Abstract
The tachykinin neuropeptides, substance P and substance K, are produced in nociceptive primary sensory neurons and in many brain regions involved in pain signaling. However, the precise role and importance of these neuropeptides in pain responses has been debated. We now show that mice that cannot produce these peptides display no significant pain responses following formalin injection and have an increased pain threshold in the hotplate test. On the other hand, the mutant mice react normally in the tail flick assay and acetic acid-induced writhing tests. These results demonstrate that substance P and/or substance K have essential functions in specific responses to pain.
The tachykinins are a family of structurally related neuropeptides. In the mouse, they are encoded by the genes Tac1 and Tac2. Tac1 produces substance P, substance P (neurokinin A), neurokinin A (3–10), neuropeptide K, and neuropeptide γ as a result of differential splicing and posttranslational processing (1–4). Tac2 produces the peptide neurokinin B.
The undecapeptide substance P was first detected by von Euler and Gaddum (5) in 1931. Its structure was revealed by Leeman and her coworkers (6, 7) in 1971. The Tac1 cDNA was cloned in 1983 by Nakanishi and his coworkers (2, 8). The Tac1 gene is expressed in many regions in the central and peripheral nervous system, as well as in nonneuronal tissues. Substance P has been implicated in a variety of physiological processes including cardiovascular, respiratory, and gastrointestinal functions; inflammatory responses; and nociception. In addition, Hunt and coworkers have suggested that substance P may be involved in axon guidance during embryonic development (9).
The precise role of substance P in these processes is unclear. For example, substance P is synthesized in nociceptive primary sensory neurons, which send C- and Aδ fibers to dorsal horn projection neurons in lamina I and IV-V, and to nociception-specific interneurons in lamina II-III of the spinal cord. Axons of projection neurons terminate in many supraspinal nuclei that are involved in pain transmission (10, 11). Nociceptive stimulation triggers the release of substance P from C-afferent terminals in the marginal layers of the spinal cord (12), evokes slow excitatory postsynaptic potentials in second-order sensory neurons in the dorsal horn, and facilitates their activation (13). These data, together with other functional evidence (11, 14), indicated an important role for substance P in the processing of nociceptive signals.
We have begun to use a genetic approach to study the functions of tachykinin peptides. As a first step, we have generated mice with a targeted mutation in the Tac1 gene. These mice are viable and fertile, but exhibit striking defects in nociceptive behaviors.
MATERIALS AND METHODS
Generation and Breeding of Tac1−/− Mice.
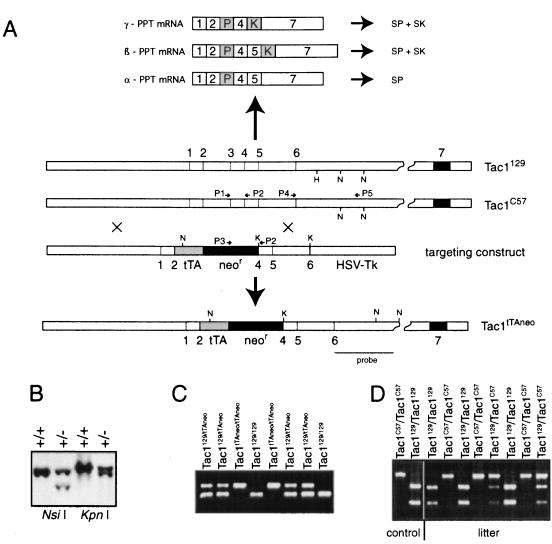
Tac1 mutations were established by homologous recombination in MPI2 embryonic stem (ES) cells according to standard protocols (15). One mutant ES cell line was used to derive chimeras by morula aggregation and blastocyst injection. These chimeras were crossed with C57BL/6J mice. ES cell-derived F1 offsprings were identified by their agouti coat color and genotyped by PCR (see Fig. 1). Chimeric animals were crossed to C57BL/6J mice to obtain F1 offspring with a mixed 129 × C57BL/6J genetic background that were either wild type or heterozygous for the Tac1 mutation. Three Tac1 alleles can be distinguished in these F1 animals: the 129-derived mutant TactTAneo allele, the 129-derived Tac1129 allele, and the C57BL/6J-derived Tac1C57 allele (Fig. 1A). Tac1129 and Tac1C57 could be discriminated by using a HpaII polymorphism in intron 6 (Fig. 1D). Heterozygous (TactTAneo/Tac1129) and wild-type (Tac1C57/Tac1129) F1 mice were subsequently interbred to establish homozygous Tac1-deficient animals (TactTAneo/TactTAneo) and wild-type (Tac1129/Tac1129) control animals of a similar haplotype. These genotypes will henceforth be referred to as Tac1−/− and Tac1+/+, respectively.
