Abstract
One of the fundamental principles of visual cortical organization is that neurons form a “map” in which neighboring cells have similar orientation preferences. Previous anatomical and imaging studies have shown that although the exact layouts of these orientation preference maps vary between individuals, features of iso-orientation domains such as width and spacing appear constant within a species. Using chronic optical imaging of intrinsic signals we now demonstrate that in ferret area 17 a larger proportion of cortical surface is dominated by responses to horizontal and vertical contours than to the two oblique orientations. This was true for all ferrets studied both during development and in adulthood. Interestingly, however, we found that the degree of the overrepresentation varied significantly between individual animals. In some young ferrets, responses to horizontal and vertical stimuli developed faster than responses to oblique stimuli, and a much larger percentage of the cortex responded preferentially to horizontal and vertical stimuli. In other individuals, responses to all stimuli developed at roughly the same rate, and there was relatively little overrepresentation of horizontal and vertical preferences.
Keywords: oblique effect, activity maps, orientation maps, optical imaging
We have used chronic optical imaging of intrinsic signals (1) to examine developing orientation activity maps in ferret primary visual cortex. The ferret was chosen for these studies because it has a visual system similar to the cat’s (2) but is born approximately 3 weeks earlier in development (3), thus providing a robust physiological preparation during the time that orientation-specific responses develop (ref. 4; see also refs. 5 and 6).
The technique of optical imaging permits observation of the overall spatial organization of orientation preference across a region of visual cortex, information not easily obtained from single-unit studies. Furthermore, by measuring the collective response of a large number of neurons simultaneously, optical imaging avoids many of the sampling problems that can occur in studies of single-cell responses. Chronic imaging allows the development of orientation maps to be followed over time in individual animals, removing the necessity to pool data across animals. This technique therefore is not only ideal for assessing the proportion of cortical surface devoted to processing different orientations during development, but it can also reveal interanimal variability in the representation of orientation preferences, which would be missed by conventional electrophysiological recordings.
All of the ferrets in this study (eight developing and three adult) showed a significant overrepresentation of horizontal and vertical versus oblique orientation preferences in their cortical maps. In the eight developing ferrets, there were large differences in the magnitude of this anisotropy. Furthermore, in the developing animals with larger anisotropies, the horizontal and vertical activity maps also tended to develop significantly faster than the oblique maps.
The bias toward horizontal and vertical preferences seen in all of the ferrets studied is consistent with electrophysiological studies in the cat (7–14) and kitten (15), which suggest that there may be more cortical cells with horizontal and vertical preferences. The observed interanimal variability in the degree of the bias is surprising, as previous studies have suggested that basic features of iso-orientation domains such as hypercolumn spacing are relatively constant between animals (refs. 16–24; but see also ref. 25). The variability may, however, help to explain why some laboratories have failed to demonstrate the horizontal/vertical bias electrophysiologically in either cats (26–28) or kittens (29–31). Finally, this variability might underlie the interindividual variation that has been observed in psychophysical studies of the “oblique effect” in cats, monkeys, and humans (32–36).
MATERIALS AND METHODS
Optical Imaging of Intrinsic Signals.
Eight young (31–35 days old at the beginning of the experiments) and three adult (4.5 months to 1 year old) ferrets (Marshall Farms, New Rose, NY) were used in this study. Experiments were performed under aseptic conditions by using standard procedures for optical imaging (37). Anesthesia was induced by using a mixture of xylazine and ketamine. Animals were intubated and mechanically ventilated. Anesthesia was maintained by using 1–3% halothane delivered in a 3:1 mixture of nitrous oxide:oxygen. End-tidal carbon dioxide was maintained at 3.8–4.2%. Subcutaneous injections of ≈1 ml/hr 5% dextrose Ringer’s solution were administered to prevent dehydration. During the initial imaging session, the scalp was incised and retracted and a craniotomy was performed over the caudal pole of the left hemisphere. The dura remained intact. Two percent agar and a glass coverslip were applied over the craniotomy. Animals were fitted with contact lenses to focus the eyes on the monitor placed 33 cm in front of the animal. The brain was illuminated through the dura by using a halogen lamp and fiber optics, with the illumination band-pass filtered at 707 ± 10 nm, a wavelength that provides good penetration of the dura. The signals were recorded with a cooled charge-coupled device camera (Princeton Instruments, Trenton, NJ) and a macroscope (38) focused approximately 500 μm below the cortical surface. Five frames of 600-msec duration were collected during each 3-sec stimulus presentation. Following each recording session, the agar and coverslip were removed, and the craniotomy was flushed with sterile saline and covered with a 3% agar plug containing chloramphenicol antibiotic (Paraxin, Bayer). The fascia and scalp were sutured and the scalp wound was infused with lidocaine and covered with a topical antibiotic. The animal was then allowed to recover from the anesthesia before being returned to its mother.
