Abstract
Lithium is the most commonly used drug for the treatment of manic depressive illness. The precise mechanisms underlying its clinical efficacy remain unknown. We found that long-term exposure to lithium chloride dramatically protects cultured rat cerebellar, cerebral cortical, and hippocampal neurons against glutamate-induced excitotoxicity, which involves apoptosis mediated by N-methyl-d-aspartate (NMDA) receptors. This neuroprotection is long-lasting, occurs at therapeutically relevant concentrations of lithium with an EC50 of approximately 1.3 mM, and requires treatment for 6–7 days for complete protection to occur. In contrast, a 24-h treatment with lithium is ineffective. The protection in cerebellar neurons is specific for glutamate-induced excitotoxicity and can be attributed to inhibition of NMDA receptor-mediated calcium influx measured by 45Ca2+ uptake studies and fura-2 fluorescence microphotometry. The long-term effects of lithium are not caused by down-regulation of NMDA receptor subunit proteins and are unlikely related to its known ability to block inositol monophosphatase activity. Our results suggest that modulation of glutamate receptor hyperactivity represents at least part of the molecular mechanisms by which lithium alters brain function and exerts its clinical efficacy in the treatment for manic depressive illness. These actions of lithium also suggest that abnormality of glutamatergic neurotransmission as a pathogenic mechanism underlying bipolar illness warrants future investigation.
Lithium was introduced into psychiatry almost half a century ago and remains the most important drug for the treatment and prophylaxis of mania and depression (1–3). It has been estimated that approximately one person per thousand in highly industrialized countries is undergoing lithium therapy. Despite intensive research, the molecular mechanisms underlying the therapeutic actions of lithium are still obscure. Critical to the attribution of any observed biochemical effect to therapeutic relevance is the observation that the action of lithium to stabilize mood cycling of bipolar illness requires a lag period for the efficacy to occur and is not immediately reversed upon discontinuation of treatment (1–3). Thus, the mechanisms of action of lithium appears to involve its long-term effects in stabilizing mood in manic depressive patients, perhaps by resetting ion homeostasis or neurotransmitter receptor balance.
Glutamate is a major excitatory amino acid neurotransmitter that plays a prominent role in synaptic plasticity, learning, and memory (4–6). Glutamate is also a potent neuronal excitotoxin under a variety of experimental conditions, triggering either rapid or delayed neurotoxicity (7–9). Glutamate-induced neuronal death in discrete brain areas has been implicated in neurodegenerative diseases such as Huntington’s chorea, Alzheimer’s disease, and Parkinsonism (10–12). An abnormality in glutamate-dopamine neurotransmission has also been proposed to be the basis of some forms of schizophrenia (13, 14). In addition, the psychotropic drugs, such as carbamazepine, imipramine, and related tricyclic antidepressants, have been found to have antagonistic properties on the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors (15, 16). Thus, it seemed logical to investigate the action of lithium on NMDA receptor-mediated response in neurons. In this study, we examined the long-term effects of lithium on NMDA receptor-mediated excitotoxicity in cultured neurons and explored its possible underlying mechanisms.
MATERIALS AND METHODS
Cell Cultures.
Cerebellar granule cells were prepared from 8-day-old Sprague–Dawley rats (Taconic Farms) as described (17). The cells were maintained in basal modified Eagle’s medium containing 10% fetal calf serum, 2 mM glutamine, gentamicin (50 μg/ml), and 25 mM KCl. The cells were seeded at a density of 2.7 × 105 cells per cm2 in 24-well plates, 96-well plates, or 100-mm Petri dishes precoated with poly-(l-lysine), depending on the purpose of the experiments. The cells were maintained at 37°C in the presence of 6% CO2/95% air in a humidified incubator. Cytosine arabinofuranoside (10 μM) was added to the cultures approximately 24 h after plating to arrest the growth of nonneuronal cells. The culture medium was not changed until the cerebellar granule cell cultures were used to avoid the neurotoxicity elicited by glutamate present in fresh medium. Routinely, the cultures were pretreated with indicated concentration of LiCl for 6–7 days, starting from days 1 to 2 in vitro. Cells were then exposed to 100 μM glutamate for 24 h. Neuron-enriched cerebral cortical and hippocampal cells were prepared from the brains of 17- to 18-day-old Sprague–Dawley rat embryos (Taconic Farms) as described (18). The cells were maintained in Neurobasal medium (GIBCO/BRL) containing B-27 serum-free supplement (GIBCO/BRL). The cells were seeded at a density of 2 × 105 cells per cm2 (cortical culture) or 103 cells per cm2 (hippocampal culture) in 96-well plates precoated with poly-(l-lysine) and laminin. The cells were maintained at 37°C in the presence of 6% CO2/95% air in a humidified incubator. Cultures were used after 9 days in vitro for assessment of glutamate-induced neurotoxicity in the absence or presence of lithium pretreatment.
