Abstract
Learning to read requires an awareness that spoken words can be decomposed into the phonologic constituents that the alphabetic characters represent. Such phonologic awareness is characteristically lacking in dyslexic readers who, therefore, have difficulty mapping the alphabetic characters onto the spoken word. To find the location and extent of the functional disruption in neural systems that underlies this impairment, we used functional magnetic resonance imaging to compare brain activation patterns in dyslexic and nonimpaired subjects as they performed tasks that made progressively greater demands on phonologic analysis. Brain activation patterns differed significantly between the groups with dyslexic readers showing relative underactivation in posterior regions (Wernicke’s area, the angular gyrus, and striate cortex) and relative overactivation in an anterior region (inferior frontal gyrus). These results support a conclusion that the impairment in dyslexia is phonologic in nature and that these brain activation patterns may provide a neural signature for this impairment.
Speech enables its users to create an indefinitely large number of words by combining and permuting a small number of phonologic segments, the consonants and vowels that serve as the natural constituents of the biologic specialization for language. An alphabetic transcription brings this same ability to readers but only as they connect its arbitrary characters (letters) to the phonologic segments they represent. Making that connection requires an awareness that all words, in fact, can be decomposed into phonologic segments. Thus, it is this awareness that allows the reader to connect the letter strings (the orthography) to the corresponding units of speech (phonologic constituents) that they represent. As numerous studies have shown, however, such awareness is largely missing in dyslexic children and adults (1–4). Not surprisingly, then, perhaps the most sensitive measure of the reading problem in dyslexia is inability to read phonologically legal nonsense words (5–7). As for why dyslexic readers should have exceptional difficulty developing phonologic awareness, there is support for the notion that the difficulty resides in the phonologic component of the larger specialization for language (8–10). If that component is imperfect, its representations will be less than ideally distinct and, therefore, harder to bring to conscious awareness.
Previous efforts using functional imaging methods to examine brain organization in dyslexia have been inconclusive (11–17) largely, we think, because the experimental tasks tapped the several aspects of the reading process in somewhat unsystematic ways. Our aim therefore was to develop a set of hierarchically structured tasks that control the kind of language-relevant coding required, including especially the demand on phonologic analysis, and then to compare the performance and brain activation patterns (as measured by functional MRI) of dyslexic (DYS) and nonimpaired (NI) readers. Thus, proceeding from the base of the hierarchy to the top, the tasks made demands on visual–spatial processing, orthographic processing, simple phonologic analysis, complex phonologic analysis, and lexical–semantic judgment. We hypothesized that differences in brain activation patterns would emerge as DYS and NI readers were asked to perform tasks that make progressively greater demands on phonologic analysis.
METHODS
Tasks.
Both the decision and response components of the tasks were comparable; in each instance the subject viewed two simultaneously presented stimulus displays, one above the other, and was asked to make a same/different judgment by pressing a response button if the displays matched on a given cognitive dimension, such as line orientation judgment, letter case judgment, single-letter rhyme, nonword rhyme, and category judgment. As noted above, the five tasks were ordered hierarchically. (i) At the lowest level, the line orientation (L) judgment task (e.g., Do [\\\/] and [\\\/] match?) taps visual–spatial processing but makes no orthographic demands. (ii) Next, the letter-case (C) judgment task (e.g., Do [bbBb] and [bbBb] match in the pattern of upper- and lowercase letters?) adds an orthographic processing demand but makes no phonologic demands, because the stimulus items that consist entirely of consonant strings are, therefore, phonotactically impermissible. (iii) The third task, single letter rhyme (SLR; e.g., Do the letters [T] and [V] rhyme?), although orthographically more simple than C, adds a phonologic processing demand, requiring the transcoding of the letters (orthography) into phonologic structures and then requiring a phonologic analysis of those structures sufficient to determine that they do or do not rhyme. (iv) The fourth task, nonword rhyme (NWR; e.g., Do [leat] and [jete] rhyme?), requires analysis of more complex structures. (v) The final task, semantic category (SC) judgment (e.g., Are [corn] and [rice] in the same category?), also makes substantial demands on transcoding from print to phonology (18, 19) but requires in addition that the printed stimulus items activate particular word representations in the reader’s lexicon to arrive at the word’s meaning. Stimulus pairs in all five tasks were presented at a rate of 1 every 5.5 sec, a rate that pilot studies suggested was comfortable for DYS readers. A common baseline subtraction condition was used in analysis: C, SLR, NWR, and SC tasks contrasted with the nonlanguage line orientation judgment (L) baseline condition.