Figure 1.
Targeted mutagenesis of the Tac1 gene. (A), Map of the wild-type genomic locus and cDNA from the mouse strains 129SV/J (Tac1129) and the C57BL/6J (Tac1C57), the targeting construct, and the recombinant locus (Tac1tTAneo). The DNA probe used for Southern blot analysis and the primers used for PCR analysis are indicated. H, HpaII; N, Nsi I; K, KpnI. (B) Southern blot analysis of a recombinant ES cell after digestion with Nsi I or KpnI. (C) Genotyping of offspring from Tac1tTAneo/C57 × Tac1tTAneo/C57 matings by PCR analysis using primer P1, P2, and P3. (D), Genotyping of offspring from Tac1129/C57 × Tac1129/C57 matings by PCR amplification with primers P4 and P5, and subsequent digestion with HpaII. Note that HpaII cuts only the PCR fragment derived from the 129 allele, but not the C57BL/6 allele. Homozygous mutant Tac1tTAneo/tTAneo and wild-type Tac1129/129 are referred to as Tac1−/− and Tac1+/+ mice, respectively, throughout the text.
RIAs.
Radioimunoassays were performed on brain-extracts using a substance P RIA kit (RIK-7451, Peninsula Laboratories) according to the recommendation of the manufacturer. Substance K and neurokinin B levels were determined after HPLC fractionation by RIA using an antibody that binds both peptides (RIK-7359, Peninsula Laboratories).
Immunohistochemistry.
Immunohistochemistry was performed as described (16) by using primary antibodies from different species [calcitonin gene-related peptide (CGRP) Peninsula Laboratories, catalog no. IHC 6009, 1:1000, raised in rabbit, and substance P, Chemicon, catalog no. MAB356, 1:1000, rat monoclonal). All fluorescent secondary antibodies were affinity purified Fab2 fragments of the IgG that have been previously cross-absorbed with IgG from the other species (rabbit and rat, respectively) and produced for the specific purpose of multiple labelings (Jackson ImmunoResearch).
In Situ Hybridization.
Twelve μm thick sections were processed for in situ hybridization histochemistry as described (17) from two animals of each genotype. A full-length cDNA clone (a gift of J. Krause) was used to produce a riboprobe template containing T3 and T7 polymerase sites between nucleic acids 386–776 (GenBank accession no. M64236). Riboprobes were generated by using 35S-UTP. For more details on the procedures see: http://www.nimh.nih.gov/lcmr/snge/Protocol.html. Grain density was measured from a total of 59 Tac1−/− cells and 38 Tac1+/+ cells in several sections by using the National Institutes of Health image program.
Behavioral Experiments.
Animals were 8–20 weeks old when tested and were housed in a temperature- and humidity-controlled vivarium that was kept on a 12-h dark-light cycle (light on 7:00 EST). Animals had free access to food and water. All behavioral experiments were performed between 9:00 EST and 12:00 EST.
The tail flick assay was performed by using an automated tail flick apparatus (Columbus Instruments, Columbus, OH) by using standard procedures. The cut-off time was 12 s. Stress-induced analgesia was assessed after baseline tail flick testing. To produce stress-induced analgesia, individual mice swam for 90 s in a 4-l glass beaker filled with water at a temperature of 4°C (±0.5°C). After each swim, mice were patted dry and allowed to rest for 10 min before the tail flick test was repeated.
The hotplate test was performed by using an electronically controlled hotplate analgesia meter (Columbus Instruments, Columbus, OH) heated to 52°C (±0.1°C). The cut-off time was 120 s. The latency until mice showed first signs of discomfort (paw-lifting, -licking or -shaking; jumping) was recorded.
For the abdominal constriction assay, mice were separated into individual cages and injected i.p. with 10 ml/kg 0.6% (wt/vol) acetic acid. A few minutes after the injection, animals showed typical abdominal constrictions—lengthwise stretches of the torso with a concomitant concave arching of the back. These were counted from 2–12 min after the injection.