Measurement of Neurotoxicity.
The mitochondrial dehydrogenase activity that cleaves 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was used to determine cell survival in a quantitative colorimetric assay (19). The tetrazolium ring of MTT is cleaved by various dehydrogenase enzymes in active mitochondria, forming a blue-colored insoluble product, formazan. Cerebellar granule cells were incubated with MTT (125 μg/ml) added to the growth medium for 1 h at 37°C. The medium was then aspirated and the formazan product was dissolved in dimethyl sulfoxide and quantified spectrophotometrically at 540 nm. The results are expressed as a percentage of control culture viability.
Analysis of DNA Fragmentation.
DNA fragmentation was assessed by using a soluble DNA preparation as described (17). Cerebellar granule cells (2 × 107 cells) grown on a 100-mm dish were lysed in 10 mM Tris⋅HCl (pH 7.5) containing 10 mM EDTA and 0.2% Triton X-100. The lysate was centrifuged at 12,000 × g for 10 min. The supernatant was treated with proteinase K (0.3 mg/ml) and RNase A (0.3 mg/ml) and then extracted in the presence of NaI. The DNA was precipitated with isopropanol and dissolved in 10 mM Tris⋅HCl (pH 8.0) containing 1 mM EDTA. The DNA was electrophoresed in 1.5% agarose gel in TBE buffer. The DNA bands were then visualized by ethidium bromide-staining and photographed.
Analysis of Chromatin Condensation.
Chromatin condensation was detected by nucleus staining with Hoechst 33258 as described (17). Cerebellar granule cells (2.5 × 106 cells) grown on a 35-mm dish were washed with ice-cold PBS and fixed with 4% formaldehyde in PBS. Cells were then stained with Hoechst 33258 (5 μg/ml) for 5 min at 4°C. Nuclei were visualized by using a Zeiss Axiophot fluorescence microscope at ×1,000 magnification.
Measurement of Intracellular Free Calcium.
Measurement of intracellular free calcium concentration ([Ca2+]i) was carried out as described by using fluorescence microphotometry and the Ca2+-sensitive indicator fura-2 (15). Cerebellar granule cells (2.5 × 106 cells) grown in dishes containing glass bottoms were loaded with 2.5 μM fura-2 tetrakis(acetoxymethyl) ester for 30–60 min. The cells were washed three times with an external salt solution (145 mM NaCl/2.5 mM KCl/1 mM CaCl2/10 mM Hepes, pH 7.4/10 mM glucose) at 37°C. The fura-2 fluorescence was measured by using a SPEX AR-CM fluorescence photometric apparatus and Nikon microscope. Selected clumps of cells were perfused with warmed (37°C) external solution containing 100 μM glutamate and 10 μM glycine without added LiCl for 10-sec followed by a 60-sec wash. In each dish, four clumps of cells, selected at random, were tested. Within a single experiment, each lithium pretreatment was tested in two dishes. The fura-2 measurements were calibrated by measurements taken in external salts containing 20 μM ionomycin, 40 μM carbonyl cyanide m-chlorophenyl-hydrazone, and either 10 mM CaCl2 (for Rmax) or 20 mM EGTA (for Rmin). Calcium concentrations were calculated by use of the ratio method, taking 224 nM as the Kd value of fura-2 (20).
Immunoblotting.
Cells (2 × 107 cells) grown on a 100-mm dish were rinsed with PBS, scraped off the dish, and lysed by boiling in a buffer containing 10 mM Tris (pH 7.4), 1% SDS, and 1 mM sodium orthovanadate. The lysates were centrifuged at 10,000 × g for 5 min and an aliquot containing 20 μg of protein was electrophoresed in SDS/PAGE gels. The proteins were electrophoretically transferred onto a poly(vinylidene difluoride) membrane at 25 V for 2 h. Blots were blocked by incubation for 1 h with 5% nonfat milk in PBS/0.1% Tween 20 and then incubated with primary antibodies for 1 h at room temperature. The following antibodies were used: anti-NMDAR1 rabbit polyclonal antibodies (Chemicon), anti-NMDAR2A rabbit polyclonal antibodies (Chemicon), anti-NMDAR2B goat polyclonal antibodies (Santa Cruz Biotechnology), and anti-NMDAR2C goat polyclonal antibodies (Santa Cruz Biotechnology). Blots were then incubated with horseradish peroxidase-conjugated secondary antibody. Detection was made by the enhanced chemiluminescence method using ECL Western blotting reagents (Amersham).