Subjects.
We studied 61 right-handed subjects, 29 DYS readers (14 men and 15 women, ages 16–54 years) and 32 NI readers (16 men and 16 women, ages 18–63 years) after informed consent had been obtained. Both groups were in the average range for IQ; DYS readers had a full-scale IQ (mean ± SEM) of 91 ± 2.3 and NI readers had an IQ of 115 ± 2.2. Other than requiring that all subjects have an IQ in the average range (80 or above), we elected not to match subjects on IQ so as not to bias our sample selection in favor of less impaired readers because in adults IQ is known to be influenced by reading ability. One of the 29 DYS and none of the NI readers met Diagnostic and Statistical Manual of Mental Disorders-IV criteria for attention-deficit/hyperactivity disorder.
MRI.
Functional imaging was performed on a 1.5-Tesla Signa MR imaging system from General Electric equipped with echo-planar imaging (EPI) hardware from Advanced NMR Systems (Wilmington, MA). Activation images were collected by using an EPI gradient echo sequence (flip angle, 60°; echo time, 60 msec; repetition time, 2,000 msec; field of view, 40 × 20 cm; 9 mm, slice thickness; 128 × 64 × 1 number of excitations). Thirty images per slice location were collected while the subject performed one of the five (L, C, SLR, NWR, or SC) activation tasks. Each task was run four times with the order of successive tasks randomized, a total of 120 images per slice per task being collected.
Image Analysis.
We focused on 17 brain regions of interest (ROI) that previous research had implicated in reading and language (20–23) and examined these for evidence of differences between the two reading groups in patterns of activation across the series of tasks. Before statistical analysis, the images from each run were motion-corrected for three translation directions and for the three possible rotations by using the spm-96 program (24). One hundred eight images of the 120 images per slice per task were analyzed; 12 images were discarded (3 images at the beginning of each task trial and four trials per task) to account for variation of hemodynamic changes that occur initially in response to a task. The remaining 108 images were thresholded, normalized, and median-filtered. Activated pixels in ROIs were detected for each pair of activation tasks by using the split t test, which divides the data into two parts and performs a separate t test on each half data set. The pixel was considered to be activated if the t value for a given pixel from both t maps was more than 2. Images were cluster filtered so that isolated activated pixels without at least two activated neighbors were discarded. The anatomic images and activation maps from individual subjects were transformed by in-plane transformation and slice interpolation into a proportional three-dimensional grid defined by Talairach and Tournoux (25). The following 17 ROIs were specified in each hemisphere: Brodmann’s area (BA) 47/11/46; inferior frontal gyrus (IFG), BA 44/45; posterior superior temporal gyrus (STG), posterior BA 22; angular gyrus, BA 39; inferior lateral extrastriate, ILES; BA 17, calcarine cortex; anterior cingulate gyrus; BA 6 superior-medial; BA 6 superior-lateral; BA 21; anterior STG, BA 22/41/42; supramarginal gyrus, BA 40; BA 37; insula; superior medial extrastriate; superior lateral extrastriate; and lingual gyrus.
Statistical Analysis.