In the formalin assay, 20 μl of a 5% formalin or 0.9% saline solution were injected subcutaneously under the dorsal surface of the right hindpaw. The animals’ responses were recorded between 1–10 min and 21–30 min after the injection as described (15). All animal experiments were approved by the National Institute of Mental Health Animal Care and Use Committee.
RESULTS
Mice with a Targeted Disruption of the Tachykinin 1 Gene.
We disrupted the Tac1 gene in ES cells by replacing parts of exons two and three with a tTA-neo cassette (Fig. 1A), and obtained germline chimeric animals from one targeted ES cell clone (Figs. 1 B and D). As genetic background may affect behavioral responses (18, 19), we used a breeding scheme to produce knockout (Tac1−/−) and wild-type (Tac1+/+) control mice with similar genetic constitution (see Materials and Methods).
Homozygous Tac1−/− mutant mice were obtained with the expected Mendelian frequency; they had no gross physical abnormalities, were similar in size and weight to Tac1+/+ mice, and appeared healthy over a period of at least 6 months. Hence, contrary to what might have been expected, substance P is not essential for survival under laboratory conditions. All animals were fertile and cared for their offspring.
Expression of Tachykinin Neuropeptides and the Substance P Receptor NK1.
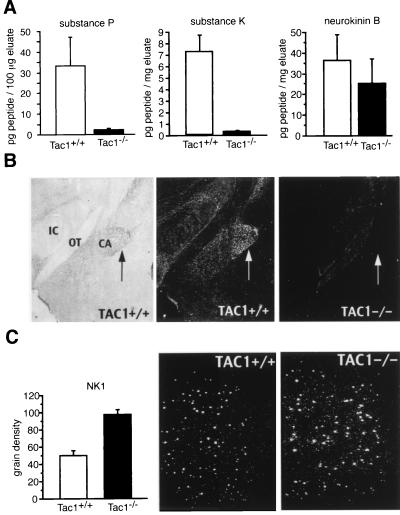
Tachykinin peptide levels were assayed in brain extracts of wild-type Tac1+/+ and homozygous Tac1−/− animals by RIA (Fig. 2A); neither substance P nor substance K immunoreactivity were detected in homozygous Tac1−/− animals. In contrast, levels of neurokinin B, which is encoded by a different gene, were similar in wild-type and mutant mice. Substance P levels in heterozygous animals were reduced by ≈50% (data not shown). Immunohistochemical staining of brain sections revealed an almost complete disappearance of substance P immunoreactivity (Fig. 2B). We did notice some residual immunostaining of nerve fibers in a few distinct brain areas, which may be due to antibody crossreactivity. Together the RIA and immunhistochemical data indicate that we have created a Tac1 null allele. The distribution and levels of the substance P receptor NK1 mRNA (20, 21) were analyzed by in situ hybridization (17). The number and distribution of NK1 receptor mRNA positive cells in the striatum were similar in wild-type and mutant mice, but the expression levels per cell were higher in mutant animals (P < 0.0005, ANOVA) (Fig. 2C), suggesting a compensatory increase in NK1 receptor mRNA, due to the lack of its natural ligand.
Figure 2.
Expression of tachykinin neuropeptides and the substance P receptor NK1 mRNA. (A) Substance P peptide levels (mean ± SEM.) were determined by RIAs in whole brain extracts after HPLC fractionation (Tac1+/+, n = 4; Tac1−/−, n = 4). (B) Immunohistochemistry reveals disappearance of SP immunoreactivity in the brain of Tac1−/− mice. The figure shows immunostaining of nerve fibers in the central amygdala (arrow) of the Tac1+/+ brain in brightfield and darkfield. The darkfield image of the Tac1−/− brain shows the complete lack of immunoreactivity in the identical region. CA, central amygdala; IC, internal capsule; OT, optic tract. (C) NK1 receptor mRNA levels were analyzed by in situ hybridization of coronal sections through the striatum and subsequent quantitation by using National Institutes of Health image (cell number: Tac1+/+, n = 37; Tac1−/−, n = 58; mRNA levels are shown on an arbitrary scale). The microscopic darkfield image also demonstrates that the area over cells covered by silver grains is larger in the striatum of Tac1−/− animals.
Nociceptive Behaviors.