RESULTS AND DISCUSSION
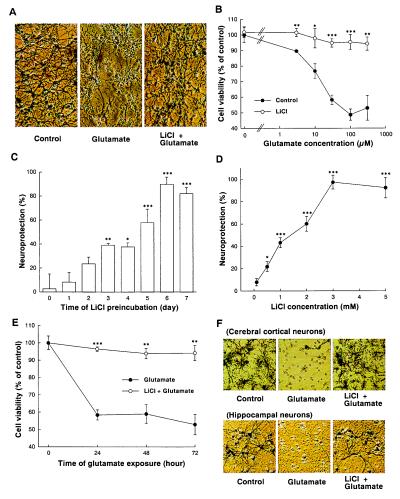
Primary cultures of cerebellar granule cells prepared from neonatal rat pups represent a nearly homogenous population of excitatory neurons that are vulnerable to glutamate insult through activation of NMDA receptors (21, 22). Exposure of rat cerebellar granule cells (cultured for 7–9 days in vitro) to glutamate (100 μM) in culture medium for 24 h resulted in the appearance of dead cells, which were round, smaller, and unable to metabolize MTT by mitochondrial dehydrogenases (Fig. 1A). Preincubation of the cultures with 2 mM LiCl for 7 days markedly protected these neurons against glutamate-induced neurotoxicity, assessed by morphological criteria. Quantification of neuronal survival by MTT metabolism revealed that lithium-induced neuroprotection was associated with a dramatic reduction in the maximal extent of excitotoxicity and appeared to be noncompetitive with respect to glutamate concentration (Fig. 1B). The degree of protection afforded by lithium was dependent on the length of preincubation time. Maximal protective effect was achieved by preincubation for 6–7 days, whereas a 24-h preincubation failed to elicit any effect (Fig. 1C). Lithium-induced excitoprotection did not require the continuous presence of this drug. When after 6 days of treatment with LiCl, the drug was removed from cerebellar granule cells by replacement of medium with sister-culture conditioned medium for 24 h, maximal protection from glutamate challenge was still observed (data not shown). The lithium-induced neuroprotection was also concentration-dependent. Although significant protection by lithium was detected at 0.5 mM, the maximal effect was observed at 3 mM with an EC50 of approximately 1.3 mM (Fig. 1D). Thus, the protection against excitotoxicity occurs within the therapeutic plasma concentration range of this drug, i.e., 0.5–1.5 mM (23). When glutamate exposure time was prolonged from 24 to 72 h, near complete neuroprotection by lithium was still persistent (Fig. 1E), indicating a long-lasting effect. Neuron-enriched cerebral cortical and hippocampal cells prepared from embryonic rats also showed lithium-induced protection against excitotoxicity. Glutamate-induced neuronal death in cortical and hippocampal cultures was robustly suppressed by a long-term preincubation (7 days) with 1 mM LiCl (Fig. 1F), indicating that the neuroprotective effect is not restricted to cerebellar granule cells.
Figure 1.