There is no a priori reason to believe that all subjects will show equally large activations when performing the same task at the same level of performance. For this reason, we have used a within-subjects hierarchical design that controls for individual differences in overall activation levels by using each subject as his or her own control. The primary dependent variable was a count for each subject of the number of activated pixels in a given ROI for a given task. Several dependent measures (e.g., spatial extent and percent signal change) can be extracted from fMRI data (26). In the current study, we focused primarily on a count of significantly activated pixels in each ROI, a measure of the spatial extent of activation within that region. We also examined the average percent signal change measure and these results were consistent with the pixel count analysis. Images were cluster-filtered so that isolated activated pixels without at least two activated neighbors were discarded. Because of concerns about random activations, we do not rely on a single activated pixel with the threshold used in these experiments. Rather, the method requires that there be contiguous clusters, a procedure that diminishes the false positive rate.
RESULTS
Behavioral.
Reading performance in the DYS subjects was significantly impaired: the mean standard score on a measure of nonword reading (27) was 81 ± 1.9 (mean ± SEM) in DYS readers compared with 114 ± 1.5 in NI readers, with no overlap between groups. Similarly, error patterns on the fMRI tasks revealed that DYS readers differed from NI readers most strikingly on the NWR task. NWR is perhaps the clearest indication of decoding ability because familiarity with the letter pattern cannot influence the individual’s response. Error rates (percent errors) during the fMRI tasks on the L, C, SLR, NWR, and SC tasks for NI subjects were 3.0 ± 0.8, 2.6 ± 0.5, 1.2 ± 0.5, 9.3 ± 1, and 4.6 ± 0.7; the corresponding mean error rates for DYS subjects were 5.1 ± 1.3, 7.6 ± 1.5, 11.0 ± 2.3, 31.5 ± 2.3, 13.8 ± 1.8. Analysis of error rate revealed a significant reading group–task interaction [F(4, 228) = 24.98; P < 0.0001]. DYS and NI readers did not differ significantly on the line judgment task.
fMRI.
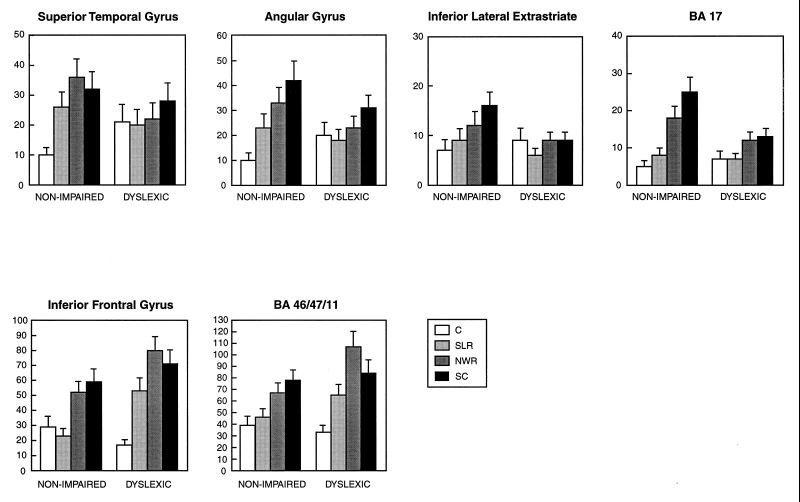
By using a standard progressive analysis procedure, we first performed an overall reading group–sex–task–ROI–hemisphere ANOVA that revealed a significant reading group (DYS vs. NI)–task–ROI interaction [F(48, 2,736) = 1.75; P < 0.03], indicating that the difference between DYS and NI readers varies depending on the ROI and demands of the task. On the basis of the results of this overall test, we then examined each of the 17 ROIs to determine whether DYS and NI readers differed in their task activation profile. Significant reading group–task interactions were noted in four regions [posterior superior temporal gyrus (posterior STG, Wernicke’s area), angular gyrus (BA 39), striate cortex (BA 17), and inferior frontal gyrus (IFG, Broca’s area)] and marginally significant interactions were found in two additional regions [ILES cortex and anterior inferior frontal gyrus (BA 46/47/11)] (Fig. 1). It is important to recognize that we were looking for patterns of activation across tasks rather than differences on a single task; hence, our emphasis on task–reading group interactions.
Figure 1.