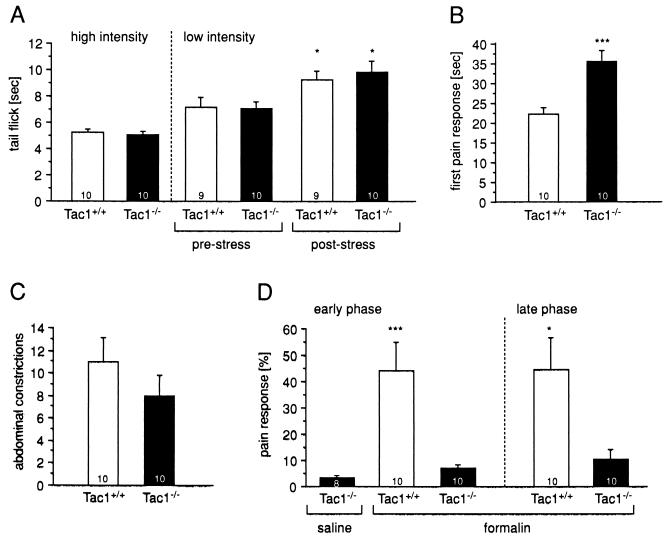
To investigate behavioral responses to pain stimuli in Tac1−/− mutant mice, homozygous Tac1−/−, and control Tac1+/+ animals were tested in several assays of nociception (22). First, responses to noxious thermal stimuli were studied. The tail flick assay was employed to measure spinal pain reflexes and the hotplate assay was used to measure pain responses that involve higher cerebral functions (supraspinal responses). Tail flick latencies were indistinguishable between Tac1−/− and Tac1+/+ animals at both light intensities used (Fig. 3A). Furthermore, animals of both genotypes exhibited similar delay in tail flick latency after analgesia was induced by swim stress. In contrast, mutant animals displayed a highly significant (P ≤ 0.0005, Mann–Whitney U test) delay in pain responses when tested with the hotplate assay (Fig. 3B).
Figure 3.
Responses to noxious heat was determined in the (A) tail flick and (B) hotplate tests. tail flick latency (mean ± SEM) were similar between the two genotypes at high- and low-light beam-intensities. After swim stress, analgesia was observed as a significant delay in tail flick latency, which also did not differ between the genotypes. In contrast, pain-response latency in the hotplate test was highly significantly increased in mice with the Tac1−/− genotype. Responses to noxious chemical stimuli were determined in the (C) acetic acid-induced abdominal constriction assay and in the (D) formalin test. No significant difference between the genotypes was found in the number of abdominal constriction, but Tac1−/− mice did not show any significant pain responses in the early or late phase of the formalin test. Data were analyzed with the Mann–Whitney U test, except the stress-induced analgesia was analyzed with the Wilcoxon signed rank test. ∗, P < 0.05; ∗∗∗, P < 0.0005. The number at the bottom of each bar indicates the number of animals analyzed.
We next studied the behavioral responses of mutant and wild-type animals to noxious chemicals. The abdominal constriction assay evaluates visceral pain responses (writhing) after i.p. injection of a dilute acetic acid solution. Writhing is known to be abolished by spinal or cerebellar transections, but not by midbrain transection and is thus thought to be a lower brainstem response (22). There was no significant difference in the number of episodes of writhing between Tac1+/+ and Tac1−/− during a 10-min observation period (Fig. 3C; P = 0.26, Mann–Whitney U test).
Subcutaneous formalin injection into the dorsal surface of the hind paw elicits a biphasic behavioral response (23, 24). The first phase starts immediately after the injection and lasts for ≈5 min (acute phase); after a short quiescent period the second phase (tonic phase) starts and lasts for ≈40 min. Both responses include behaviors such as licking, shaking, and elevating the affected paw and they are mediated by supraspinal structures. Surprisingly, mutant Tac1−/− animals showed virtually no pain responses in either phase of this test and behaved like a control group of Tac1−/− mice injected with saline (Fig. 3D).
Sensory Neurons.
