a two-site ELISA sensitive to 30 pg/ml (Boehringer Mannheim, product 1530623) using antibodies against hNGF (Boehringer Mannheim, products 1008218 and 1008234). Culture medium was harvested 72 hr after transfection or infection and was compared with the supernatant of noninfected 293T or retrovirally hNGF-infected control cells (28, 29).
NGF Biological Assay.
The ability of lentivirally generated hNGF to elicit neurite outgrowth was assessed in rat PC12 cells, with sensitivity for hNGF of 100 pg/ml. The supernatant of retrovirally infected cells was diluted 1:100 and transferred onto undifferentiated growing PC12 cells.
Bcl-xL Biological Assay.
PC12 cells were differentiated with hNGF for 7 days and infected with 0.5 μl of lentiviral vector encoding for Bcl-xL (1 × 107 IU/ml). Three days later hNGF- containing medium was removed and cells were kept in plain medium for at least 3 weeks. Differentiated PC12 cells deprived of hNGF in plain medium served as a control. Undifferentiated PC12 cells grown in serum-containing medium were also infected with lentivirus encoding for Bcl-xL (1 × 107 IU/ml) and 3 days later were serum-deprived and kept up to 3 weeks. Growing uninfected cells were serum deprived as a control.
In Vivo Experiment.
Female Fischer 344 rats were anesthetized [ketamine (75 mg/kg), acepromazine (0.75 mg/kg), and rompum (4 mg/kg)] and placed in a Kopf stereotactic apparatus for all surgical procedures. Three microliters of the viral vector concentrate (pHRCMVhNGF/Bcl-xL/GFP) or saline was stereotactically injected unilaterally into the septum (A.P. = +0.5 mm, M.L. = +0.5 mm, D.V. = 5.5 mm) at a rate of 1 μl/min. Three weeks after injection, complete unilateral aspirative lesions of the fimbria fornix were performed under visual inspection with the aid of a surgical microscope as described (30). Animals were perfused with 4% paraformaldehyde after 3 weeks. Control animals injected with viral vector stocks but not lesioned were perfused after 3 weeks as well. Brains were removed, fixed overnight, and transferred into 30% sucrose until equilibration. Fifty-micrometer sections were cut on the freezing sliding microtome.
For quantification, every third section was collected and, after washing in TBS, blocked in 0.6% H2O2, permeabilized with 0.3% Triton X-100, and blocked in 5% donkey serum in TBS (TBS++). Sections were incubated in the primary antibody goat anti-choline acetyltransferase (ChAT) in TBS++ for 48 hr at 4°C. After three washes in TBS++, sections were incubated in biotinylated donkey anti-goat secondary antibody (Jackson ImmunoResearch, 1:80 dilution) and developed by the avidin-biotin method (Vector Laboratories) with nickel intensification using 3,3-diaminobenzidine as the chromogen.
For immunofluorescent staining, primary antibodies raised in three different species were pooled in TBS containing 10% donkey serum and 0.3% Triton X-100 and incubated for 48 hr at 4°C. The antibody for ChAT [goat polyclonal antibody (Chemicon), 1:200 dilution] was combined with two of the following antibodies from different hosts: Bcl-xL [rabbit polyclonal (Santa Cruz Biotechnology), 1:500 dilution], hNGF [rabbit polyclonal (PharMingen), 1:100 dilution], or marker for postmitotic neurons (NeuN) mouse monoclonal antibody (gift from R. J. Mullin, 1:20 dilution). Sections were washed and blocked in TBS with donkey serum. Corresponding secondary antibodies [donkey anti-mouse Biotin, donkey anti-rabbit fluorescein isothiocyanate, donkey anti-guinea pig Cy5, donkey anti-goat Cy5 (Jackson ImmunoResearch), 1:250 dilution] were pooled, and sections were incubated for 2 hr at room temperature, followed by washing in TBS and a 2-hr incubation at room temperature in streptavidin Cy3 or streptavidin TR in TBS (Jackson ImmunoResearch, 1:250 dilution). 4,6-Diamidino-2-phenylindole was added in the last wash and sections were mounted and cover-slipped, as described for in vitro slides.
Morphometric Analysis.