Number of activated pixels for brain regions where activation patterns across tasks differ significantly between NI and DYS readers. Activations (mean ± SEM) are shown on ordinate and tasks are on abscissa. We performed an overall ANOVA and followed up those interactions that were significant (minimizing type I error). Data are also shown for regions with marginal P values (minimizing type II error). Significance levels of the task by group effect (Huynh–Feldt corrected P values): STG, F(3, 171) = 4.3 and P = 0.009; BA 17, F(3, 171) = 4.0 and P = 0.012; IFG, F(3, 171) = 3.8 and P = 0.012; angular gyrus, F(3, 171) = 2.7 and P = 0.054; BA 46/47/11, F(3, 171) = 2.4 and P = 0.071; ILES, F(3, 171) = 2.2 and P = 0.094. The six anatomic regions (with center or ROI given in x, y, and z coordinates of Talairach) are (i) posterior STG, BA 22 (53, −43, 11); (ii) angular gyrus, BA 39, angular gyrus of the inferior parietal lobule (47, −45, 33); (iii) ILES, BA 18, 19, inferior occipital gyrus, inferior aspect of lateral occipital gyrus (36, −80, −5); (iv) BA 17, striate cortex (8, −89, 3); (v) IFG, BA 44 posterior aspect (pars operculum) of IFG and BA 45 middle aspect (pars triangularis) of IFG (47, 18, 18); (vi) BA 47, 11, 46, anterior inferior aspect of IFG, lateral and medial orbital gyri, and superior aspect of IFG and inferior aspect of middle frontal gyrus (33, 36, 0). Coordinates are shown for right hemisphere where x is positive (x is negative for left hemisphere).
Previous investigators have assumed the existence of a posterior cortical system adapted for reading, a system including Wernicke’s area, the angular gyrus, extrastriate cortex, and striate cortex (28–30). As shown in Figs. 1 Upper and 2, we found differences between DYS and NI readers in the patterns of activation in several critical components of this system: posterior STG (Wernicke’s area), BA 39 (angular gyrus), and BA 17 (striate cortex). The pattern of group differences was similar at each of these sites: NI subjects show a systematic increase in activation in going from C to SLR to NWR, that is, as orthographic to phonologic coding demands increase, and DYS readers fail to show such systematic modulation in their activation patterns in response to the same task demands. The data from an additional posterior site, the ILES, shows marginal statistical significance. The ILES demonstrates a pattern of group differences across tasks similar to that found in other putative components of the posterior cortical system adapted for reading. In addition, an anterior region, the IFG, demonstrates significant differences in the pattern of activation between NI and DYS readers (Figs. 1 Lower and 2). However, in this case, in contrast to findings in the posterior system, DYS compared with NI readers demonstrate greater activation in response to increasing phonologic decoding demands. An additional anterior–frontal brain region, BA 46/47/11, demonstrates a pattern of activation across tasks comparable to the contiguous IFG, although the group difference was marginal.
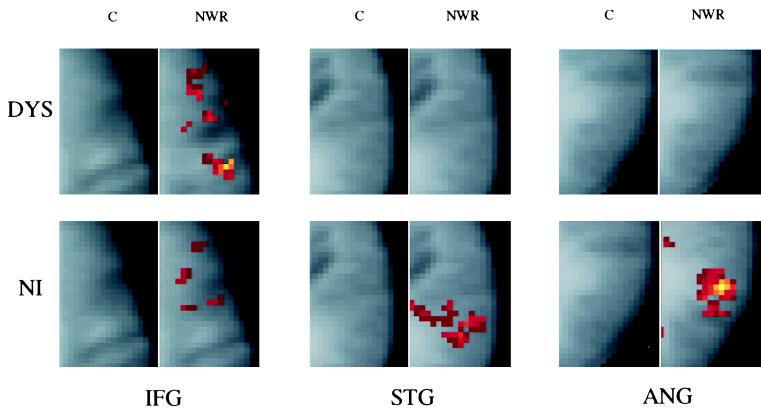
Figure 2.
