Abstract
Background
In spite of Argentina having one of the highest frequencies of haemolytic uraemic syndrome (HUS), the incidence of Escherichia coli O157:H7 is low in comparison to rates registered in the US. Isolation of several non-O157 shiga toxin-producing Escherichia coli (STEC) strains from cattle and foods suggests that E. coli O157:H7 is an uncommon serotype in Argentina. The present study was undertaken to compare the survival rates of selected non-O157 STEC strains under acidic and alcoholic stress conditions, using an E. coli O157:H7 strain as reference.
Results
Growth at 37°C of E. coli O26:H11, O88:H21, O91:H21, O111:H-, O113:H21, O116:H21, O117:H7, O157:H7, O171:H2 and OX3:H21, was found to occur at pH higher than 4.0. When the strains were challenged to acid tolerance at pH as low as 2.5, viability extended beyond 8 h, but none of the bacteria, except E. coli O91:H21, could survive longer than 24 h, the autochthonous E. coli O91:H21 being the more resistant serotype. No survival was found after 24 h in Luria Bertani broth supplemented with 12% ethanol, but all these serotypes were shown to be very resistant to 6% ethanol. E. coli O91:H21 showed the highest resistance among serotypes tested.
Conclusions
This information is relevant in food industry, which strongly relies on the acid or alcoholic conditions to inactivate pathogens. This study revealed that stress resistance of some STEC serotypes isolated in Argentina is higher than that for E. coli O157:H7.
Keywords: STEC, E. coli O157:H7, E. coli O91:H21; acid stress, ethanol, HUS, food
Background
Shiga toxin-producing E. coli (STEC) is now considered to be a common cause of haemorrhagic colitis (HC), haemolytic-uraemic syndrome (HUS) and thrombotic thrombocytopenic purpura in human [1].
Argentina has an exceptionally high frequency of HUS, with 300 to 400 cases per year, and this is the most common cause of acute and chronic renal failure in young children [2-5]. HUS is considered a foodborne disease, early linked to consumption of hamburgers, ground beef and milk derivatives contaminated with STEC [6]. Bovine meat consumption in Argentina is estimated at 60 kg/habitant/year and is considered the highest reported in the world [3].
In 1982, E. coli O157:H7 was isolated from hamburgers in the US and was considered as an etiologic agent of HC [7]. Since then, more than 100 serotypes of STEC have been isolated from animals and foods [8]. In Argentina, E. coli O157:H7 does not seem to be as common as in the US [3]. We have described many non-O157 strains, most of them isolated from cattle and foods [9].
When food products serve as the vehicle of infections, the ingested inoculum may be quite high, as bacteria may have had the opportunity to replicate in food to a high titre before consumption [10]. The importance of gastric juice to control foodborne infection is well known. Previous research with STEC and Shigella species brought out the ability of a microorganism to survive the acidic environment of the stomach as an important determinant of the infective dose [11,12]. Definitive volunteer studies to determine the infective dose experimentally have not been done and are unlikely to be conducted with STEC [10], although it is suspected to be as low as 10 cells for E. coli O157:H7. The bacterial acid tolerance may lower the infective dose when the source of infection is a mildly acidic food [13].
Several investigators have noted the ability of the human pathogen E. coli O157:H7 to survive stress conditions encountered in some foods, in the stomach and in vitro [11,14-19]. Despite this, there is still a lack of knowledge on many non-O157:H7 STEC, also described as leading cause of human illness [20].
Previous research showed that some strains of E. coli are able to survive at pH values as low as 2.5 [11,17], but it does not grow at pH values less than 4.4 [21]. Many studies were designed to examine how microorganisms cope with environmental stress and have referred to the acid survival systems as the acid tolerance response (ATR), acid habituation and acid resistance (AR). A major problem is encountered when attempting to compare acid survival results among various laboratories, which use different strategies to induce and test acid survival. Frequently, those procedures differ in the use of complex versus minimal medium, log phase versus stationary phase cells, and acid challenge at various pH and temperatures [21,22]. It appears that there are indeed numerous acid survival mechanisms, some of which have long-term dramatic effects while others have more subtle, yet significant consequences [22]. It has been difficult, if not impossible, to determine from the literature if the systems described are truly different or simply reflect different strategies of measuring the same system [21].