Visual Stimuli.
Visual stimuli were produced by using custom-made software (stim, Kaare Christian, Rockefeller University, NY) and were presented binocularly on a 21-inch monitor placed 33 cm away from the ferret. The stimuli consisted of full-screen square-wave gratings drifting at 10–15°/sec, presented at four different orientations: horizontal, vertical, and the two obliques. The spatial frequency ranged from 0.5 to 0.15 cycles/degree (this value was chosen empirically to optimize the activity signal and varied with age probably because of changing optics in the ferrets’ maturing eyes); the same spatial frequency was used for all orientations in a given recording session. The stimuli were randomly interleaved, and each was generally presented 128 times.
Calculation of Activity Maps and Polar Maps.
To reduce noise in the acquired images, signal averaging was used. To remove nonorientation-specific signals such as uneven illumination and blood-vessel artifacts from the maps, each orientation activity map was normalized to the average of maps seen in response to all orientations (for details, see ref. 9). The resulting images are termed single-condition maps.
The information from single-condition activity maps in response to all four orientations of stimuli were combined into single, color-coded “polar maps” by using vectorial addition on a pixel-by-pixel basis (for details, see ref. 9).
Calculation of Orientation Preference Areas.
The proportion of cortex that prefers each of the four orientations was determined by calculating an “orientation preference area” for each orientation from data contained in the polar maps. The first step in this analysis was to determine a region of interest (ROI) for each animal containing only the area of the map where orientation-specific activity was seen in the most mature map. Next, the data in the polar maps from each animal at each recording age were passed through a threshold procedure such that all dark pixels with little or no orientation-specific response (vector length <0.1 of maximum) were not included in any of the four orientation preference areas. The orientation preference area for each orientation was then calculated as the percentage of total pixels within the ROI whose response preference fell within the four 45° orientation ranges centered on 0, 45, 90, and 135° (i.e., those pixels within a given color range). The polar maps were chosen for this analysis because they give a better indication of the area of cortex that prefers a given orientation than do the single condition maps, which show the area that responds to a given orientation.
The threshold value of 0.1 was chosen for this analysis because it resulted in inclusion of <5% of total pixels in very early maps that appeared featureless and inclusion of >75% of pixels in the most mature maps studied in each animal. Varying the threshold value to 0.01 (resulting in inclusion of >90% of pixels in the most immature maps) to vector length 0.2 (resulting in inclusion of only 25% of pixels in the most mature maps) did not change the basic shapes of the orientation preference area graphs.
To quantify the uncertainty in this measurement, orientation preference areas were calculated for individual data blocks (each block representing the response to 64 stimulus presentations) recorded from each animal during each recording session, as well as for the entire session. The multiple blocks allowed us to estimate the standard deviation in the measurements.
To provide a single number characterizing its magnitude we defined the anisotropy from the orientation preference areas as [(horizontal + vertical) − (left oblique + right oblique)]/(total area) × 100%. A 100% anisotropy would result from maps where there was no representation of the obliques; a 0% anisotropy would result if there were equal representation of all orientations.
Determining the rate of the development of responses to each orientation from the response area data is problematic for two reasons. First, the final map recorded in each animal may not represent a completely mature map, so there is no available measure of the fully developed anisotropy in each animal. Second, any quantitative analysis of the rates of development of response areas would necessitate modeling the growth rate, and there is no theoretical or empirical reason for us to adopt any particular underlying mathematical function—in some animals (e.g., ferret 1-5-413) the development appears approximately linear whereas in others (e.g., ferret 1-2-413) it does not.
Cross-Correlation Analysis of Map Maturation.