Chronic lithium pretreatment protects neurons against glutamate toxicity in cultured cerebellar granule, cerebral cortical, and hippocampal cells. (A) Lithium-induced excitoprotection revealed by cell morphology. Cultured cerebellar granule cells were pretreated with LiCl (2 mM) for 7 days. Cultures were then exposed to glutamate (100 μM) and live cells were examined for morphology 24 h later after MTT staining. Cells were photographed with a phase-contrast microscope at a magnification of ×200. Note that the glutamate exposure resulted in the typical appearance of dead cells, which were round, smaller, translucent, and unable to metabolize MTT to blue formazan product. Chronic lithium prevented this aspect of glutamate neurotoxicity. (B) The extent of lithium-induced excitoprotection in cerebellar granule neurons at various glutamate concentrations. Cultured cells were pretreated with LiCl (3 mM) for 7 days. Cultures were then exposed to indicated concentrations of glutamate, and neuronal survival was determined 24 h after addition of glutamate by using the MTT colorimetric assay. (C) Preincubation time-dependent excitoprotection elicited by lithium. LiCl (2 mM) was added to cells at various times (0–7 days) before addition of glutamate (100 μM) on the 7th day in cultures to vary the time of lithium exposure. Neuronal survival was determined 24 h after addition of glutamate by using the MTT colorimetric assay. The results are expressed as percent of neuroprotection. Note that maximal protection was achieved by pretreatment with lithium for 6 days or longer. (D) Concentration-dependent induction of the excitoprotective state. Cells were pretreated with various concentrations of LiCl (0.1–5 mM) for 6 days and then exposed to 100 μM glutamate for 24 h before measurement of neuronal survival by MTT assays. The results are expressed as percent of neuroprotection. (E) Time course of glutamate-induced neurotoxicity. Cultured cerebellar granule cells were pretreated with LiCl (3 mM) for 7 days and then exposed to glutamate (100 μM) as indicated. Neuronal survival was determined 24–72 h after addition of glutamate by using the MTT colorimetric assay. (F) Morphology of cultured cerebral cortical and hippocampal neurons exposed to glutamate in the absence and presence of lithium pretreatment. Cultured cortical or hippocampal neurons were pretreated with 1 mM LiCl for 7 days, starting from the second day in culture. Cultures were then exposed to glutamate (100 μM), and live cells were examined for morphology 24 h later by MTT staining. Cells were photographed with a phase-contrast microscope at a magnification of ×200. Chronic lithium prevented glutamate-induced neurotoxicity. Data are the mean ± SEM of viability measurements from four cultures. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, compared with the group treated with glutamate alone (one-way ANOVA with Bonferroni–Dunn test).
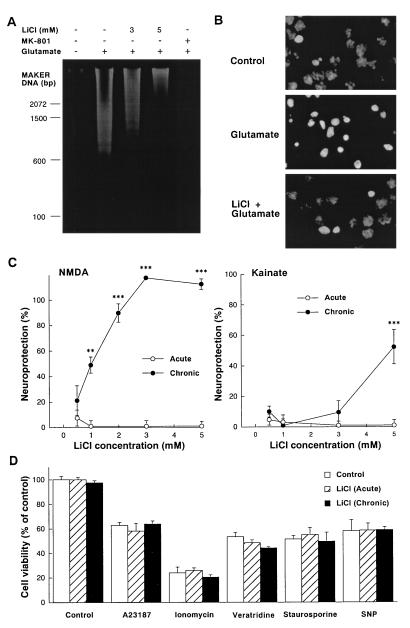
Glutamate-induced delayed neurotoxicity of cerebellar granule cells was associated with the appearance of the hallmarks of apoptosis such as internucleosomal DNA cleavage and chromatin condensation (Fig. 2 A and B). The internucleosomal cleavage, detected as a DNA ladder on an agarose gel, was completely blocked by the NMDA receptor antagonist dizocilpine (MK-801) and was dose-dependently inhibited by chronic LiCl pretreatment. The remaining DNA fragments smearing on the gel suggest that the necrotic component of glutamate-induced neurotoxicity in cerebellar granule cells (24) was unprotected by lithium pretreatment. Chronic lithium also inhibited glutamate-induced chromatin condensation detected by nuclear staining with Hoechst dye 33258. Although chronic treatment with lithium potently and completely blocked NMDA-induced neurotoxicity of cerebellar granule cells (Fig. 2C), the toxicity induced by kainate, an agonist of non-NMDA type of glutamate receptors, was only partially protected by chronic lithium even at the highest concentration (5 mM) of this ion examined (Fig. 2C). Acute treatment with LiCl was ineffective against either excitotoxin. The selectivity of the protection was further demonstrated by the observations that acute or chronic lithium was ineffective against neuronal death induced by A23187 and ionomycin (Ca2+ ionophores), veratridine (a Na+ channel opener), staurosporine (a protein kinase inhibitor), and sodium nitroprusside (a nitric oxide donor) (Fig. 2D).
Figure 2.