The volume of the septum was determined by the method of Cavalieri as described (31, 32). A point-counting grid printed on an acetate sheet was placed over the monitor, upon which the whole septum was displayed from a low-power objective (×4). The area around each point was calibrated with a stage micrometer. From the distance between the sections (d) multiplied times the area per point (Ap), every point was used as a volume probe. The total volume (Vref) of the septum was determined by the numbers of points (Q) counted overlying the medial septum in semiserial (one-in-three) sections and multiplying the sum (∑Q) with the volume of each point (Vref = ∑Q × d × Ap). Every third section through the septum stained for ChAT was selected for neuronal cell counts. The medial septum was defined as the area lying above a line between the midportion of the anterior commissure, beneath the corpus callosum and laterally limited by the ventricles. ChAT-positive cells displaying a round cell body and at least one process were counted for the lesioned and the unlesioned sites of the septum. Cell saving is represented as the percentage of ChAT-positive neurons per lesioned/unlesioned site, comparing Bcl-xL-, hNGF-, GFP-, and saline-injected groups. Statistical analysis was performed using the multiway ANOVA analysis, followed by a Fisher post hoc test, with a significance level of P < 0.05.
RESULTS
In Vitro Analysis of Bcl-xL, hNGF, and GFP Expression.
Supernatant of cells transiently transfected with a lentiviral vector encoding hNGF, diluted 1:100 in serum-free medium, elicited neurite outgrowth from PC12 cells in vitro. PC12 cells stopped dividing and differentiated into mature cells with neuronal morphology. Supplementation of serum-free medium with hNGF allowed for maintenance of these differentiated cells for more than 12 weeks.
PC12 cells pretreated with hNGF to induce differentiation died within 3 days when NGF and serum were removed and cells were grown in plain medium (Fig. 1A). Stable expression of Bcl-xL by lentiviral gene transfer in differentiated cells allowed for cells to survive more than 3 weeks without serum or hNGF; cells continued to express tyrosine hydroxylase (Fig. 1B). Undifferentiated PC12 cells also died after withdrawal of serum (Fig. 1C). Stable expression of Bcl-xL prior to serum withdrawal saved these cells from cell death (Fig. 1D).
Figure 1.
Immunocytochemistry of PC12 cells survival and differentiation. (A) PC12 cells died when they were differentiated with hNGF and subsequently deprived of serum and hNGF. When infected with a lentiviral vector encoding Bcl-xL, PC12 cells differentiated with hNGF survived for an additional 3 weeks (B) (stained blue for tyrosine hydroxylase). PC12 cells not differentiated with hNGF and deprived of serum were dead after 3 days (C). However, PC12 cells infected with a vector containing Bcl-xL expressed Bcl-xL (green) and tyrosine hydroxylase (blue) and survived for 7 days (D).
In Vivo Analysis of Transgene Expression in the Intact and Fimbria/Fornix-Lesioned Forebrain Cholinergic System.
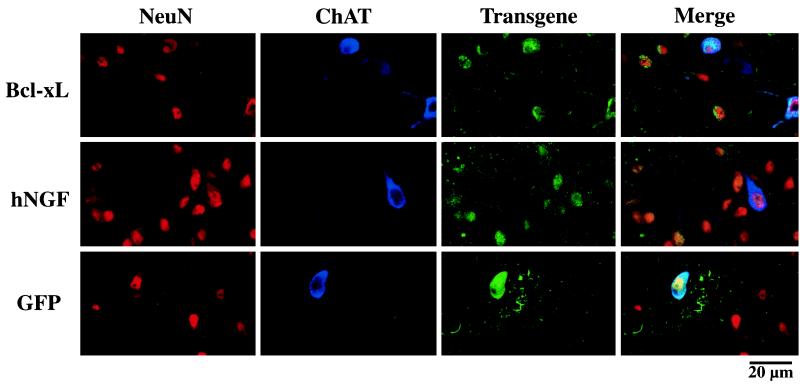
Immunostaining of coronal sections through the septum revealed transduced cells expressing GFP and hNGF around the injection site and Bcl-xL-overexpressing cells, with the majority of cells displaying neuronal morphology and coexpressing neuronal markers such as NeuN and ChAT (Fig. 2). There was no detectable difference in the septal volume of fimbria/fornix-lesioned and unlesioned animals (data not shown) or in the morphology of infected transgene-expressing cells.
Figure 2.