To quantify how mature each orientation activity map was at each age in a model-independent manner, two-dimensional (2-D) cross-correlations were performed between each recorded activity map and the most mature map recorded for that stimulus orientation in that animal. The first step in the correlation analysis was to determine an ROI for each animal as described above. To control for possible misalignment of the maps, we calculated the correlation coefficients between the two maps for x and y offsets ranging from −30 to 30 pixels (each pixel corresponds to 36 × 36 μm2 of cortex). The largest of these values was then taken as the measure of correlation between the two maps. A value of 0 indicates an immature map; a value near 1 indicates a more fully developed map. Note that no significant brain growth occurs during the period covered by these experiments (37) and thus is not a confounding factor in this analysis.
RESULTS
Optical imaging of ferret area 17 was performed through the intact dura, and activity maps in each animal at each age were generated in response to moving square-wave gratings at each of four orientations: horizontal, vertical, and the two obliques. Examination of the resulting images revealed different patterns of orientation development. In some ferrets, activation of iso-orientation domains responding to horizontal and vertical gratings developed faster than domains responding to obliquely oriented gratings. These animals also showed greater overrepresentation of cortical area preferring horizontal and vertical stimuli. In other ferrets, domains responding to all orientations developed roughly in parallel. These ferrets tended to show only a modest overrepresentation of horizontal and vertical preferences.
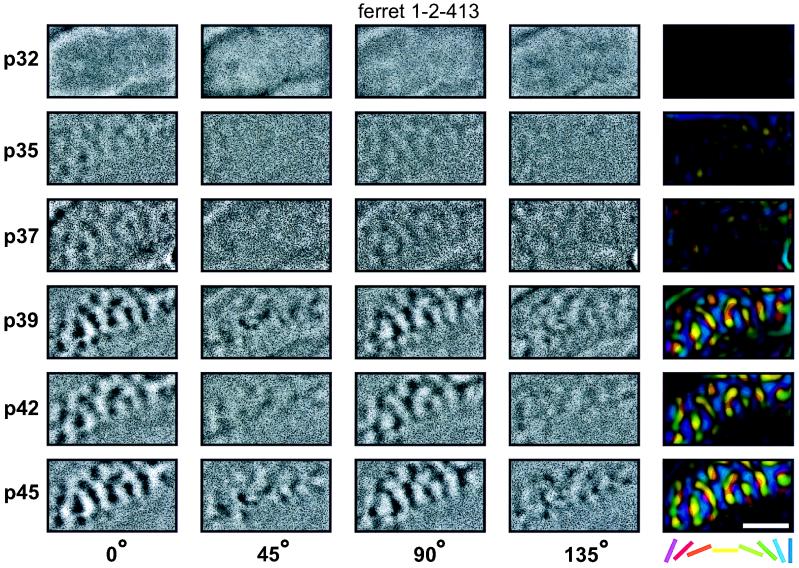
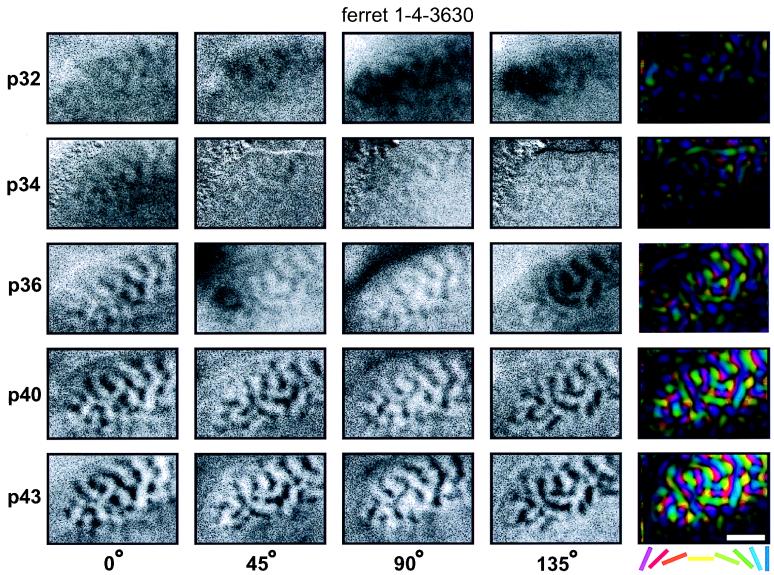
Activity maps illustrating the range of the developmental patterns observed are shown in Figs. 1 and 2. Each row of these figures shows orientation-specific maps recorded in the left primary visual cortex at the indicated age. The first four columns show single-condition activity maps recorded in response to a specific orientation. To determine the overall organization of orientation preference in the cortex, information from the four activity maps was combined into a single, color-coded “polar map” shown in the right-most columns. In the polar maps, the color of each pixel encodes the preferred orientation of cells in that region of cortex, whereas the brightness indicates the orientation selectivity (for details see refs. 9 and 39).
