Abstract
Interoceptive feedback signals from the body are transmitted to hypothalamic neurons that control pituitary hormone release. This review article describes the organization of central neural pathways that convey ascending visceral sensory signals to endocrine neurons in the paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus in rats. A special emphasis is placed on viscerosensory inputs to corticotropin releasing factor (CRF)-containing PVN neurons that drive the hypothalamic-pituitary-adrenal axis, and on inputs to magnocellular PVN and SON neurons that release vasopressin (AVP) or oxytocin (OT) from the posterior pituitary. The postnatal development of these ascending pathways also is considered.
Introduction
Interoceptive feedback signals from the body are conveyed to widely distributed regions of the central nervous system (CNS), including regions of the hypothalamus and limbic forebrain that initiate and modulate autonomic and endocrine homeostatic processes. This review article describes the organization of central neural pathways that transmit sensory signals from thoracic and abdominal viscera to endocrine neurons in the paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus. A special emphasis is placed on viscerosensory inputs to parvocellular corticotropin releasing factor (CRF)-containing PVN neurons that control anterior pituitary release of adrenocorticotropic hormone (ACTH), and on inputs to magnocellular PVN and SON neurons that release vasopressin (AVP) or oxytocin (OT) from their axon terminals in the posterior pituitary. The postnatal development of these ascending pathways also is considered. In laboratory rats, central visceral sensory pathways undergo a significant amount of synaptic assembly and refinement during the first two weeks of postnatal life. The anatomical and functional maturation of interoceptive inputs to the endocrine hypothalamus appears to parallel the organism's newly emerging ability to respond physiologically to certain environmental challenges.
Visceral sensory inputs to spinal cord and caudal brainstem
Sensory signals from the viscera are carried to the CNS by spinal and cranial afferents. In rats, spinal viscerosensory afferents terminate in laminae I–VII of the dorsal horn and intermediate zone, and in lamina X around the central canal [1]. Inputs from visceral and somatic sensory afferents converge onto common second order neurons within the spinal dorsal horn. A subset of these neurons convey the convergent somato- and viscerosensory signals to the diencephalon via the anterolateral spinothalamic tract. A separate spinal viscerosensory pathway relays through lamina X, medial lamina VII, and the dorsal gray commissure to ascend at the junction of the gracile and cuneate fasciculi [2; 3]. This viscerotopically organized pathway provides direct and relayed inputs to the medullary nucleus of the solitary tract (NST), ventrolateral medulla (VLM), pontine parabrachial nucleus (PBN), and hypothalamus [2; 3; 4; 5; 6; 7; 8]. Although spinal inputs to the hypothalamus do not appear to directly target endocrine neurons, they synapse within regions of the lateral hypothalamus that do [9].
In addition to relayed spinal sensory inputs, the NST receives direct sensory inputs from thoracic and abdominal viscera via glossopharyngeal and vagal cranial afferents. Glossopharyngeal and vagal sensory neurons innervate the heart and associated cardiovascular targets [10], and vagal sensory neurons innervate the gut and associated digestive viscera from the oral cavity through the colon [11; 12; 13]. Central glossopharyngeal and vagal afferent fibers enter the dorsolateral medulla via multiple sensory rootlets and converge within the solitary tract, analogous to the spinal trigeminal tract or Lissauer's tract, which conveys the viscerosensory axons along the rostrocaudal length of the NST [11].
Central visceral sensory pathways to the endocrine hypothalamus
As summarized in Figure 1, visceral sensory signals are relayed from the NST to the endocrine hypothalamus via direct and indirect ascending pathways [14; 15; 16; 17]. The indirect pathways include relays from the area postrema (AP) and NST to the VLM [16; 18; 19; 20] and PBN [21; 22; 23; 24]. The AP is a circumventricular organ with a reduced or absent blood-brain barrier. Projections from AP neurons to the subjacent NST and also directly to the VLM and PBN [22; 23; 25] provide a neural route for blood-borne signals (e.g., cytokines, osmolytes, hormones, toxins) to affect central viscerosensory processing.
Figure 1.