Immnohistochemical staining of coronal sections through the septal area. Confocal microscopic images show in vivo transduction of adult rat septal neurons expressing neuronal markers (NeuN; red), cholinergic markers (ChAT; blue), and the hNGF or Bcl-xL transgene (hNGF/Bcl-xL; green). The images obtained from each individual staining and from the merged images are shown. Representative fields of the area surrounding the injections site are shown with several cells triple labeled for hNGF, NeuN, and ChAT, as well as for Bcl-xL, NeuN, and ChAT.
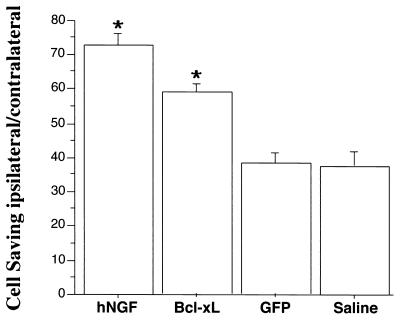
Light microscopic analysis of septal sections stained for ChAT revealed 48% of cholinergic septal neurons expressed GFP in unlesioned animals. In GFP lentiviral vector- and saline-injected control groups, 38% (±3.4%) of septal cholinergic neurons survived the transection of the fimbria fornix. Lentiviral transfer and expression of hNGF in the septum resulted in the survival of 73% (±3.6%) of cholinergic neurons after lesion of the fimbria fornix (Figs. 3 and 4). The injection of Bcl-xL-expressing viral vector resulted in the survival of 59% (±2.2%) of cholinergic neurons. The cell savings elicited by lentiviral gene transfer of hNGF was significantly different from that of Bcl-xL (P = 0.016), GFP (P < 0.001), and saline (P < 0.001) injections. The overexpression of Bcl-xL ipsilateral to the lesion resulted in less of a cell saving compared with expression of NGF; however, the cell saving was significantly higher than in the GFP (P = 0.019) and saline (P = 0.016) injection groups.
Figure 3.
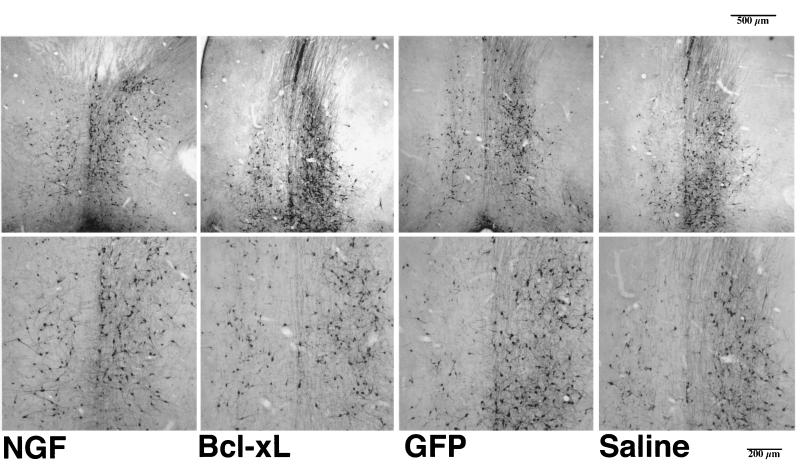
Coronal sections (50 μm) through the medial septum stained for ChAT immunoreactivity 6 weeks after hNGF, Bcl-xL, or GFP vector injection or saline control injection and 3 weeks after unilateral aspirative lesion of the fimbria fornix, ipsilateral to the injection. The medial septum was defined as the area lying above a line between the midportion of the anterior commissure, beneath the corpus callosum, and laterally limited by the ventricles. ChAT-positive cells displaying a round cell body and at least one process were counted for the lesioned and the unlesioned site of the septum.
Figure 4.
Percentage of ipsilateral/contralateral septal neurons quantified after intraseptal vector injections and lesion of the fimbria fornix. The percentage of ChAT-immunoreactive cells (mean ± SEM) are shown for hNGF-, Bcl-xL-, and GFP-expressing vector and saline control. hNGF and Bcl-xL viral vector-injected animals presented significantly higher numbers of surviving cholinergic septal neurons compared with GFP viral vector or saline-injected control animals (∗, P < 0.05, ANOVA, Fischer post hoc test).