Figure 1.
Faster development of horizontal and vertical than of oblique orientation maps. Each row of the figure shows orientation maps recorded in the left primary visual cortex of one ferret at the age (postnatal day, p) indicated at the left of the row. The first four columns show orientation maps recorded in response to a particular orientation of moving square-wave grating (0° = horizontal). The fifth column shows the polar map calculated from those four single-condition maps. For each map, caudal is up and medial is to the left. The curve in the upper left corner of each map indicates the location of the caudal pole of the cortex behind which the skull remained intact over the cerebellum. The approximate location of the area 17/18 border can be seen in each image as a line rostral to which orientation activity is not seen. Single-unit recordings were not performed in this study to precisely locate the area of the visual field studied in each animal. However, the craniotomies were always performed at the same location on the skull with respect to skull sutures, exposing what has previously been found to be the representation of area centralis (2, 3). In this example the first orientation maps can be seen at postnatal day 35. At this age only horizontal and vertical maps are clearly seen. The orientation maps for the two oblique orientations develop considerably slower (they are first clearly seen at p37) and the difference in the strength of the maps remains present throughout the experiment. (Bar = 2 mm.)
Figure 2.
Concurrent development of all orientation maps. This figure shows data from an animal in which all the orientations developed simultaneously. Note also that in this case the first orientation maps are visible at a substantially earlier time (p32) than in the example shown in Fig. 1. All other conventions are as in Fig. 1.
Maps from a ferret showing faster development of horizontal and vertical domains are shown in Fig. 1. In this animal, there was little or no orientation-specific response to any stimulus during the earliest imaging session on postnatal day 32. By postnatal day 35, however, clear horizontal and vertical activity maps were present, although weak if any maps were seen with oblique stimuli. Oblique maps became obvious, though still weak, by postnatal day 37. Horizontal and vertical maps appear to be fully developed (i.e., no longer changing) by postnatal day 42, whereas oblique maps remain weaker and continue to mature through postnatal day 45, the last imaging session for this animal.
Fig. 2 shows data from an animal where the development of orientation maps appears to have progressed at a more or less equal pace for all orientations tested. In this animal faint maps are already visible at postnatal day 32. At this and all subsequent ages, the strength of the activity maps in response to all four orientations appears approximately equal on visual inspection.
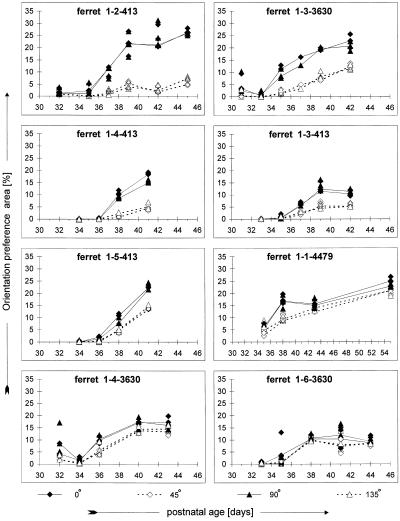
To obtain quantitative measures of these results, the development of orientation maps in individual animals was studied by using two different methods. First, the “orientation preference area” (see Materials and Methods) was determined for each stimulus orientation at each age. The results of this analysis are shown in Fig. 3. At early ages in all animals, there are no obvious orientation-specific domains in cortex. As the animals mature, a larger proportion of the cortex becomes orientation-selective. All eight ferrets tested showed significantly larger orientation preference areas for horizontal and vertical during development (two-factor ANOVA, P < 0.001 for all, except P < 0.01 for ferret 1-6-3630; posthoc one-tailed t tests of time-averaged data, P < 0.02 for all animals). The magnitude of the anisotropy at the final experimental time point (see Materials and Methods) ranged from 3.5 to 61.6% across animals. Although this measure appeared to vary along a continuum, the interanimal variability is highly significant (one-factor ANOVA, P < 0.001).