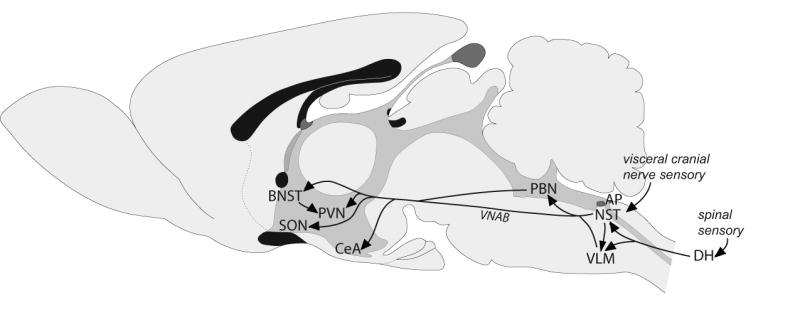
Schematic of ascending pathways (arrows) through which visceral sensory signals from the spinal cord and caudal brainstem reach the hypothalamus and limbic forebrain. Multiple interconnections among these brain regions are not shown, including reciprocal connections between the CeA and BNST, and descending projections from the hypothalamus and limbic forebrain to the PBN, NST and VLM. See abbreviation list.
The NST, VLM and PBN each contain neurons that project directly to the hypothalamus [14; 16; 26; 27; 28], although PBN inputs to the PVN and SON are relatively sparse. PVN and SON endocrine neurons receive direct synaptic input from the NST and VLM, but receive little or no direct input from the PBN [28; 29; 30]. The PBN instead provides robust inputs to the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) [28; 31; 32], both of which also receive direct inputs from the NST and VLM. The CeA and BNST are highly interconnected [33], and the ventrolateral BNST densely innervates the medial parvocellular PVN (PVNmp) [34; 35]. Caudal VLM neurons that receive indirect input from arterial baroreceptors and from the cervical vagus nerve (via the NST) have long ascending axons that terminate directly on neurohypophyseal and tuberoinfundibular PVN neurons [30].
Viscerosensory recruitment of neural inputs to the endocrine hypothalamus
The ability of visceral sensory stimuli to affect pituitary hormone release has been appreciated for many years. Early studies demonstrated posterior pituitary release of antidiuretic and milk-ejection factors (i.e., AVP and OT, respectively) in rats after electrical stimulation of the central end of the cut vagus nerve [36; 37; 38; 39; 40]. A series of studies by Ueta and colleagues subsequently demonstrated that gastrointestinal and vagal stimulation promotes significant activation of magnocellular and parvocellular endocrine neurons within the PVN and SON in anesthetized rats [41; 42; 43]. Some of these experiments involved mechanical gastric distension or electrical stimulation of vagal afferents, while others involved pharmacological stimulation of gastrointestinal vagal afferents via systemic administration of cholecystokinin octapeptide (CCK).
Exogenously administered CCK provides a useful experimental tool with which to activate ascending visceral sensory pathways. Systemic CCK increases pituitary hormone release via CCK-1 receptor-mediated activation of vagal afferent inputs to the caudal medulla that recruit central ascending viscerosensory pathways to the hypothalamus [43; 44; 45; 46; 47; 48]. In 1986 it was reported that synthetic CCK administered peripherally at supraphysiological doses potently stimulates pituitary OT (but not AVP) secretion in rats [49]. The OT secretory responses to CCK administration were markedly attenuated after bilateral subdiaphragmatic vagotomy, after capsaicin-induced sensory vagotomy, or after systemic blockade of CCK-1 receptors [49; 50]. In rats, systemic CCK increases the firing rate of magnocellular OT neurons, and transiently inhibits the firing of magnocellular AVP neurons [51]. Plasma OT levels also are increased by gastric distension, which synergizes with exogenous CCK to further elevate plasma OT in rats [49]. In addition to increasing plasma OT levels, systemic CCK administration alters pituitary release of growth hormone and thyrotropin in adult male rats [52; 53; 54]. Interestingly, systemic CCK increases plasma levels of AVP but not OT in humans [55] and ferrets [56], and increases plasma levels of AVP, luteinizing hormone, gonadotropin-releasing hormone, and ACTH in rhesus macaques [57; 58; 59]. It is unclear why rats and mice release OT after CCK administration, whereas ferrets, primates, and rabbits (Dr. Loretta Flanagan-Cato, personal communication) release AVP instead. Although the reason for the species difference is unclear, the pattern of hormone release is consistent with known species differences in endocrine responses to stress. The doses of synthetic CCK used to stimulate endocrine secretion are nauseogenic and even emetic in humans, and promote sickness-like behavior and HPA axis activation in rats and other experimental animals. Rats and mice release OT along with ACTH during stress responses, whereas humans and non-human primates release ACTH and AVP.