Ethanol has previously been implicated in the low level of survival of E. coli O157:H7 in certain foods. Therefore, we investigated the potential ability of this acceptable food additive to kill selected STEC strains [23].
Stationary phase bacteria are 100 to 10000 times more resistant to acid than exponentially growing organisms and do not need prior exposure to a low pH to exhibit acid resistance [10].
The objective of this study was to determine: (i) the pH required to inhibit the growth of autochthonous non-O157:H7 STEC in a liquid medium acidified with HCl; (ii) the survival characteristics of STEC exposed to the following stress conditions in independent experiments: pH 2.5, pH 3.0, ethanol 6% and ethanol 12% at 37°C, and to compare them to an E. coli O157:H7 strain isolated from a human patient, which is presumably resistant to food preparation and the acidic environment in the stomach.
Results
Acid growth limit and viability of STEC at acidic pH
The minimum growth pH for STEC strains was 4 (Fig 1). The pH of the culture media was raised to neutrality during the experiment at pH 4 (Fig. 2) allowing all cultures to reach the stationary phase within 24 h (data no shown).
Figure 1.
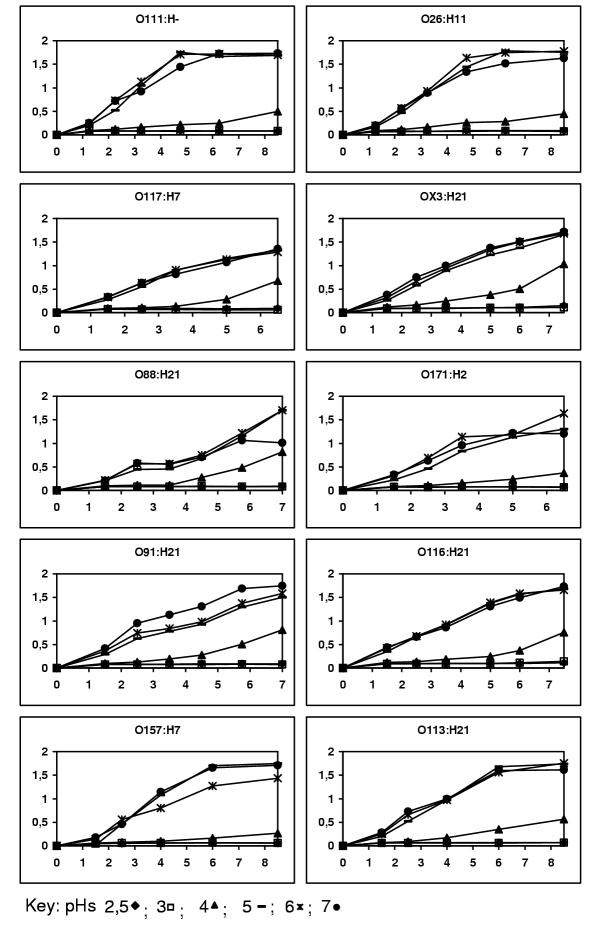
Growth of ten STEC serotypes at different pH. Ordinates correspond to Optical Density and abscises correspond to time in hours.
Figure 2.
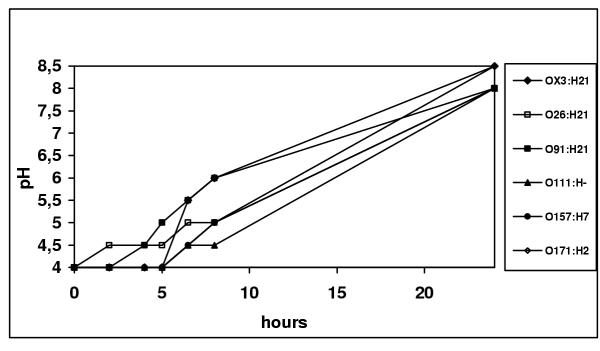
pH variation during the growth of STEC serotypes in acid LB broth (starting pH = 4).