Figure 3.
Quantification of orientation preference areas. Orientation-specific response areas were calculated in each animal at each age as described in Materials and Methods. Values calculated for blocks of data from individual trials are shown to provide information on the noise in the measurement; combined data for all trials at a given orientation in a given animal at a given age are indicated by the symbols connected by lines. In all eight animals studied, horizontal and vertical response areas are significantly larger than oblique response areas (two-factor ANOVA, P < 0.01 for ferret 1-6-3630; P < 0.001 for all other animals). However, the magnitude of the anisotropy varies between animals. Note that ferrets 1-2-413 through 1-5-413 were littermates, as were ferrets 1-3-3630, 1-4-3630, and 1-6-3630. Ferrets 1-4-413 and 1-4-3630 were females.
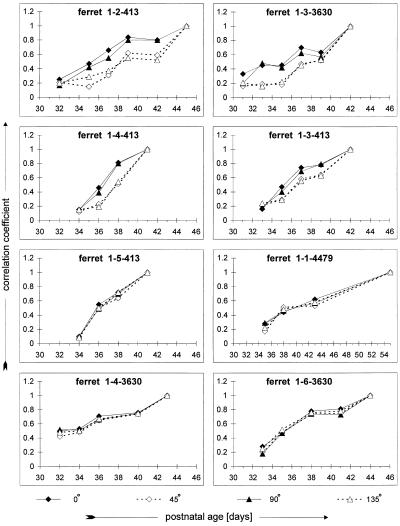
To provide a measure of the rate of orientation-map development in each animal, early single-condition maps were compared with the most mature map by using cross-correlation. Because we have previously shown that the structure of each orientation activity map in an individual animal is stable during development (37), this correlation coefficient provides a good measure of map maturity. The results of this analysis are shown in Fig. 4. The four animals with the largest anisotropies (Fig. 3, upper four panels) also show faster maturation of their horizontal and vertical activity maps than of their oblique maps (two-factor ANOVA, P < 0.001; Fig. 4, upper four panels). The four animals with smaller anisotropies (Fig. 3, lower four panels) showed roughly parallel maturation of their activity maps for all orientations (two-factor ANOVA, P > 0.05, except P = 0.023 for 1-4-3630; Fig. 4, lower four panels).
Figure 4.
Cross-correlation analysis of map maturation. Correlation coefficients indicate the similarity between each developing map and the most mature map recorded for that orientation in that animal. Larger correlation coefficients indicate a more mature map (note that in all animals at the oldest age the mature map is correlated with itself, necessitating a coefficient of 1). Panels present data from the same animals illustrated in Fig. 3, presented in the same order. The animals illustrated in the upper four panels show faster development of horizontal and vertical preferences, whereas those in the lower four show parallel development of all orientations.
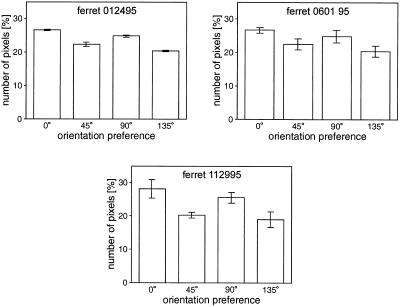
For comparison, we also measured the anisotropy in three adult ferrets. A significant overrepresentation of horizontal and vertical responses was seen in all three (Fig. 5). The magnitudes of the anisotropy seen in the adults ranged from 8.9 to 17.0%. These values are in the range seen in the developing ferrets.
Figure 5.
Orientation preference areas in adult ferrets. The three panels show the orientation preference areas calculated for three adult animals. Error bars indicate the standard deviation of values calculated from individual trials. In each of these adults the cortical area devoted to horizontal and vertical stimuli was significantly larger than that devoted to the obliques (one-tailed t test, P < 0.001 for each animal).