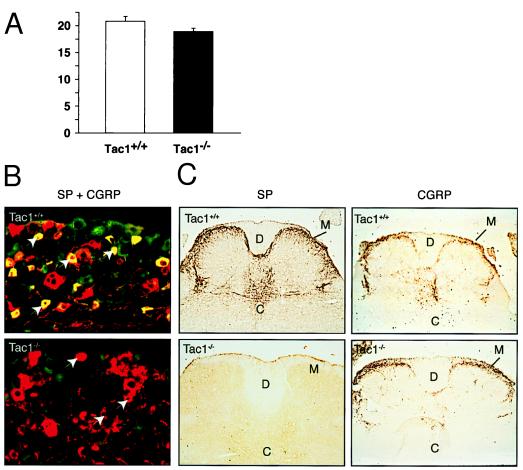
The increased pain threshold of Tac1−/− mice in the hotplate and formalin assays cannot be attributed to the loss of primary sensory neurons in Tac1−/− mice, since dorsal root ganglia (DRGs) from Tac1+/+ and Tac1−/− were very similar in size and contained a similar number of neurons (Fig. 4A). Moreover, we found no difference in the number and distribution of small-diameter CGRP-immunoreactive cells in DRGs from Tac1+/+ and Tac1−/− mice (Fig. 4B). CGRP-containing nerve terminals were also similarly distributed throughout the dorsal horn in mice of both genotypes. As almost all of the small-diameter primary sensory neurons coexpress substance P and the CGRP (25, 26), these data indicate that there was no significant loss of primary sensory neurons that normally produce substance P.
Figure 4.
Substance P producing cells in DRGs and spinal cord. (A) Neurons were marked using a specific neuronal nuclear marker (NeuN) [chemicon (1:2000)], and the number of positive nuclei in several sections of 4–6 DRGs per mouse were counted using one square inch grid. The number of neurons in the DRGs from Tac1+/+ (n = 3) and Tac1−/− (n = 3) mice. (B) Double staining of DRGs from Tac1+/+ and Tac1−/− mice with substance P and CGRP specific immunosera. Substance P-positive cells typically have small diameters and are labeled green (fluorescein isothiocyanate), while CGRP positive cells are labeled red (CY3). Double-positive cells therefore appear yellow. Arrows point to small-diameter CGRP-positive and substance P-negative cells in the DRG of Tac1−/− mice. (C) Analysis of substance P and CGRP immunostaining in the spinal cord. The presence of both peptides, mainly in lamina I and II, overlaps in wild-type Tac1+/+ mice. Note the absence of substance P immunoreactivity in Tac1−/− animals, while the CGRP-staining is normal. C, central canal; D, dorsal columns; M, marginal zone of the dorsal horn.
DISCUSSION
Here we show that mice with a targeted mutation in the Tac1 gene fail to produce the tachykinin neuropeptides substance P and substance K. Tac1 mutant animals develop normally, but they are hypoalgesic in two assays for nociception, the hotplate and the formalin tests. In other nociceptive tests, tail flick assay and acetic acid induced writhing, there was no difference between wild-type and knockout mice. Our results clearly demonstrate the essential role of these neuropeptides in some, but not all, nociceptive responses.
Paw-licking and paw-lifting were most affected by the Tac1 mutation. These behaviors involve supraspinal mechanisms, while tail flick and writhing, which were normal in Tac1−/− mice are thought to involve spinal or brain stem reflexes (22). On the other hand, the nociceptive defect in Tac1 knockout mice did not relate to the nature of the nociceptive stimulus (thermal vs. chemical), its duration (acute vs. tonic), or its escapability (tail flick/hotplate vs. acetic acid/formalin).
Could the deficiency in nociception result primarily from functional alterations in substance P-containing supraspinal sites involved in the processing of painful stimuli, such as thalamic nuclei, the periaequaductal gray, or the locus coeruleus? At present, we cannot rule out this possibility, but it is hard to ignore the body of data suggesting that substance P plays a significant role in the modulation of nociceptive signals at the level of the spinal cord. Substance P is expressed in ≈6- 20% of all DRG cells, depending on the segmental level (27). Functional characterization of DRG neurons by electrophysiological means, followed by immunohistochemical analysis revealed that 50% of C-fiber neurons and 20% of Aδ-fiber neurons, but no Aα/Aβ-fiber neurons produce substance P (28). A major subset of substance P positive DGR neurons also produce neurokinin A (29). Substance P containing afferents from primary sensory neurons project to second order neurons in the superficial layers of the spinal cord that normally receive nociceptive inputs (30, 31). Release of substance P produces a strong and long lasting excitation of these spinal neurons and facilitates their activation by noxious stimulation (32–35). Intrathecal injection of substance P causes scratching responses and caudally directed biting (36, 37).