The results indicated that all STEC strains examined, were acid tolerant at pH as low as 2.5. In all cases, except E. coli O26:H11, it took more than 8 h until viability disappeared (Fig 3A). Only E. coli O91:H21 was able to survive longer than 24 h at pH 3.0 (data not shown). Various serotypes showed different behaviour throughout the experiments at pH 2.5 and pH 3.0 (p < 0.05) (Fig 3A and 3B). E. coli O91:H21 was the most resistant autochthonous serotype after 510 minutes exposure to pH 2.5 and pH 3.0. On the contrary, E. coli O111:H- and O26:H11 were the most sensitive strains at these acidic conditions.
Figure 3.
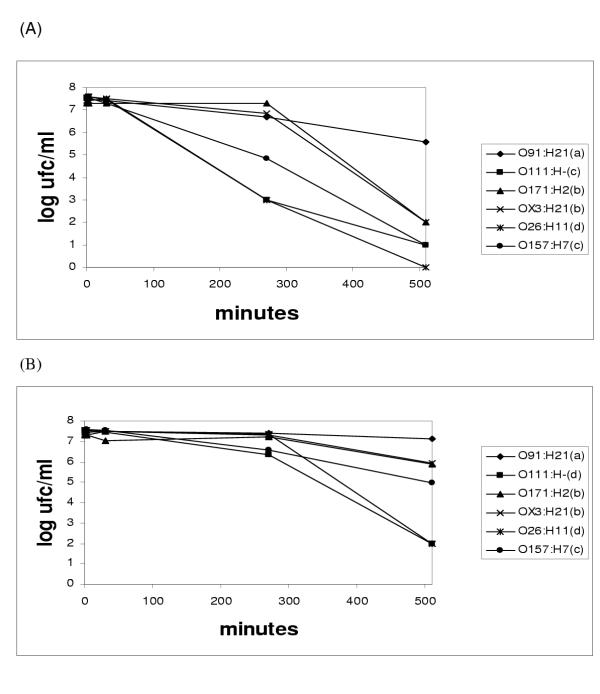
Survival of six STEC serotypes after 510 min exposure at pH 2.5 (A) and 3.0 (B) in LB broth. Strains followed by the same letter are not significantly different (p < 0.05). Resistance: a>b>c>d.
Effect of alcohol on viability of STEC
Within 24 h, 12% ethanol dramatically decreased the viability of all STEC tested. Viability of E. coli O91:H21 decreased at a slower rate (p < 0.05) than the remaining serotypes. All strains were resistant to 6% ethanol beyond 24 h (Fig. 4).
Figure 4.
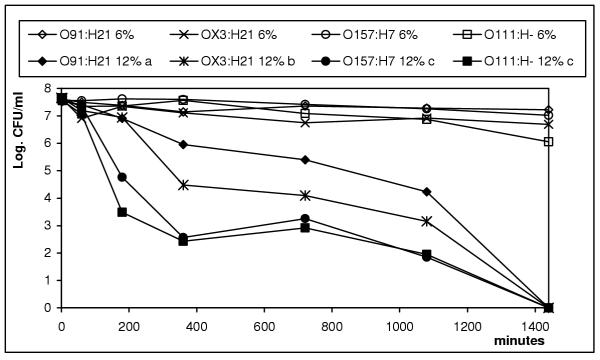
Survival of four STEC serotypes in 6% and 12% ethanol. Strains followed by the same letter are not significantly different (p < 0.05). Resistance: a>b>c.
Discussion
Argentina has the highest frequency of HUS (300–400 cases/year). Nevertheless, the incidence of E. coli O157:H7 is low in comparison to rates in other countries such as US and Canada, suggesting that O157:H7 is an uncommon serotype in the Argentina [3]. On the other hand, we have found a high percentage of non-O157 autochthonous strains, most of them isolated from cattle and foods [9].
The ability of the human pathogen E. coli O157:H7 to survive stress conditions encountered in various foods, in the stomach and in vitro assays, was studied by several researchers [13-19]. Despite this, there is still a lack of knowledge on several non-O157:H7 STEC known as the leading cause of HUS [20].
The general stress response of E. coli is characterised by numerous alterations in cellular physiology and even morphology, which enhance survival by increasing cellular stress resistance and preventing cellular damage rather than by repairing it. The concept of general stress response in E. coli has emerged from the analysis of the molecular process in stationary-phase cells [24].