DISCUSSION
Fimbria/fornix lesion causes the death of approximately 62% of the NGF-dependent cholinergic neurons. Thirty-eight percent of ChAT-positive neurons survive in both the saline and the GFP groups after fornix transection. Lentiviral gene transfer and overexpression of Bcl-xL within the cholinergic septal neuron result in 59% cell saving, providing an additional cell saving of 21%. However, in our experiments, hNGF rescues 73% of cholinergic neurons, 35% more than the baseline survival of GFP or saline control groups. Lentiviral gene transfer was achieved in approximately 48% septal cholinergic neurons with a 3-μl injection of highly concentrated viral vector stock. If only half of the cholinergic neurons are infected and the level of increased survival in both Bcl-xL and hNGF expressing groups is considered, we conclude that the Bcl-xL viral vector was highly efficient and the transgene expression was sufficient to produce a biological effect. The saving of more than 68% of the cholinergic neurons by hNGF provides additional evidence that NGF acts, in part, by a paracrine mechanism to support more ChAT-positive neurons than were originally infected with the virus (Fig. 5 shows a schematic of mechanism).
Figure 5.
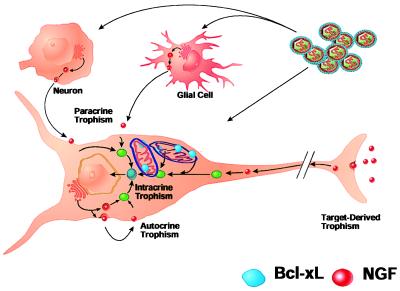
In vivo model for the role of hNGF and Bcl-xL in neuronal survival. Lentiviral transfer and expression of hNGF and Bcl-xL by lentiviral vectors is obtained in target and adjacent glial and neuronal cells. Bcl-xL stably expressed is localized within the target cell in mitochondrial and nuclear membranes; however, it is not secreted. Only infected cholinergic neurons can survive axotomy-induced cell death and trophic factor withdrawal by interfering with the cell death cascade by overexpression of Bcl-xL. Surrounding cells do not contribute to cell survival and, therefore, cell saving is less than that obtained with overexpression of hNGF. hNGF is expressed by the target neuron and adjacent glial and neuronal cells as a secreted protein; the autocrine and paracrine effects on the target cell allow for a higher number of cells saved by hNGF.
Rodent and primate cholinergic adult septal neurons require exogenous NGF for maintenance in the adult and after axotomy (3–5, 33–36). NGF produced in the hippocampal postsynaptic neurons is thought to be released and interacts with presynaptic neuronal receptors, resulting in support of cell survival. After fimbria/fornix lesion, levels of NGF are increased in the hippocampus as the result of accumulation due to the absence of retrograde transport of the septal cholinergic neurons (37). hNGF delivered to the ventricle or parenchyma by osmotic minipumps rescues up to 90% of cholinergic neurons after lesion (38). Whether the effect of NGF in this model is due to necrosis or apoptosis has not been investigated in this model. The present results confirm the ability of hNGF to protect cholinergic cells from axotomy-induced cell death. Further, these results demonstrate that lentiviral delivery of hNGF can have a prophylactic effect on cholinergic neurons. The results with NGF do not allow for the interpretation of the mechanism, because the virus infects other neurons in addition to cholinergic neurons and hNGF is a secreted molecule. Therefore, survival could be due to either autocrine or paracrine effects.
In contrast, the survival effect of Bcl-xL overexpression in axotomized cholinergic neurons suggests that these neurons die by an apoptotic mechanism or programmed cell death, which can be halted by an overexpression of Bcl-xL in the target cell. Because neither Bcl-xL nor any product of Bcl-xL is secreted that might indirectly support neurons (as evidenced by the in vitro experiments above), Bcl-xL acts directly on the axotomized cholinergic neuron. This cell-specific effect also explains the difference in survival percentage between Bcl-xL and hNGF, because hNGF can potentially act in both autocrine and paracrine manners. Importantly, Bcl-xL not only protected the cells from cell death but also permitted the maintenance of cell-specific expression of the essential enzyme ChAT and a marker for differentiated neuronal cells, NeuN.
The Bcl-2 family of proteins includes apoptosis-promoting (Bax, Bak, and Bcl-xS) and survival-maintaining (Bcl-2 and Bcl-xL) members that function at least in part through competing homo- and heterodimer formation (39); of them, Bcl-x is expressed in greatest amount (in both mRNA and protein) in the central nervous system of adult rats, mice, and primates (19, 20, 40, 41).