The virtual absence of pain-related behaviors in both phases of the formalin test in Tac1−/− mice was striking and unexpected. The early (acute) phase is thought to be caused by a direct stimulation of primary afferents fibers by formalin, while the second (tonic) phase is thought to involve inflammatory events and ongoing activation of nociceptors. Formalin-induced release of substance P into the dorsal horn parallels behavioral nociceptive responses and electrical activity of dorsal horn neurons (38–41), suggesting that substance P may be important in the transmission of the nociceptive signal. On the other hand, tachykinin receptor antagonists have not consistently shown a clear involvement of substance P in formalin-induced pain; some investigators found antagonist-effects primarily during the early phase (42, 43), while others found effects during the late phase (44–47), or both phases (48, 49). The interpretation of the pharmacological data is further complicated by the fact that some antagonists exhibit NK1 receptor independent effects (50) and may also affect motor behaviors that can interfere with the behavioral test (51).
In addition to substance P, formalin injection also triggers the release of excitatory amino acids (52) and other neuropeptides including CGRP, neurotensin, and somatostatin (53). It has been shown that substance P interacts synergistically with these transmitters in the excitation of second order neurons (54–56). Although primary (glutamatergic) transmission may persist, it is conceivable that the lack of substance P and substance K in Tac1 knockout animals results in alterations of ascending pain signals at the level of the spinal cord in such a manner that fewer supraspinal pain responses are elicited.
Interestingly, we have previously shown that mice that cannot produce the opioid peptide enkephalin, also exhibit defects in supraspinal assays while spinal responses were unaffected (15). Enkephalin, which is synthesized by inhibitory interneurons in lamina III (and to some extent in II) of the dorsal horn, inhibits the release of substance P (57, 58) and glutamate (59) from primary afferents. Mice that make no enkephalin exhibited shorter response latencies in the hotplate assay and highly abnormal responses in the formalin test. Thus, mutations in the Tac1 and enkephalin-genes, had opposite effects on pain responses. It is tempting to speculate that the enkephalin and Tac1-derived neuropeptides modulate nociceptive inputs antagonistically and influence whether a nociceptive stimulus is experienced as pain.
Acknowledgments
We thank Mike Brownstein for support, Jennifer Hall for excellent animal care, Jim Krause for the tac1 cDNA, and all our colleagues for valuable discussions and comments on the manuscript.
ABBREVIATIONS
- ES
embryonic stem
- CGRP
calcitonin gene-related peptide
- DRG
dorsal root ganglia
References
- 1.Nawa H, Doteuchi M, Igano K, Inouye K, Nakanishi S. Life Sci. 1984;34:1153–60. doi: 10.1016/0024-3205(84)90087-0. [DOI] [PubMed] [Google Scholar]
- 2.Nawa H, Kotani H, Nakanishi S. Nature (London) 1984;312:729–734. doi: 10.1038/312729a0. [DOI] [PubMed] [Google Scholar]
- 3.Krause J E, Chirgwin J M, Carter M S, Xu Z S, Hershey A D. Proc Natl Acad Sci USA. 1987;4:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundelin J B, Provvedini D M, Wahlestedt C R, Laurell H, Pohl J S, Peterson P A. Eur J Biochem. 1992;203:625–631. doi: 10.1111/j.1432-1033.1992.tb16592.x. [DOI] [PubMed] [Google Scholar]
- 5.von Euler U S, Gaddum J H. J Physiol (London) 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M M, Leeman S E. J Biol Chem. 1970;245:4784–4790. [PubMed] [Google Scholar]