DISCUSSION
Using chronic optical imaging based on intrinsic signals to study the maturation of orientation activity maps in ferret primary visual cortex, we have demonstrated that there is a remarkable degree of interanimal variability in the representation of orientation during development. In half of the young ferrets studied, horizontal and vertical orientation domains developed faster, and there was a large overrepresentation of the cortical area preferring horizontal and vertical stimuli (anisotropies in the final map of 28.5–61.6%). In the other half of the ferrets, all orientations developed roughly in parallel, and there was a smaller overrepresentation of horizontal and vertical preferences (anisotropies in the final map of 3.5–22.0%).
One might be concerned that artifacts in the imaging system or the visual stimulus display might have caused the overrepresentation of horizontal and vertical preferences observed here. We think we can rule out this possibility because such artifactual anisotropies would be expected to be constant over time and thus could not account for the observed changes in the magnitude of the anisotropy during development within animals. More importantly, such artifacts could not explain why the anisotropy varied so dramatically between animals, especially because animals with large and small anisotropies were often recorded on the same day.
Also, measurement uncertainty or sampling problems cannot account either for the observed interanimal variability or the anisotropy itself. The uncertainty in our measurements of orientation preference area for each orientation is generally smaller than the observed anisotropies and interanimal differences (see variation across multiple measures in Fig. 3 and statistical tests in Results).
Previous electrophysiological studies have reported that the overrepresentation of horizontal and vertical preferences is stronger in simple cells (7, 9, 10) and in the central visual representation (7, 9–13). Optical imaging signals are primarily derived from responses in the upper layers of cortex (42), where there are few simple cells. So, if anything, our data might have produced an overall underestimation of the anisotropy in the area devoted to different orientations. The number of simple cells sampled by imaging, however, should be similar from animal to animal, so it seems unlikely that this could explain the observed interanimal variability. That the data for all our animals were collected across a wide area of cortex approximately centered on area centralis also makes it improbable that visual field effects could account for the observed interanimal variability.
Sampling problems have always been a major concern in electrophysiological studies of orientation anisotropy. Although a larger proportion of cells with horizontal and vertical preferences has been reported (7–15), many of these results may not be statistically significant (for review see ref. 15), and similar studies in other laboratories have failed to find a horizontal/vertical bias (26–31). The problem arises because it is indeed difficult to demonstrate the significance of a small deviation from equal representation. For example, if a population of neurons falls into two categories (horizontal/vertical vs. oblique) with an anisotropy magnitude equal to the mean of our adults (12%), then to demonstrate that this distribution is significantly different (P < 0.05) from random would require a sample size of ≈275 neurons. Furthermore, the nonrandom spatial distribution of orientation preferences across cortex exacerbates the problem by biasing the sampling within electrode tracks. All these problems are much less severe in optical imaging studies where orientation preferences across a large area of cortex are studied in every cortical map. Single-unit studies, of course, have their own advantages in that they provide critical data on receptive field properties, such as the orientation tuning of individual neurons, which cannot be obtained by using optical imaging techniques.
A number of psychophysical “oblique effects” have been described (32–36) with better performance on perceptual tasks for horizontal and vertical stimuli than for the obliques. This may be a direct consequence of the overrepresentation of horizontal and vertical orientation preferences. Like the electrophysiologically determined bias, the oblique effect is strongest in the central visual field (40, 41). More interestingly with respect to our results, the magnitude of the oblique effect in humans has also been found to vary between individuals. This interindividual difference in orientation anisotropy has been attributed to a wide variety of factors, including gender (43), visual experience (44, 45), and genetic differences (46, 47). Gender cannot explain the interanimal differences seen in our study, because both males and females showed both patterns of orientation development. Visual experience also seems unlikely to have caused the interanimal differences, because all of the ferrets in this study were raised in the same environment, and the bias was present as soon as orientation selectivity could be seen in cortex at or near the time of natural eye-opening. In our present approach, genetic components cannot be ruled out. Individuals in the same litter, however, did show both developmental patterns.
It has been clear since ocular dominance columns were first visualized by using 2-deoxyglucose (16–21) that cortical maps between individuals vary in their exact layout. More recently, significant interanimal differences in the periodicity of ocular dominance columns (25) and the related measure of blob density (48) have been seen. These studies, however, do not show any differences between animals in the proportion of cortex devoted to processing information from the two eyes.