Composite activation maps in DYS and NI readers for the C and NWR judgment tasks. As shown, DYS and NI readers differ in the degree of activation produced in different brain regions during phonologic (NWR) compared with orthographic (C) coding; DYS readers demonstrate a pattern of relative overactivation anteriorly in IFG in contrast to relative underactivation posteriorly, in STG and the angular gyrus. Composite maps (with z-axis Talairach position) are shown for the left anterior region (IFG, z = 33) and two regions in the left posterior system [post STG (STG, z = 12) and the angular gyrus (ANG, z = 23)]. Composite maps are based on brain activations representing C and NWR. The median t value was obtained for each pixel in each of the Talairach-transformed images of the 29 DYS and 32 NI readers, respectively. Those t values greater than 0.2 were cluster-filtered (cluster size = 3) and overlaid on composite anatomic images that were obtained by adding Talairach-transformed anatomical images from the two groups. The cluster criterion used in this composite differs from that used in the statistical analysis; when combining multiple activation maps from different subjects, it is necessary to change the threshold and cluster criterion to compensate for imprecise overlap of activation regions between subjects.
Hemispheric differences between NI and DYS readers have long been suspected (13, 14, 31, 32), and these were found in two regions: the angular gyrus and BA 37. According to the logic of the statistical analytic strategy, we first looked for and found an overall significant reading group–hemisphere–ROI interaction. We then looked for reading group–hemisphere interactions in each ROI. First an overall significant reading group–hemisphere–ROI interaction was obtained [F(16, 912) = 2.14; P < 0.05] and then significant reading group–hemisphere interactions were found in two regions: the angular gyrus (BA 39) [F(1, 57) = 5.04; P < 0.05] and BA 37 [F(1, 57) = 7.88; P < 0.01]. The task–reading group interaction in the angular gyrus described above (showing anomalous activity across tasks for DYS readers) was not further qualified by hemispheric differences; hence, these two different reading group effects in this ROI appear to be orthogonal to one another. BA 37 encompasses the posterior aspect of the inferior and middle temporal gyri and anterior aspect of the lateral occipital gyrus (Talairach coordinates 44, −66, 21). In each case, activations in NI readers were greater in left hemisphere and, in contrast, in DYS readers activations in these regions were greater in the right hemisphere. This pattern was observed across all tasks. On the basis of our earlier work (33), we examined for hemispheric differences between males and females. In the IFG, a significant sex difference was found [sex–hemisphere–task interaction: F(3, 171) = 3.37; P < 0.025]. During NWR, men showed significantly greater activation in the left hemisphere compared with right, and women showed relatively greater right hemisphere activation, consistent with previous observations.
DISCUSSION
In this study we found significant differences in brain activation patterns between DYS and NI readers, differences that emerge during tasks that make progressive demands on phonologic analysis. These findings relate the cognitive/behavioral deficit characterizing DYS readers to anomalous activation patterns in both posterior and anterior brain regions (Fig. 3).Thus, within a large posterior cortical system including Wernicke’s area, the angular gyrus, the extrastriate and striate cortex, DYS readers fail to systematically increase activation as the difficulty of mapping print onto phonologic structures increases. In contrast, in anterior regions including the IFG and BA 46/47/11, dyslexic readers show a pattern of overactivation in response to even the simplest phonologic task (SLR; Fig. 1). For NI readers, these data provide functional evidence of a widely distributed computational system for reading characterized by specialization and reciprocity: within the system, task-specific responses vary from region to region. For example, in the IFG only the complex phonologic task (NWR) produced a significant increase in activation relative to the orthographic (C) task, suggesting that this region is engaged in letter to sound transcoding; in Wernicke’s area both simple (SLR) and more complex (NWR) phonologic tasks produced significant increases in activation relative to the orthographic task, implying that this region processes information in a more abstract phonological form (Fig. 1).