- 7.Chang M M, Leeman S E, Niall H D. Nat New Biol. 1971;232:86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- 8.Nawa H, Hirose T, Takashima H, Inayama S, Nakanishi S. Nature (London) 1983;306:32–36. doi: 10.1038/306032a0. [DOI] [PubMed] [Google Scholar]
- 9.De Felipe C, Pinnock R D, Hunt S P. Science. 1995;267:899–902. doi: 10.1126/science.7531367. [DOI] [PubMed] [Google Scholar]
- 10.Harlan R E, Garcia M M, Krause J E. J Comp Neurol. 1989;287:179–212. doi: 10.1002/cne.902870204. [DOI] [PubMed] [Google Scholar]
- 11.Otsuka M, Yoshioka K. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 12.Duggan A W, Morton C R, Zhao Z Q, Hendry I A. Brain Res. 1987;403:345–349. doi: 10.1016/0006-8993(87)90073-4. [DOI] [PubMed] [Google Scholar]
- 13.De Koninck Y, Henry J L. Proc Natl Acad Sci USA. 1991;88:11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otsuka M, Yanagisawa M. Cell Mol Neurobiol. 1990;10:293–302. doi: 10.1007/BF00711176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.König M, Zimmer A M, Steiner H, Holmes P V, Crawley J N, Brownstein M J, Zimmer A. Nature (London) 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 16.Hunyady B, Krempels K, Harta G, Mezey É. J Histochem Cytochem. 1996;44:1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 17.Bradley D J, Towle H C, Young W S. J Neurosci. 1992;12:2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmer A. Trends Neurosci. 1996;19:470. doi: 10.1016/S0166-2236(96)20053-0. [DOI] [PubMed] [Google Scholar]
- 19.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 20.Yokota Y, Sasai Y, Tanaka K, Fujiwara T, Tsuchida K, Shigemoto R, Kakizuka A, Ohkubo H, Nakanishi S. J Biol Chem. 1989;264:17649–17652. [PubMed] [Google Scholar]
- 21.Hershey A D, Krause J E. Science. 1990;247:958–962. doi: 10.1126/science.2154852. [DOI] [PubMed] [Google Scholar]
- 22.Franklin K B J, Abbott F V. In: Techniques for Assessing the Effects of Drugs on Nociceptive Responses. Boulton A A, Baker G B, Greenshaw A J, editors. Vol. 13. Clifton, NJ: Humana; 1989. pp. 145–216. [Google Scholar]
- 23.Tjolsen A, Berge O G, Hunskaar S, Rosland J H, Hole K. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 24.Abbadie C, Taylor B K, Peterson M A, Basbaum A I. Pain. 1997;69:101–110. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- 25.Skofitsch G, Jacobowitz D M. Peptides (Tarrytown, NY) 1985;6:747–754. doi: 10.1016/0196-9781(85)90179-2. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y, Takami K, Kawai Y, Girgis S, Hillyard C J, Macintyre I, Emson P C, Tohyama M. Neuroscience. 1985;15:1227–1237. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- 27.Tuchscherer M M, Seybold V S. Neuroscience. 1985;14:593–605. doi: 10.1016/0306-4522(85)90313-6. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy P W, Lawson S N. Neuroscience. 1989;28:745–753. doi: 10.1016/0306-4522(89)90019-5. [DOI] [PubMed] [Google Scholar]
- 29.Dalsgaard C J, Haegerstrand A, Theodorsson-Norheim E, Brodin E, Hökfelt T. Histochemistry. 1985;83:37–39. doi: 10.1007/BF00495297. [DOI] [PubMed] [Google Scholar]
- 30.Henry J L. Brain Res. 1976;114:439–451. doi: 10.1016/0006-8993(76)90965-3. [DOI] [PubMed] [Google Scholar]
- 31.Hökfelt T, Kellerth J O, Nilsson G, Pernow B. Brain Res. 1975;100:235–252. doi: 10.1016/0006-8993(75)90481-3. [DOI] [PubMed] [Google Scholar]
- 32.Murase K, Ryu P D, Randic M. J Neurophysiol. 1989;61:854–865. doi: 10.1152/jn.1989.61.4.854. [DOI] [PubMed] [Google Scholar]
- 33.Randic M, Jeftinija S, Urban L, Raspantini C, Folkers K. Peptides (Tarrytown, NY) 1988;9:651–660. doi: 10.1016/0196-9781(88)90178-7. [DOI] [PubMed] [Google Scholar]