The six STEC serotypes here assayed for viability at pH 2.5 and pH 3.0, were described as enterohaemorrhagic E. coli strains isolated from patients with HUS or HC [1,25]. Their extreme acid survival, which is defined as survival at pH below the growth range [21], and the acid limit for growth are considered to be two relevant properties for a bacterium to resist environment aggression.
We compared the abilities of E. coli serotypes to either grow or survive at low pH in Luria Bertani (LB) broth acidified with HCl. Our study showed that all STEC tested can grow at pH higher than 4.0. These findings differ from those obtained by Lin et al. [21] possibly because we did not buffer the LB broth, allowing pH to change freely. The pH of the culture medium rose to neutrality during the experiment at pH 4, and the growth reached stationary phase within 24 h. In each case, rising pH was preceded by biomass increase; in agreement with Lazar et al. [26], since the main carbon source in LB medium are peptides and amino acids, these cultures become alkaline in the stationary phase due to the release of excess amine-containing compounds.
The results indicated that the six STEC strains were acid tolerant at pH as low as 2.5 and in all cases, it took at least 8 h until viability disappeared. Normal fasting stomach conditions are pH below 3 and gastric emptying time is less than 2 h. As a consequence, all serotypes tested here would probably resist those conditions.
None of the serotypes assayed, except O91:H21, was able to survive longer than 24 h at pH 3. These findings are relevant to the food industry, which frequently relies upon the acid condition to inactivate food pathogens.
After 510 minutes exposure to pH 2.5 or pH 3.0, E. coli O91:H21 was the most resistant serotype. The autochthonous most sensitive strain corresponded to E. coli O26:H11 and O111:H-, in coincidence with the results obtained by Benjamin and Datta [11], who found two O111:H- strains with a very low acid stress resistance.
At 12% ethanol that is a common level in many beverages such as wine, the viability of all STEC strains was dramatically reduced within 24 h. Under this level, E. coli O91:21 was the most resistant strain. Viability however was not greatly affected by 6% ethanol.
In this study we noted resistance of some autochthonous STEC strains to acidic and alcoholic stress. The stress response of some serotypes isolated in Argentina was shown to be higher than the corresponding to E. coli O157:H7.
Finally, our results demonstrated the importance of STEC serotypes other than O157:H7 for laboratory food challenge studies, HUS prevention and control strategies in Argentina.
Methods
Bacterial strains and growth conditions
E. coli strains are listed in Table 1. They had been isolated from cattle or meat in Argentina [9] except an O157:H7 strain that was isolated from a human stool sample. Bacteria were incubated for 16 to 18 h at 37°C in LB broth medium (Yeast extract, Casein peptone, Sodium chloride, Merck) reaching the stationary phase under these conditions.
Table 1.
Sources of Escherichia coli strains
| Serotype | stx | Source |
| O157:H7a | stx2 | Human |
| O113:H21a | stx2 | Hamburger |
| O88:H21 | stx2 | Hamburger |
| O91:H21a | stx1/stx2 | Hamburger |
| O171:H2a | stx2 | Hamburger |
| O116:H21a | stx2 | Hamburger |
| OX3:H21a | stx2 | Hamburger |
| O117:H7a | stx2 | Hamburger |
| O26:H11 | stx2 | Bovine |
| O111:H- | stx1 | Bovine |
a Shared between cattle and meat. stx: Shiga toxin gene.
Acid growth limit and viability of STEC at acidic pH
The kinetics of acid sensitivity of STEC was studied in culture medium. LB broth was adjusted to the desired pH with HCl (pH 2.5; 3.0; 4.0; 5.0; 6.0 and 7.0) and autoclaved. Fresh overnight cultures were diluted 1:50 in 25 ml of this medium and incubated at 37°C with gentle shaking. From the start of incubation (time 0), samples were periodically withdrawn, and the optical density (OD) determined. The pH of LB broth was measured through the experiment.
Acid resistance was studied for six STEC strains (O26:H11, O91:H21, O111:H-, O157:H7, O171:H2 and OX3:H21) that were selected on the basis of their behaviour at pH 4. The bacterial population at pH 2.5 and 3.0 was determined by plating on LB-agar plates 0.1 ml portions of appropriately diluted cultures and incubating at 37°C for 24 h. Finally, the end of viability was determined by extending the time of the experiment until no CFU were detected.