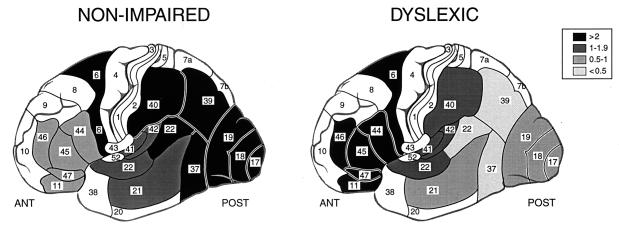
Figure 3.
Relative increase in activation during phonologic compared with orthographic coding in different brain regions in NI and DYS readers. As shown in the key, the shadings represent the relative magnitude of the increase in activation (mean pixel counts) for a given ROI calculated as: (NWR − C/C) = R. In posterior regions [e.g., posterior BA 22 (STG) and BA 39 (angular gyrus)], the relative change in activation is large (>2, shown in black) in NI readers but very small in DYS readers (<0.5, shown as lightest gray). A contrasting pattern is shown in anterior regions, for example, in BA 44 and 45 (IFG), where NI readers demonstrate an increase in activation (0.5–1) and DYS readers demonstrate an even greater increase (>2). There are regions where NI and DYS readers show similar increases in activation, for example, BA 6 and anterior STG (BA 41, BA 42, anterior BA 22). Brain regions shown in white were not part of the 17 ROIs examined; numbers represent BAs.
These data help to reconcile the seemingly contradictory findings of previous imaging studies of dyslexia, some of which involved anomalous findings in the visual system (15) and others indicated abnormal activation within components of the language system (11–14, 16, 17). Our data indicate that dyslexic readers demonstrate a functional disruption in an extensive system in posterior cortex encompassing both traditional visual and traditional language regions and a portion of association cortex. The involvement of this latter region centered about the angular gyrus is of particular interest because this portion of association cortex is considered pivotal in carrying out those cross-modal integrations necessary for reading [i.e., mapping the visual percept of the print onto the phonologic structures of the language (28–30)]. Consistent with this study of developmental dyslexia, a large literature on acquired inability to read (alexia) describes neuroanatomic lesions most prominently centered about the angular gyrus (34–36). We suppose that it is no coincidence that both the acquired and the developmental disorders affecting reading have in common a disruption within the neural systems serving to link the visual representations of the letters to the phonologic structures they represent. Although reading difficulty is the primary symptom in both acquired alexia and developmental dyslexia, associated symptoms and findings in the two disorders would be expected to differ somewhat, reflecting the differences between an acquired and a developmental disorder. In acquired alexia, a structural lesion resulting from an insult (e.g., stroke or tumor) disrupts a component of an already functioning neural system, and the lesion may extend to involve other brain regions and systems. In developmental dyslexia, as a result of a constitutionally based functional disruption, the system never develops normally so that the symptoms reflect the emanative effects of an early disruption to the phonologic system. In either case, the disruption is within the same neuroanatomic system.
These results extend data from more recent studies that found anomalous activation in one or another of these regions but did not map out the full extent of the functional disruption. For example, Rumsey et al. (17), with positron-emission tomography and a more limited range of tasks than ours, found differences in posterior but not in anterior brain regions in DYS compared with NI readers. In the present study, the use of tasks that systematically varied demands on phonologic mapping of print and a larger subject sample may have increased our ability to discern a pattern of relative overactivation in IFG in DYS readers. Paulesu et al. (16) have reported underactivation in the insula in dyslexia, but Rumsey (17) and we find no support for this claim. Our findings implicating the IFG in developmental dyslexia are consonant with reports of acquired alexia accompanying some aphasias of the Broca type that have also involved this anterior region (37).
Our findings of these two contrasting patterns of activation, that is, relative underactivation in posterior regions and relative overactivation in anterior regions, cannot be accounted for by the same explanation—for example, that DYS readers are using greater effort. True, the pattern of relative overactivation observed in anterior brain may represent the neural consequences of the increased effort required of dyslexic readers in carrying out phonologic analysis, an increase in effort measured behaviorally as an increased error rate on tasks that make demands on such analysis. In posterior regions, on the other hand, the absence of task-related increases in activation, both in response to tasks that require relatively little and to tasks that demand relatively more phonologic analysis cannot be ascribed to effort. Instead, they plausibly reflect a functional disruption in a system critical for carrying out such operations.