The natural ligand for endogenous CCK-1 receptors is CCK that is released from the gut in a nutrient-dependent manner. Circulating plasma levels of endogenous CCK increase transiently in rats and other mammals (including humans) after food intake, representing peptide “spillover” into the systemic circulation after CCK is released locally near its sites of action within digestive tissues and along gastrointestinal vagal afferent fibers. Under normal circumstances, endogenous CCK acts at its receptors to initiate and maintain various digestive processes, and also to promote satiety. Although a very large or calorically dense meal might produce homeostatic stress, it is unlikely that endogenous CCK released after a typical meal activates the same ascending visceral pathways as are activated by synthetic CCK, and normal meal-related satiety may have nothing to do with the ability of CCK or gastric distension to stimulate hypothalamic endocrine neurons. Indeed, the ability of CCK to promote satiety seems to require only neural circuits that are contained within the caudal brainstem [47; 60; 61]. Thus, it remains unclear whether either endogenously released CCK or natural meal-related gastric distension contributes to recruitment of hypothalamic endocrine neurons. As discussed further, below, it has been shown that voluntary intake of a large meal by rats activates only a subset of the NST visceral sensory neurons that are activated after systemic CCK or other “interoceptive stress” treatments [62]. Nevertheless, the ability of synthetic CCK, mechanical gastric distension, and vagal nerve stimulation to alter pituitary hormone secretion provides compelling evidence for the sufficiency of visceral sensory pathways to recruit the endocrine hypothalamus.
Viscerosensory inputs to the endocrine hypothalamus are primarily noradrenergic
Viscerosensory projections from the NST and VLM to the PBN, hypothalamus, CeA, and BNST are primarily (although not exclusively) catecholaminergic, arising from nor/adrenergic A2/C2 neurons within the NST and A1/C1 neurons within the VLM [19; 26; 63]. For convenience, in this article catecholaminergic NST and VLM projection neurons are referred to collectively as noradrenergic (NA) neurons, because they all are immunoreactive for the NA synthetic enzyme dopamine beta hydroxylase (DbH). Various peptides are co-expressed by these NA neurons, including those that project directly to the PVN and SON [48; 64; 65; 66].
Dense NA inputs to the PVN (Figure 2) and SON arise almost exclusively from the NST and VLM, with an additional restricted input to the periventricular PVN that arises from the pontine locus coeruleus [19; 26; 67]. The PVN and SON contain high densities of adrenergic receptors in subregions that receive input from the NST and VLM [19; 67; 68]. Ascending NA projections from the NST and VLM course primarily through the ventral noradrenergic ascending bundle (VNAB) (Figure 1) [19; 67]. Electrical stimulation of the VNAB elicits CRF secretion through an adrenergic receptor-mediated mechanism [69], and increases plasma levels of ACTH. Similarly, direct stimulation of the NST and VLM increases PVN neuronal firing and pituitary secretion [70; 71], and these effects are attenuated by prior destruction of NA terminals within the PVN [70].
Figure 2.