Effects of chronic lithium pretreatment on neurotoxicity of cerebellar granule cells. (A) Chronic lithium prevents glutamate-induced internucleosomal DNA fragmentation of cerebellar granule cell. Cells were pretreated with various concentrations of LiCl (3–5 mM) for 7 days and then exposed to glutamate (100 μM) as described in Fig. 1. Soluble DNA was extracted from cells 24 h after the addition of glutamate, subjected to agarose gel electrophoresis, stained with ethidium bromide, and photographed. (B) Chronic lithium inhibits glutamate-induced chromatin condensation of cerebellar granule cell. Cells were pretreated with LiCl (3 mM) for 7 days and then exposed to glutamate (100 μM). Chromatin condensation was detected by nucleus staining with Hoechst 33258. Nuclei were photographed with a Zeiss Axiophot fluorescence microscope at a magnification of ×1,000. (C) Chronic lithium pretreatment protects cells against NMDA and kainate toxicity. Lithium chloride (0.5–5 mM) was added to cultured neurons 1 h (acute) or 7 days (chronic) before NMDA (1 mM) or kainate (100 μM). Additionally MK-801 (1 μM) was added to the kainate-treated groups. After 24 h of stimulation, neuronal viability was measured with the MTT colorimetric assay. The results are expressed as percent of neuroprotection. (D) Effects of lithium on A23187, ionomycin, veratridine, staurosporine, and sodium nitroprusside (SNP)-induced neurotoxicity. Lithium chloride (3 mM) was added to cultured cerebellar granule neurons 1 h (acute) or 7 days (chronic) prior to their exposure to A23187 (300 nM), ionomycin (3 μM), veratridine (1 μM), staurosporine (30 nM), and SNP (100 μM). Neuronal survival was determined 24 h after addition by using the MTT colorimetric assay. Data are the mean ± SEM of viability measurements from four or five cultures. ∗∗, P < 0.01; ∗∗∗, P < 0.001, compared with the group of control (one-way ANOVA with Bonferroni–Dunn test).
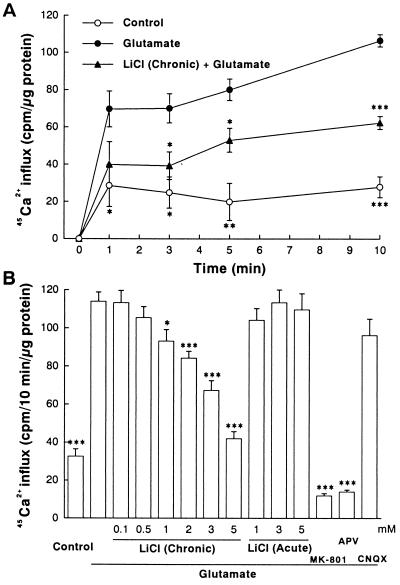
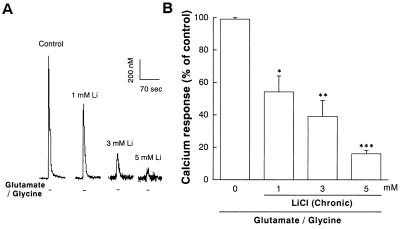
Calcium is a key messenger in glutamate receptor-mediated synaptic plasticity and neurotoxicity (7, 25). To examine the possibility that lithium exerts its neuroprotective effects by modulating NMDA receptor-mediated Ca2+ entry, we initially performed 45Ca2+ influx studies. Application of glutamate (100 μM) into the culture medium elicited a time-dependent increase in 45Ca2+ influx into cerebellar granule cells and the glutamate-induced increase was inhibited by approximately 50% at all time points by a 7-day pretreatment with LiCl (Fig. 3A). The inhibition was enhanced by increasing the concentrations of lithium (1–5 mM) during preincubation (Fig. 3B). In contrast, a 1-h lithium treatment failed to influence glutamate-induced 45Ca2+ entry. In the presence of glutamate, 45Ca2+ influx was unaffected by an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptor antagonist, CNQX, but was reduced by NMDA receptor antagonists, MK-801 and 2-amino-5-phosphonopentanoate (APV), to a level below the basal value. This suggests that glutamate-induce 45Ca2+ influx is entirely mediated by NMDA receptors and that even basal 45Ca2+ entry is partially caused by activation of NMDA receptors by endogenous glutamate released from these cells under depolarizing conditions (21). The chronic effect of lithium on glutamate-induced increase of [Ca2+]i was further examined by using fura-2 fluorescence microphotometry, which measures changes in [Ca2+]i in a clump of neurons during a 10-sec pulse of glutamate in a physiological buffer solution without Mg2+ (Fig. 4). During this short exposure to glutamate, there is little contribution by release from intracellular stores to the calcium increase measured by this method (26). Similar to the results of 45Ca2+ uptake studies, chronic lithium (1–5 mM) preincubation robustly inhibited the increase in [Ca2+]i measured during the 10-sec perfusion with glutamate (100 μM)/glycine (10 μM).
Figure 3.