Effect of alcohol on STEC viability
E. coli O157:H7, O91:H21, O111:H-, and OX3:H21, strains were used in this experiment. Stationary phase fresh overnight cultures were diluted 1:50 in 25 ml of sterile LB broth supplemented with ethanol up to either 6% vol/vol or 12% vol/vol, and incubated at 37°C with gentle shaking. Aliquots were taken from all cultures at appropriate intervals and serially diluted in saline, plated on LB-agar and incubated 24 h at 37°C with the aim to detect survival limit.
Statistical analysis
Data from each independent experiment (pH 2.5; pH 3.0; ethanol 6%; ethanol 12%) were analysed using the General Lineal Models procedures of SAS [27]. Each experiment was performed in triplicate. The least significant difference test was used to determine significant (p < 0.05) differences between STEC serotypes.
Authors' contributions
P. M. Molina carried out the survival studies and participated in drafting the first version of the manuscript.
A. M. Parma contributed in the design of this study and drafted the final version of the manuscript.
M. E. Sanz carried out the isolation and genetic characterization of the bacterial strains.
Acknowledgments
Acknowledgements
Authors thank María R. Ortiz for her technical assistance, Claudia Marinelli for her statistical support and Dr. Michael Wade for commenting on the manuscript. This work was supported by SECYT-UNCPBA, CIC and FONCYT. P. M. Molina is a holder of a fellowship from FONCYT. A. E. Parma is a member of the Scientific Researcher Career of CIC Prov. Buenos Aires.
Contributor Information
Pablo M Molina, Email: pablo-m@vet.unicen.edu.ar.
Alberto E Parma, Email: aparma@vet.unicen.edu.ar.
Marcelo E Sanz, Email: msanz@vet.unicen.edu.ar.
References
- Nataro JP, Kaper B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianantonio C, Vitacco M, Mendilaharzu FG, Gallo E, Sojo ET. The hemolytic uremic syndrome. Nephron. 1973;11:92–174. doi: 10.1159/000180229. [DOI] [PubMed] [Google Scholar]
- López EL, Contrini MM, de Rosa MF. Epidemiology of shiga toxin-producing Escherichia coli in South America. In: Kaper JB, O'Brien AD, editor. In Escherichia coli O157:H7 and other shiga toxin-producing E coli strains. Washington DC. American Society for Microbiology; 1998. pp. 30–37. [Google Scholar]
- Parma AE, Viñas MR, Sanz ME. Improvement of the polymerase chain reaction to detect Escherichia coli Shiga-like toxin II gene from clinical isolates. J Microbiol Methods. 1996;26:81–85. [Google Scholar]
- Rivas M, Voyer L, Tous M, de Mena MF, Leardini N, Wainztein R, Callejo R, Quadri B, Corti B, Prado V. Verocytotoxin-producing Escherichia coli infection in family members of children with hemolytic uremic syndrome. Medicina. 1996;56:119–125. [PubMed] [Google Scholar]
- Riley LW. The epidemiological, clinical, and microbiologic features of haemorrhagic colitis. Ann Rev Microbiol. 1987;41:383–407. doi: 10.1146/annurev.mi.41.100187.002123. [DOI] [PubMed] [Google Scholar]
- Riley LW, Remis RS, Helgerson SD. Haemorrhagic colitis associated with a rare Escherichia coli serotypes. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Clarke RC, Wilson JB, Read SC, Rahn K, Renwick SA, Sandhu KA, Alves D, Karmali MA, Lior H, et al. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J Food Prot. 1996;59:1112–1122. doi: 10.4315/0362-028X-59.10.1112. [DOI] [PubMed] [Google Scholar]
- Parma AE, Sanz ME, Blanco JE, Blanco J, Viñas MR, Blanco M, Padola NL, Etcheverría AI. Virulence genotypes and serotypes of verotoxigenic Escherichia coli isolated from cattle and foods in Argentina. Importance in public health. Eur J Epidemiol. 2000;16:757–762. doi: 10.1023/a:1026746016896. [DOI] [PubMed] [Google Scholar]