- 34.Radhakrishnan V, Henry J L. Neuroscience. 1995;64:943–958. doi: 10.1016/0306-4522(94)00440-g. [DOI] [PubMed] [Google Scholar]
- 35.Randic M, Ryu P D, Urban L. Brain Res. 1986;383:15–27. doi: 10.1016/0006-8993(86)90003-x. [DOI] [PubMed] [Google Scholar]
- 36.Hylden J L K, Wilcox G L. Brain Res. 1981;217:212–215. doi: 10.1016/0006-8993(81)90203-1. [DOI] [PubMed] [Google Scholar]
- 37.Piercey M F, Dobry P J K, Schoeder L A, Einspahr F J. Brain Res. 1981;210:407–412. doi: 10.1016/0006-8993(81)90918-5. [DOI] [PubMed] [Google Scholar]
- 38.Kuraishi Y, Hirota N, Sato Y, Hino Y, Satoh M, Takagi H. Brain Res. 1985;325:294–298. doi: 10.1016/0006-8993(85)90326-9. [DOI] [PubMed] [Google Scholar]
- 39.Kantner R M, Kirby M L, Goldstein B D. Brain Res. 1985;338:196–199. doi: 10.1016/0006-8993(85)90268-9. [DOI] [PubMed] [Google Scholar]
- 40.Holland L N, Goldstein B D. Brain Res. 1990;537:287–292. doi: 10.1016/0006-8993(90)90370-q. [DOI] [PubMed] [Google Scholar]
- 41.McCarson K E, Goldstein B D. Brain Res. 1991;568:109–115. doi: 10.1016/0006-8993(91)91385-e. [DOI] [PubMed] [Google Scholar]
- 42.Ohkubo T, Shibata M, Takahashi H, Inoki R. J Pharmacol Exp Ther. 1990;252:1261–1268. [PubMed] [Google Scholar]
- 43.Ono M, Satoh T. Jpn J Pharmacol. 1991;55:523–530. doi: 10.1254/jjp.55.523. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto T, Yaksh T L. Life Sci. 1991;49:1955–1963. doi: 10.1016/0024-3205(91)90637-q. [DOI] [PubMed] [Google Scholar]
- 45.Sakurada T, Katsumata K, Yogo H, Tan-No K, Sakurada S, Ohba M, Kisara K. Pain. 1995;60:175–180. doi: 10.1016/0304-3959(94)00107-P. [DOI] [PubMed] [Google Scholar]
- 46.Iyengar S, Hipskind P A, Gehlert D R, Schober D, Lobb K L, Nixon J A, Helton D R, Kallman M J, Boucher S, Couture R, et al. J Pharmacol Exp Ther. 1997;280:774–785. [PubMed] [Google Scholar]
- 47.Rupniak N M, Carlson E, Boyce S, Webb J K, Hill R G. Pain. 1996;67:189–195. doi: 10.1016/0304-3959(96)03109-0. [DOI] [PubMed] [Google Scholar]
- 48.Chapman V, Dickenson A H. Neurosci Lett. 1993;157:149–152. doi: 10.1016/0304-3940(93)90724-y. [DOI] [PubMed] [Google Scholar]
- 49.Garret C, Carruette A, Fardin V, Moussaoui S, Peyronel J F, Blanchard J C, Laduron P M. Proc Natl Acad Sci USA. 1991;88:10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt A W, McLean S, Heym J. Eur J Pharmacol. 1992;219:491–492. doi: 10.1016/0014-2999(92)90498-s. [DOI] [PubMed] [Google Scholar]
- 51.Sakurada T, Manome Y, Katsumata K, Tan-No K, Sakurada S, Ohba M, Kisara K. Eur J Pharmacol. 1994;261:85–90. doi: 10.1016/0014-2999(94)90304-2. [DOI] [PubMed] [Google Scholar]
- 52.Skilling S R, Smullin D H, Beitz A J, Larson A A. J Neurochem. 1988;51:127–132. doi: 10.1111/j.1471-4159.1988.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang R X, Mi Z P, Qiao J T. Regul Pept. 1994;51:25–32. doi: 10.1016/0167-0115(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 54.Dougherty P M, Willis W D. Pain. 1991;47:85–93. doi: 10.1016/0304-3959(91)90015-P. [DOI] [PubMed] [Google Scholar]
- 55.Rusin K I, Ryu P D, Randic M. J Neurophysiol. 1992;68:265–286. doi: 10.1152/jn.1992.68.1.265. [DOI] [PubMed] [Google Scholar]
- 56.Rusin K I, Bleakman D, Chard P S, Randic M, Miller R J. J Neurochem. 1993;60:952–960. doi: 10.1111/j.1471-4159.1993.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 57.Jessell T M, Iversen L L. Nature (London) 1977;268:549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- 58.Hirota N, Kuraishi Y, Hino Y, Sato Y, Satoh M, Takagi H. Neuropharmacology. 1985;24:567–570. doi: 10.1016/0028-3908(85)90065-6. [DOI] [PubMed] [Google Scholar]
- 59.Ueda M, Sugimoto K, Oyama T, Kuraishi Y, Satoh M. Neuropharmacology. 1993;34:303–308. doi: 10.1016/0028-3908(94)00160-t. [DOI] [PubMed] [Google Scholar]