The Bcl-xL gene, which peaks in concentration in the adult central nervous system, is believed to be necessary for neuronal survival in vitro (42). Bcl-xL-deficient mice died around embryonic day 13, based on extensive apoptotic cell death, as evident in postmitotic immature neurons of the developing brain, spinal cord, and dorsal root ganglia and the defect of hematopoietic cells (43). This early lethality is in sharp contrast to Bcl-2 ablation in mice that survive at least 6 weeks (44, 45). Surprisingly, the nervous system, intestines, and skin of these mice appear normal, despite the fact that these organs show high levels of endogenous Bcl-2 expression in normal mice (44). Bcl-2-deficient mice have normal numbers of developing neurons after the period of target-dependent neuronal death but subsequently show degeneration (46). In our study we were able to show an increased survival of cholinergic neurons after axotomy after lentiviral gene transfer and stable transgene expression of Bcl-xL.
This study provides in vivo experimental evidence that the stable gene transfer of antiapoptotic factors (Bcl-xL) and neurotrophins (hNGF) via lentiviral vectors is sufficient to rescue cholinergic neurons after axotomy in vivo. Neurons undergo cell death because of traumatic injury or exposure to neurotoxins including excitotoxicity or withdrawal of trophic factor support. Most neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and Huntington’s diseases are progressive disorders, with protracted cell death over many years. Although it has been demonstrated that neurotrophic factor intervention soon after a traumatic injury can protect a cell from degeneration, the results in the present experiment demonstrate that overexpression of either NGF or the antiapoptotic gene Bcl-xL into healthy cholinergic neurons can act prophylactically to protect cells from a later challenge.
Acknowledgments
We thank J. C. Reed for the Bcl-xL cDNA, M. L. Gage, E. A. Markakis, and T. D. Palmer for their critical review of the manuscript, and Hiroyuki Miyoshi for help with the in vitro work. This work was supported by the National Institute for Aging, American Paralysis Association, and the Hollfelder Foundation. U.B. was supported by National Institutes of Health Grant AGO 8514. We also thank the Francis Berger Foundation for their support. I.M.V. is an American Cancer Society Professor of Molecular Biology. T.K. was supported by a grant from the Cystic Fibrosis Society.
Footnotes
Abbreivations: NGF, nerve growth factor; hNGF, human NGF; GFP, green fluorescent protein; ChAT, choline acetyltransferase; NeuN, marker for postmitotic neurons.
References
- 1.Korschung S. J Neurosci. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bothwell M. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 3.Williams L R, Varon S, Peterson G M, Wictorin K, Fischer W, Bjorklund A, Gage F H. Proc Natl Acad Sci USA. 1986;83:9231–9235. doi: 10.1073/pnas.83.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kromer L F. Science. 1987;235:214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- 5.Hefti F. J Neurosci. 1986;6:2155–2162. doi: 10.1523/JNEUROSCI.06-08-02155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 7.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 8.Kane D J, Ord T, Anton R, Bredesen D E. J Neurosci Res. 1995;40:269–275. doi: 10.1002/jnr.490400216. [DOI] [PubMed] [Google Scholar]
- 9.Nunez G, Merino R, Grillot D, Gonzalez-Garcia M. Immunol Today. 1994;15:582–588. doi: 10.1016/0167-5699(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 10.Vaux D L, Cory S, Adams J M. Nature (London) 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 11.Zhong L T, Sarafian T, Kane D J, Charles A C, Mah S P, Edwards R H, Bredesen D E. Proc Natl Acad Sci USA. 1993;90:4533–4537. doi: 10.1073/pnas.90.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troy C M, Shelanski M L. Proc Natl Acad Sci USA. 1994;91:6384–6387. doi: 10.1073/pnas.91.14.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troy C M, Stefanis L, Greene L A, Shelanski M L. J Neurosci. 1977;17:1911–1918. doi: 10.1523/JNEUROSCI.17-06-01911.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenlund L J, Korsmeyer S J, Johnson E M., Jr Neuron. 1995;15:649–661. doi: 10.1016/0896-6273(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 15.Tsujimoto Y, Shimizu S, Eguchi Y, Kamiike W, Matsuda H. Leukemia. 1997;3:380–382. [PubMed] [Google Scholar]