Our results demonstrate that a fundamental feature of cortical organization, the proportion of cortex that prefers a particular orientation, can show profound interanimal differences. Closely related individuals, raised in identical visual environments, have fundamentally different cortical mapping of orientation during the developmental period when orientation-specific responses in cortex are maturing. Whether the differences between individuals in the degree of bias toward horizontal and vertical orientation preferences is intrinsically programmed or whether it is due to the visual environment remains to be determined in future investigations. However, the interanimal differences in the development of orientation maps have profound consequences for both electrophysiological and computational studies of orientation development. Electrophysiological studies can no longer assume that there are no significant differences between individual animals in the representation of orientation, and computational models must be able to account for the range of patterns of orientation development seen in this study.
Acknowledgments
We are grateful to Michael Stryker for the initiation of this project and for his constant support. Petra Stawinski kindly provided unpublished data for the adult controls. Frank Brinkmann gave expert technical assistance and Lee Stone provided helpful comments. This work was supported in part by a grant from the Human Frontier Science Program.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviation: ROI, region of interest.
References
- 1.Grinvald A, Lieke E, Frostig R D, Gilbert C D, Wiesel T N. Nature (London) 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- 2.Law M I, Zahs K R, Stryker M P. J Comp Neurol. 1988;278:157–180. doi: 10.1002/cne.902780202. [DOI] [PubMed] [Google Scholar]
- 3.Linden D C, Guillery R W, Cucciaro J. J Comp Neurol. 1981;203:189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- 4.Chapman B, Stryker M P. J Neurosci. 1993;13:5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim D-S, Bonhoeffer T. Soc Neurosci Abstr. 1993;737:5. [Google Scholar]
- 6.Gödecke I, Kim D S, Bonhoeffer T, Singer W. Eur J Neurosci. 1997;9:1754–1762. doi: 10.1111/j.1460-9568.1997.tb01533.x. [DOI] [PubMed] [Google Scholar]
- 7.Pettigrew J D, Nikara T, Bishop P O. Exp Brain Res. 1968;6:373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- 8.Albus K. Exp Brain Res. 1975;24:181–202. doi: 10.1007/BF00234062. [DOI] [PubMed] [Google Scholar]
- 9.Leventhal A G, Hirsch H V B. Proc Natl Acad Sci, USA. 1977;74:1272–1276. doi: 10.1073/pnas.74.3.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansfield R J W, Ronner S F. Brain Res. 1978;149:229–234. doi: 10.1016/0006-8993(78)90603-0. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy H, Orban G A. J Physiol (London) 1979;296:61P–62P. [PubMed] [Google Scholar]
- 12.De Valois R L, Yund E W, Helper N. Vision Res. 1982;22:531–544. doi: 10.1016/0042-6989(82)90112-2. [DOI] [PubMed] [Google Scholar]
- 13.Payne B R, Berman N. J Neurophysiol. 1983;49:1051–1072. doi: 10.1152/jn.1983.49.4.1051. [DOI] [PubMed] [Google Scholar]
- 14.Bauer R, Jordan W. Vision Res. 1993;33:1447–1450. doi: 10.1016/0042-6989(93)90138-m. [DOI] [PubMed] [Google Scholar]
- 15.Fregnac Y, Imbert M. J Physiol (London) 1978;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubel D H, Wiesel T N, Stryker M P. Nature (London) 1977;269:328–330. doi: 10.1038/269328a0. [DOI] [PubMed] [Google Scholar]
- 17.Albus K. Exp Brain Res. 1979;37:609–613. doi: 10.1007/BF00236828. [DOI] [PubMed] [Google Scholar]
- 18.Schoppmann A, Stryker M P. Nature (London) 1981;293:574–576. doi: 10.1038/293574a0. [DOI] [PubMed] [Google Scholar]