Chronic lithium exposure inhibits glutamate-induced 45Ca2+ influx into cerebellar granule cells. (A) Time course of basal and glutamate-induced 45Ca2+ influx. Cells were treated with LiCl (3 mM) for 7 days before adding 100 μM glutamate and 45CaCl2 (1 μCi/ml) to the culture medium. At indicated times, the influx was terminated by rapid washing of the culture dishes three times with ice-cold buffer (154 mM choline chloride/2 mM EGTA/10 mM Hepes, pH 7.4) and then solubilized in 0.6 ml of 0.5 M NaOH. An aliquot of 0.5 ml was used for measuring 45Ca2+ radioactivity and the remainder was used for protein determination. (B) Concentration dependence of inhibition by lithium of the 45Ca2+ influx. Cells were pretreated chronically (7 days) or acutely (1 h) with various concentrations of LiCl (0.1–5 mM) and then exposed to glutamate (100 μM) in the presence of 45Ca2+. The 45Ca2+ influx was determined 10 min after glutamate addition. When used, the concentrations of MK-801, 2-amino-5-phosphonopentanoate (APV), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) were 1 μM, 200 μM, and 20 μM, respectively. Data are the mean ± SEM of 45Ca2+ uptake measurements from three cultures. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, compared with the group treated with glutamate alone (one-way ANOVA with Bonferroni–Dunn test).
Figure 4.
Chronic lithium inhibits the peak elevation in [Ca2+]i after exposure to glutamate in cerebellar granule neurons. Cerebellar granule neurons were pretreated with LiCl (1–5 mM) for 7 days and [Ca2+]i was then measured by using microfluorimetry in cells prelabeled with the fluorescent Ca2+ indicator fura-2. Glutamate-induced [Ca2+]i increase was elicited by a 10-sec pulse with glutamate (100 μM)/glycine (10 μM) followed by a 60-sec wash. (A) Graphic tracing of glutamate/glycine-induced [Ca2+]i in untreated and lithium-treated cerebellar granule cells. (B) Averaged peak of [Ca2+]i from three cultures of cells pretreated for 7 days with various concentrations of LiCl (1–5 mM). Data are the mean ± SEM of percent of glutamate/glycine response in untreated cells. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001, compared with the group treated with glutamate/glycine alone without lithium pretreatment (one-way ANOVA with Bonferroni–Dunn test). The 100% values are 645 ± 20 nM.
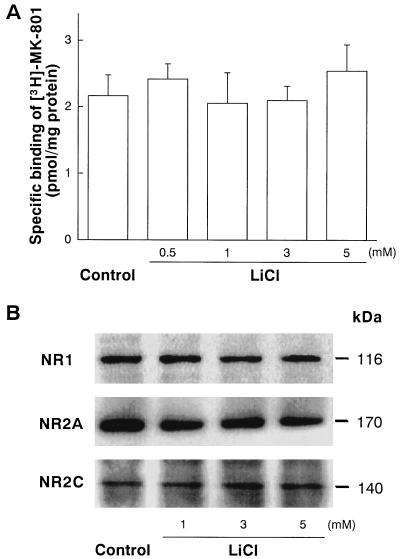
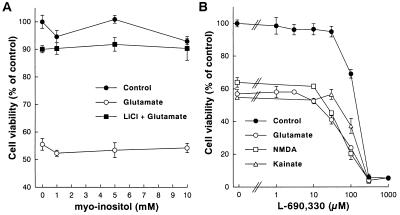
Our results show a robust chronic effect of lithium on NMDA receptor-mediated Ca2+ response and excitotoxicity. To examine whether the lithium’s effect is caused by down-regulation of NMDA receptor site, measurements of [3H]MK-801 binding to NMDA receptors and levels of immunoreactive NMDA receptor subunit proteins were performed. Neither [3H]MK-801 binding to receptors in intact cerebellar granule cells (Fig. 5A) nor the level of immunoreactive NR1, NR2A, or NR2C proteins in these cells (Fig. 5B) was affected by lithium pretreatment for 7 days. The level of NR2B protein was too low to be detected by immunoblotting in both untreated and lithium-pretreated cells, consistent with a previous report that NR2B mRNA and protein levels are very low in the rat cerebellum throughout development (28). These results also suggest that lithium-induced suppression of NMDA receptor function is unlikely caused by changes in relative NMDA receptor composition resulting from differential expression of receptor subunit proteins. The neuroprotection could be caused by lithium’s ability to block inositol monophosphatase, causing depletion of intracellular inositol. However, the presence of excess (1–10 mM) myo-inositol during lithium preincubation failed to affect neuroprotection against glutamate excitotoxicity (Fig. 6A). Moreover, the protective actions against glutamate, NMDA, and kainate insults were not mimicked by L-690,330 (Fig. 6B), an inositol monophosphatase inhibitor with a potency 1,000 times greater than that of lithium (29). Additionally, the lithium suppression of glutamate-induced 45Ca2+ influx was neither affected by myo-inositol supplement nor mimicked by L-690,330 (data not shown). Thus, these results suggest that inositol monophosphatase blockade is unlikely to participate in lithium-induced neuroprotection.