In summary, for dyslexic readers, these brain activation patterns provide evidence of an imperfectly functioning system for segmenting words into their phonologic constituents; accordingly, this disruption is evident when dyslexic readers are asked to respond to increasing demands on phonologic analysis. These findings now add neurobiologic support for previous cognitive/behavioral data pointing to the critical role of phonologic analysis and its impairment in dyslexia. The pattern of relative underactivation in posterior brain regions contrasted with relative overactivation in anterior regions may provide a neural signature for the phonologic difficulties characterizing dyslexia.
Acknowledgments
We acknowledge the contributions of the late Isabelle Liberman to this program of investigation. We thank Carmel Lepore, Hedy Sarofin, and Terry Hickey for their invaluable help in imaging subjects. This work was supported by grants from the National Institute of Child Health and Human Development (PO1 HD 21888 and P50 HD25802).
ABBREVIATIONS
- fMRI
functional MRI
- DYS
dyslexic
- NI
nonimpaired
- L
line
- C
case
- SLR
single letter rhyme
- NWR
nonword rhyme
- SC
semantic category
- ROI
regions of interest
- BA
Brodmann’s area
- IFG
inferior frontal gyrus
- STG
superior temporal gyrus
- ILES
inferior lateral extrastriate
References
- 1.Brady S A, Shankweiler D P, editors. Phonological Processes in Literacy: A Tribute to Isabelle Y. Liberman. Hillsdale, NJ: Lawrence Erlbaum; 1991. [Google Scholar]
- 2.Rieben L, Perfetti C A. Learning to Read: Basic Research and its Implications. Hillsdale, NJ: Lawrence Erlbaum; 1991. [Google Scholar]
- 3.Bruck M. Dev Psychol. 1992;28:874–886. [Google Scholar]
- 4.Shankweiler D, Crain S, Katz L, Fowler A E, Liberman A M, Brady S A, Thornton R, Lundquist E, Dreyer L, Fletcher J M, et al. Psychol Sci. 1995;6:149–156. [Google Scholar]
- 5.Gough P, Tunmer W. Remedial and Special Education. 1986;7:6–10. [Google Scholar]
- 6.Fletcher J M, Shaywitz S E, Shankweiler D P, Katz L, Liberman I Y, Steubing K K, Shaywitz B A. J Ed Psychol. 1994;86:6–23. [Google Scholar]
- 7.Stanovich K E, Siegel L S. J Ed Psychol. 1994;86:24–53. [Google Scholar]
- 8.Liberman A M. In: Reading and Spelling: Development and Disorders. Hulme C, Joshi R M, editors. Mahwah, NJ: Lawrence Erlbaum; 1998. pp. 5–18. [Google Scholar]
- 9.Liberman A M. In: Speech: A Special Code. Liberman A M, editor. Cambridge, MA: MIT Press; 1996. pp. 1–44. [Google Scholar]
- 10.Liberman I Y, Shankweiler D, Liberman A M. In: Phonology and Reading Disability: Solving the Reading Puzzle. Shankweiler D, Liberman I Y, editors. Ann Arbor, MI: Univ. of Michigan Press; 1989. pp. 1–33. [Google Scholar]
- 11.Gross-Glenn K, Duara R, Barker W W, Loewenstein D, Chang J Y, Yoshii F, Apicella A M, Pascal S, Boothe T, Sevush S, et al. J Clin Exp Neuropsychol. 1991;13:531–544. doi: 10.1080/01688639108401069. [DOI] [PubMed] [Google Scholar]
- 12.Flowers D L, Wood F B, Naylor C E. Arch Neurol. 1991;48:637–643. doi: 10.1001/archneur.1991.00530180095023. [DOI] [PubMed] [Google Scholar]
- 13.Rumsey J M, Zametkin A J, Andreason P, Hanahan A P, Hamburger S D, Aquino T, King C, Pikus A, Cohen R M. Arch Neurol. 1992;49:527–534. doi: 10.1001/archneur.1992.00530290115020. [DOI] [PubMed] [Google Scholar]