- Watterman SR, Small PLC. Characterization of the acid resistance phenotype and rpoS alleles of Shiga-Like Toxin-Producing Escherichia coli. Infect Immun. 1996;64:2808–2811. doi: 10.1128/iai.64.7.2808-2811.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin MM, Datta AR. Acid tolerance of enterohaemorrhagic Escherichia coli. Appl Environ Microbiol. 1995;61:1669–1672. doi: 10.1128/aem.61.4.1669-1672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden J, Small PLC. Acid resistance in enteric bacteria. Infect Immun. 1993;61:364–367. doi: 10.1128/iai.61.1.364-367.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyer GJ, Wang L, Johnson EA. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl Environ Microbiol. 1995;61:3752–3755. doi: 10.1128/aem.61.10.3752-3755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Raouf UM, Beuchant LR, Ammar MS. Survival and growth of Escherichia coli O157:H7 on salad vegetables. Appl Environ Microbiol. 1993;59:1999–2006. doi: 10.1128/aem.59.7.1999-2006.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzinski L, Harrison M. Infuence of incubation conditions on survival and acid tolerance response of Escherichia coli O157:H7 and non-O157:H7 isolates exposed to acetic acid. J Food Prot. 1998;61:542–546. doi: 10.4315/0362-028x-61.5.542. [DOI] [PubMed] [Google Scholar]
- Miller LG, Kaspar CW. Escherichia coli O157:H7 Acid Tolerance and Survival in Apple Cider. J Food Prot. 1994;57:460–464. doi: 10.4315/0362-028X-57.6.460. [DOI] [PubMed] [Google Scholar]
- Small P, Blankenhorn P, Welty D, Zinser E, Slonczewski J. Acid and base resistance in Escherichia coli and Shigella flexneri: role or rpo S and growth pH. J Bacteriol. 1994;176:1729–1737. doi: 10.1128/jb.176.6.1729-1737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Doyle M, Besser R. Fate of Enterohemorrhagic Escherichia coli O157:H7 in apple cider with and without preservatives. Appl Environ Microbiol. 1993;59:2526–2530. doi: 10.1128/aem.59.8.2526-2530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Doyle M. Fate of Enterohemorrhagic Escherichia coli O157:H7 in commercial mayonnaise. J Food Prot. 1994;57:780–783. doi: 10.4315/0362-028X-57.9.780. [DOI] [PubMed] [Google Scholar]
- Duffy G. Survival and growth of VTEC, an EU concerted action meeting held in Athens. Notiziario dell'Instituto Superiore di Sanità. 1999;12:3. [Google Scholar]
- Lin J, Lee IS, Frey J, Slonczewski JL, Foster JW. Comparative analysis of extreme acid survival in Salmonella typhymurium, Shigella flexneri and Escherichia coli. J Bacteriol. 1995;177:4097–4104. doi: 10.1128/jb.177.14.4097-4104.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW. Microbial responses to acid stress. In: Storz G, Hengge-Aronis R, editor. In Bacterial Stress Response. Washington DC: American Society for Microbiology; 2000. pp. 99–116. [Google Scholar]
- Jordan SL, Glover J, Malcom L, Thomson-Carter FM, Booth IR, Park. SF. Augmentation of killing of Escherichia coli O157 by combinations of lactate, ethanol and low-pH conditions. Appl Environ Microbiol. 1999;65:1308–1311. doi: 10.1128/aem.65.3.1308-1311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henge-Aronis R. The general stress response in Escherichia coli. In: Storz G, Hengge-Aronis R, editor. In Bacterial Stress Response. Washington DC: American Society for Microbiology; 2000. pp. 99–116. [Google Scholar]
- Whittam TS. Evolution of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. In: Kaper JB, O'Brien AD, editor. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington DC. American Society for Microbiology; 1998. pp. 189–209. [Google Scholar]
- Lazar SW, Almiron M, Tormo A, Kolter R. Role of the Escherichia coli SurA Protein in Stationary-Phase Survival. J Bacteriol. 1998;180:5704–5711. doi: 10.1128/jb.180.21.5704-5711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc . Cary, NC:SAS Institute Inc. Fourth Vol. 2. 1989. SAS/STAT User's Guide, Version 6. [Google Scholar]