- 16.Merry D E, Veis D J, Hickey W F, Korsmeyer S J. Development. 1994;120:301–311. doi: 10.1242/dev.120.2.301. [DOI] [PubMed] [Google Scholar]
- 17.Asahi M, Hoshimaru M, Uemura Y, Tokime T, Kojima M, Ohtsuka T, Matsuura N, Aoki T, Shibahara K, Kikuchi H. J Cereb Blood Flow Metab. 1997;17:11–18. doi: 10.1097/00004647-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Garcia M, Perez-Ballestero R, Ding L, Duan L, Boise L H, Thompson C B, Nunez G. Development. 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Garcia M, Garcia I, Ding L, O’Shea S, Boise L H, Thompson C B, Nunez G. Proc Natl Acad Sci USA. 1995;92:4304–4308. doi: 10.1073/pnas.92.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankowski H, Missotten M, Fernandez P A, Martinou I, Michel P, Sadoul R, Martinou J C. Neuroreport. 1995;6:1917–1921. doi: 10.1097/00001756-199510020-00023. [DOI] [PubMed] [Google Scholar]
- 21.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi H, Takahashi M, Gage F H, Verma I M. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blömer U, Naldini L, Kafri T, Trono D, Verma I, Gage F. J Virol. 1997;71:6642–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 25.Naldini L, Blömer U, Gage F H, Trono D, Verma I M. Proc Natl Acad Sci USA. 1996;93:11382–11387. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naviaux R K, Costanzi E, Haas M, Verma I M. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaja M, Rosenberg M, Yoshida K, Gage F H. J Neurosci. 1992;12:2849–2864. doi: 10.1523/JNEUROSCI.12-07-02849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg M B, Friedmann T, Robertson R C, Tuszynski M, Wolff J A, Breakefield X O, Gage F H. Science. 1988;242:1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- 30.Gage F H, Bjorklund A, Stenevi U. Brain Res. 1983;268:27–37. doi: 10.1016/0006-8993(83)90387-6. [DOI] [PubMed] [Google Scholar]
- 31.Peterson D A, Lucidi-Phillipi C A, Eagle K L, Gage F H. J Neurosci. 1994;14:6872–6885. doi: 10.1523/JNEUROSCI.14-11-06872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuszynski M, U, H S, Amarald D G, Gage F H. J Neurosci. 1990;10:3604–3614. doi: 10.1523/JNEUROSCI.10-11-03604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koliatsos V, Nauta H J, Clatterbuck R E, Holtzmann D M, Mobley W C, Price D L. J Neurosci. 1990;10:3801–3813. doi: 10.1523/JNEUROSCI.10-12-03801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gage F H, Bjorklund A. CIBA Found Symp. 1987;126:143–159. doi: 10.1002/9780470513422.ch9. [DOI] [PubMed] [Google Scholar]
- 36.Hefti F, Knusel B. Neurobiol Aging. 1988;9:689–690. doi: 10.1016/s0197-4580(88)80133-7. [DOI] [PubMed] [Google Scholar]
- 37.Schwab M, Otten U, Agid Y, Thoenen H. Brain Res. 1979;168:473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- 38.Tuszynski M, U, H S, Gage F H. Ann Neurol. 1991;30:635–636. doi: 10.1002/ana.410300502. [DOI] [PubMed] [Google Scholar]
- 39.Korsmeyer S J, Yin X M, Oltvai Z N, Veis-Novack D J, Linette G P. Biochim Biophys Acta. 1995;1271:63–66. doi: 10.1016/0925-4439(95)00011-r. [DOI] [PubMed] [Google Scholar]
- 40.Krajewski S, Krajewska M, Shabaik A, Wang H G, Irie S, Fong L, Reed J C. Cancer Res. 1994;54:5501–5507. [PubMed] [Google Scholar]
- 41.Krajewski S, Mai J K, Krajewska M, Sikorska M, Mossakowski M J, Reed J C. J Neurosci. 1995;15:6364–6376. doi: 10.1523/JNEUROSCI.15-10-06364.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth K A, Motoyama N, Loh D Y. J Neurosci. 1996;16:1753–1758. doi: 10.1523/JNEUROSCI.16-05-01753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motoyama N, Wang F, Roth K A, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh D Y. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 46.Michaelidis T M, Sendtner M, Cooper J D, Airaksinen M S, Holtmann B, Meyer M, Thoenen H. Neuron. 1996;17:75–89. doi: 10.1016/s0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]