- 14.Salmelin R, Service E, Kiesila P, Uutela K, Salonen O. Ann Neurol. 1996;40:157–162. doi: 10.1002/ana.410400206. [DOI] [PubMed] [Google Scholar]
- 15.Eden G F, VanMeter J W, Rumsey J M, Maisog J M, Woods R P, Zeffiro T A. Nature (London) 1996;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- 16.Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak R S J, Frith C D. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- 17.Rumsey J M, Nace K, Donohue B, Wise D, Maisog J M, Andreason P. Arch Neurol. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- 18.Van Orden G C, Pennington B F, Stone G O. Psychol Rev. 1990;97:488–522. doi: 10.1037/0033-295x.97.4.488. [DOI] [PubMed] [Google Scholar]
- 19.Lukatela G, Turvey M T. J Exp Psychol Gen. 1994;123:107–128. doi: 10.1037//0096-3445.123.2.107. [DOI] [PubMed] [Google Scholar]
- 20.Demonet J F, Price C, Wise R, Frackowiak R S J. Brain. 1994;117:671–682. doi: 10.1093/brain/117.4.671. [DOI] [PubMed] [Google Scholar]
- 21.Henderson V W. Brain Lang. 1986;29:119–133. doi: 10.1016/0093-934x(86)90037-4. [DOI] [PubMed] [Google Scholar]
- 22.Petersen S E, Fox P T, Snyder A Z, Raichle M E. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- 23.Pugh K, Shaywitz B, Constable R T, Shaywitz S, Skudlarski P, Fulbright R, Bronen R, Shankweiler D, Katz L, Fletcher J, Gore J. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- 24.Friston K J, Ashburner J, Frith C D, Poline J-B, Heather J D, Frackowiak R S J. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- 25.Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- 26.Bavelier D, Corina D, Jezzard P, Padmanabhan S, Clark V P, Karni A, Prinster A, Braun A, Lalwani A, Rauschecker J P, et al. J Cognitive Neurosci. 1997;9:664–686. doi: 10.1162/jocn.1997.9.5.664. [DOI] [PubMed] [Google Scholar]
- 27.Woodcock R W. Woodcock Reading Mastery Tests, Revised. Circle Pines, MI: American Guidance Service; 1987. [Google Scholar]
- 28.Black S E, Behrmann M. In: Localization and Neuroimaging in Neuropsychology. Kertesz A, editor. New York: Academic; 1994. pp. 331–376. [Google Scholar]
- 29.Geschwind N. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 30.Benson D F. The Neurology of Thinking. New York: Oxford Univ. Press; 1994. [Google Scholar]
- 31.Galaburda A M, Sherman G F, Rosen G D, Aboitiz F, Geschwind N. Ann Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- 32.Geschwind N. In: Dyslexia: A Neuroscientific Approach to Clinical Evaluation. Duffy F H, Geschwind N, editors. Brown, Boston: Little; 1985. pp. 195–211. [Google Scholar]
- 33.Shaywitz B A, Shaywitz S E, Pugh K R, Constable R T, Skudlarski P, Fulbright R K, Bronen R T, Fletcher J M, Shankweiler D P, Katz L, et al. Nature (London) 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- 34.Dejerine J. C R Societe du Biologie. 1891;43:197–201. [Google Scholar]
- 35.Damasio A R, Damasio H. Neurology. 1983;33:1573–1583. doi: 10.1212/wnl.33.12.1573. [DOI] [PubMed] [Google Scholar]
- 36.Friedman R F, Ween J E, Albert M L. In: Clinical Neuropsychology. Heilman K M, Valenstein E, editors. New York: Oxford Univ. Press; 1993. pp. 37–62. [Google Scholar]
- 37.Benson D F. Arch Neurol. 1977;34:327–331. doi: 10.1001/archneur.1977.00500180021004. [DOI] [PubMed] [Google Scholar]