- 19.Singer W. Exp Brain Res. 1981;44:431–436. doi: 10.1007/BF00238836. [DOI] [PubMed] [Google Scholar]
- 20.Thompson I D, Kossut M, Blakemore C. Nature (London) 1983;301:712–715. doi: 10.1038/301712a0. [DOI] [PubMed] [Google Scholar]
- 21.Löwel S, Freeman B, Singer W. J Comp Neurol. 1987;255:401–415. doi: 10.1002/cne.902550307. [DOI] [PubMed] [Google Scholar]
- 22.Blasdel G G, Salama G. Nature (London) 1986;321:579–585. doi: 10.1038/321579a0. [DOI] [PubMed] [Google Scholar]
- 23.Bonhoeffer T, Grinvald A. Nature (London) 1991;353:429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- 24.Bonhoeffer T, Grinvald A. J Neurosci. 1993;13:4157–4180. doi: 10.1523/JNEUROSCI.13-10-04157.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hocking D R, Horton J C. J Neurosci. 1996;16:7228–7239. doi: 10.1523/JNEUROSCI.16-22-07228.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell F W, Cleland B G, Cooper G F, Enroth-Cugell C. J Physiol (London) 1968;198:237–250. doi: 10.1113/jphysiol.1968.sp008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noda H, Freeman R B, Gies B, Creutzfeldt O D. Exp Brain Res. 1971;12:389–405. doi: 10.1007/BF00234494. [DOI] [PubMed] [Google Scholar]
- 28.Rose D, Blakemore C. Exp Brain Res. 1974;20:1–17. doi: 10.1007/BF00239014. [DOI] [PubMed] [Google Scholar]
- 29.Blakemore C, Van Sluyters R C. J Physiol (London) 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buisseret P, Imbert M. J Physiol (London) 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buisseret P, Gary-Bobo E, Imbert M. Dev Brain Res. 1982;4:417–426. doi: 10.1016/0165-3806(82)90185-7. [DOI] [PubMed] [Google Scholar]
- 32.Higgins G C, Stultz K. J Opt Soc Am. 1950;40:135–137. [Google Scholar]
- 33.Appelle S. Psychol Bull. 1972;78:266–278. doi: 10.1037/h0033117. [DOI] [PubMed] [Google Scholar]
- 34.Bauer J A, Owens D A, Thomas J, Held R. Perception. 1979;8:247–253. doi: 10.1068/p080247. [DOI] [PubMed] [Google Scholar]
- 35.Boltz R L, Harwerth R S, Smith E L. Science. 1979;205:511–513. doi: 10.1126/science.109923. [DOI] [PubMed] [Google Scholar]
- 36.Vandenbussche E, Orban G A. Arch Int Physiol Biochem. 1980;88:P11–P12. [PubMed] [Google Scholar]
- 37.Chapman B, Stryker M P, Bonhoeffer T. J Neurosci. 1996;16:6443–6453. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratzlaff H E, Grinvald A. J Neurosci Methods. 1991;36:127–137. doi: 10.1016/0165-0270(91)90038-2. [DOI] [PubMed] [Google Scholar]
- 39.Bonhoeffer T, Kim D S, Malonek D, Shoham D, Grinvald A. Eur J Neurosci. 1995;7:1973–1988. doi: 10.1111/j.1460-9568.1995.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 40.Orban G A, Kennedy H. Brain Res. 1981;208:203–208. doi: 10.1016/0006-8993(81)90633-8. [DOI] [PubMed] [Google Scholar]
- 41.Campbell F W, Kulikowski J J, Levinson J. Physiology. 1966;187:427–436. doi: 10.1113/jphysiol.1966.sp008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonhoeffer T, Grinvald A. In: Brain Mapping: The Methods. Toga A W, Mazziotta J C, editors. San Diego: Academic; 1996. pp. 55–97. [Google Scholar]
- 43.Berkely M A, Kitterle F, Watkins D W. Vision Res. 1975;15:239–244. doi: 10.1016/0042-6989(75)90213-8. [DOI] [PubMed] [Google Scholar]
- 44.Brabyn L B, McGuiness D. Percept Psychophysics. 1979;26:319–324. [Google Scholar]
- 45.Annis R C, Frost B. Science. 1973;182:729–731. doi: 10.1126/science.182.4113.729. [DOI] [PubMed] [Google Scholar]
- 46.Timney B N, Muir D W. Science. 1976;193:699–701. doi: 10.1126/science.948748. [DOI] [PubMed] [Google Scholar]
- 47.Ross H E, Woodhouse J M. Perception. 1979;8:507–521. doi: 10.1068/p080507. [DOI] [PubMed] [Google Scholar]
- 48.Purves D, LaMantia A. J Comp Neurol. 1993;334:169–175. doi: 10.1002/cne.903340202. [DOI] [PubMed] [Google Scholar]