Figure 5.
Chronic lithium treatment does not affect the levels of NMDA receptor binding and immunoreactive NMDA receptor subunit protein in cerebellar granule cells. (A) Specific binding of [3H]MK-801 to NMDA receptors in intact cells was performed essentially as described (27). Briefly, cells grown on 24-well plates were incubated with indicated concentration of LiCl for 7 days, washed, and incubated with 1 nM [3H]MK-801 (24 Ci/mmol, New England Nuclear) in Na+-free PBS containing 100 μM glutamate, 10 μM glycine, and 30 μM MgSO4, at 2°C for 2 h. Nonspecific binding was determined in the presence of 100 μM unlabeled MK-801. Data are the amount of [3H]MK-801 bound (pmol/mg of protein) from three experiments (mean ± SEM). Note that level of [3H]MK-801 specific binding to NMDA receptors was unaffected by pretreatment with 0.5–5 mM LiCl. (B) Cells were pretreated with 1–5 mM for 7 days and Western immunoblotting for NR1, NR2A, and NR2C proteins was performed by using their specific antibodies. Note that the levels of NR1 (116 kDa), NR2A (170 kDa), and NR2C (140 kDa) immunoreactive proteins were not changed by lithium pretreatment. The levels of NR2B protein was not detected in untreated and lithium-treated cells. The immunoblots shown are representative of three experiments.
Figure 6.
myo-Inositol does not affect and L-690,330 does not mimic lithium protection. (A) Cerebellar granule cells were pretreated with various concentrations of myo-inositol (1–10 mM) in the absence or presence of LiCl (3 mM) for 7 days and then exposed to 100 μM glutamate. (B) Cerebellar granule cells were pretreated with various concentrations of L-690,330 (a potent inositol monophosphatase inhibitor; 1–1,000 μM) for 7 days and then exposed to glutamate (100 μM), NMDA (1 mM), or kainate (100 μM). Neuronal survival was determined at 24 h after addition of drugs by using the MTT colorimetric assay. Data are the mean ± SEM of viability measurements from three cultures and are expressed as percent of untreated control. Note that myo-inositol (1–10 mM) did not affect and L-690,330 (1–30 μM) did not mimic LiCl-induced neuroprotection. At concentrations in the range of 100–1,000 μM, L-690,330 induced a dose-dependent neurotoxicity.
Recently, it has been shown that lithium is a potent and selective inhibitor of glycogen synthase kinase 3β, a highly conserved serine/threonine kinase implicated in cell-fate determination during development (30–32). Lithium inhibition of this kinase mimics the effects of the serine/threonine kinase Akt on glycogen synthase kinase 3β after activation of Akt by receptor tyrosine kinases through phosphatidylinositol 3-kinase (33). It is conceivable that NMDA receptor subunits are a target for glycogen synthase kinase and that chronic lithium treatment suppresses NMDA receptor function through modulation of the receptor phosphorylation state mediated by this kinase. Alternatively, persistent inhibition of glycogen synthase kinase activity by chronic lithium treatment may enhance gene expression through reduced phosphorylation of c-Jun, causing disinhibition of the DNA binding activity of c-Jun (34). Although the precise mechanism whereby lithium regulates glutamate-induced Ca2+ homeostasis is unknown, the dose and time requirements of this action of lithium is consistent with the clinical profile of this drug. Our results suggest that modulation of glutamate receptor hyperactivity represents, at least in part, the molecular mechanisms by which lithium exerts its clinical actions in the treatment of manic depressive illness. The possibility that bipolar affective disorder is the result of some abnormality of glutamatergic neurotransmission warrants further investigation.
Acknowledgments
We thank Dr. Xiao-ming Gao for the preparation of rat embryonic hippocampal cell cultures. Support from the Stanley Foundation for the work of C.J.H. is gratefully acknowledged.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: NMDA, N-methyl-d-aspartate; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; [Ca2+]i, intracellular free calcium concentration.
References
- 1.Goodwin F K, Jamison K R. Manic-Depressive Illness. New York: Oxford Univ. Press; 1990. [Google Scholar]
- 2.Birch N J. Lithium and the Cell: Pharmacology and Biochemistry. San Diego: Academic; 1991. [Google Scholar]
- 3.Post R M, Weiss S R B, Chuang D-M. J Clin Psychopharmacol. 1992;12:23S–35S. doi: 10.1097/00004714-199202001-00005. [DOI] [PubMed] [Google Scholar]
- 4.Collingridge G L, Watkins J C. The NMDA Receptor. New York: Oxford Univ. Press; 1994. [Google Scholar]
- 5.Collingridge G L, Bliss T V. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- 6.Bannerman D M, Good M A, Butcher S P, Ramsay M, Morris R G. Nature (London) 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- 7.Choi D W. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 8.Whetsell W O. J Neuropathol Exp Neurol. 1996;55:1–13. doi: 10.1097/00005072-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Greene J G, Greenamyre J T. Prog Neurobiol. 1996;48:613–634. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 10.Meldrum B, Garthwaite J. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 11.Bowen D M. Br J Psychiatry. 1990;157:327–330. doi: 10.1192/bjp.157.3.327. [DOI] [PubMed] [Google Scholar]
- 12.Blandini F, Greenamyre J T, Nappi G. Funct Neurol. 1996;11:3–15. [PubMed] [Google Scholar]
- 13.Kerwin R, Patel S, Meldrum B. Neuroscience. 1990;39:25–32. doi: 10.1016/0306-4522(90)90219-t. [DOI] [PubMed] [Google Scholar]
- 14.Toru M, Kurumaji A, Ishimaru M. Life Sci. 1994;55:1683–1699. doi: 10.1016/0024-3205(94)00337-8. [DOI] [PubMed] [Google Scholar]
- 15.Hough C J, Irwin R P, Gao X-M, Rogawski M A, Chuang D-M. J Pharmacol Exp Ther. 1996;276:143–149. [PubMed] [Google Scholar]
- 16.Tohda M, Urushihara H, Nomura Y. Neurochem Int. 1995;26:53–58. doi: 10.1016/0197-0186(94)00101-y. [DOI] [PubMed] [Google Scholar]
- 17.Gao X-M, Margolis R L, Leeds P, Hough C, Post R M, Chuang D-M. Brain Res. 1995;703:63–71. doi: 10.1016/0006-8993(95)01066-1. [DOI] [PubMed] [Google Scholar]
- 18.Brewer G J. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T R. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 21.Gallo V, Ciotti M T, Coletti A, Aloisi F, Levi G. Proc Natl Acad Sci USA. 1982;79:7919–7923. doi: 10.1073/pnas.79.24.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuang D-M, Gao X-M, Paul S M. Mol Pharmacol. 1993;42:210–216. [PubMed] [Google Scholar]
- 23.Johnson F N. Depression and Mania: Modern Lithium Therapy. Oxford: IRL; 1987. [Google Scholar]
- 24.Ankarcrona M, Dypbukt J M, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton S A, Nicotera P. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 25.Mayer M L, Miller R J. Trends Pharmacol Sci. 1990;11:254–260. doi: 10.1016/0165-6147(90)90254-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Peng L. Synapse. 1993;13:315–321. doi: 10.1002/syn.890130404. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman P L, Iorio K R, Snell L D, Tabakoff B. Alcohol Clin Exp Res. 1995;19:721–726. doi: 10.1111/j.1530-0277.1995.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel A, Fritschy J M, Mohler H, Benke D. J Neurochem. 1997;68:469–478. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- 29.Atack J R, Cook S M, Watt A P, Fletcher S R, Ragan C I. J Neurochem. 1993;60:652–658. doi: 10.1111/j.1471-4159.1993.tb03197.x. [DOI] [PubMed] [Google Scholar]
- 30.Siegfried E, Perkins L A, Capaci T M, Perrimon N. Nature (London) 1990;345:825–829. doi: 10.1038/345825a0. [DOI] [PubMed] [Google Scholar]
- 31.Plyte S E, Hughes K, Nikolakaki E, Pulverer B J, Woodgett J R. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- 32.Klein P S, Melton D A. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemmings B A. Science. 1997;275:1899. doi: 10.1126/science.275.5308.1899. [DOI] [PubMed] [Google Scholar]
- 34.Woodgett J R. Trends Biochem Sci. 1991;16:177–181. doi: 10.1016/0968-0004(91)90071-3. [DOI] [PubMed] [Google Scholar]