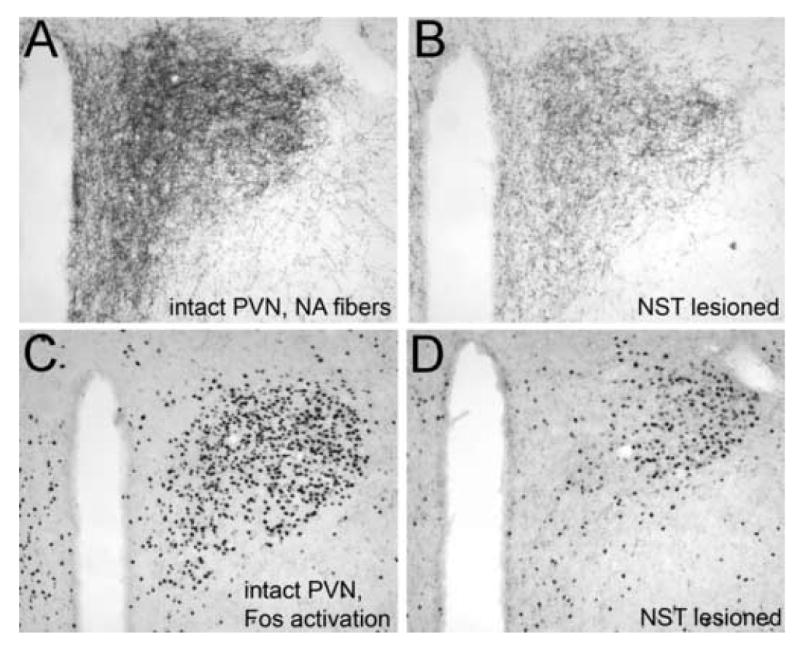
Immunoperoxidase labeling of DbH-positive NA fibers within the PVN in an intact adult rat (A) and in a rat following toxin-induced destruction of NA neurons within the NST (B). Systemic administration of LiCl (0.15M, 2% BW, i.p.) induces robust neural Fos expression within the medial parvocellular and lateral magnocelluar PVN of the intact rat (C), but attenuated Fos expression within the medial parvocellular PVN of the toxin-lesioned rat (D). Remaining NA fibers presumably arise from the VLM and from residual non-lesioned NST neurons, although the LC may contribute NA inputs to the periventricular region. See abbreviation list.
Peripheral visceral stimuli also recruit activation of medullary NA neurons that project to the hypothalamus and limbic forebrain, coincident with increased NA release at target sites. For example, extracellular NA content increases in the PVN after systemic administration of CCK, which, as reviewed above, activates vagal sensory inputs to the NST and stimulates pituitary release of several hormones. Systemic CCK activates NA neurons within the NST and VLM in rats [48], ferrets [56], and rhesus macaques [72]. In rats, NA neurons activated after CCK administration include those that project directly to the PVN [46], and immunotoxin-induced destruction of these NA neurons attenuates the ability of systemic CCK to activate Fos expression within PVN endocrine neurons [27]. A CCK-induced increase in NA content measured within the PVN directly parallels increased plasma levels of ACTH and OT in rats, consistent with the predominantly excitatory effects of systemic CCK and synaptic effects of NA on hypothalamic CRF and OT neurons [73; 74; 75; 76]. Voluntary food intake also promotes NA neural activation within the NST and VLM in rats, and the proportion of NA neurons activated in both regions increases in proportion with the size of the meal consumed [77]. However, it is unclear whether food intake and exogenous CCK activate the same medullary NA neurons, including those that project to the hypothalamus.
Within the PVN neuropil, NA terminals have been reported to form both symmetric- and asymmetric-type synapses with the dendrites and somata of presumptive endocrine neurons, including ones immunoreactive for OT, AVP, CRF, and thyrotropin releasing hormone [78; 79; 80; 81; 82; 83; 84; 85; 86; 87; 88; 89]. Magnocellular subregions of the PVN that control AVP and OT release from the posterior pituitary appear to be preferentially targeted by NA inputs arising in the VLM, whereas NA projections arising in the NST appear to preferentially target magnocellular OT neurons within the PVN and SON [90], and CRF neurons within the PVNmp [30; 91]. CRF neurons summate excitatory and inhibitory inputs into a net secretory signal to drive ACTH release from anterior pituitary corticotrophs [92; 93]. Thus, CRF neurons within the PVNmp control basal, circadian, and stress-induced glucocorticoid secretion via the HPA axis [94; 95; 96; 97]. Ample evidence indicates that NA inputs provide critical control over the activity of these stress-responsive CRF neurons [95; 98; 99; 100; 101].
Recent findings indicate that NA inputs to the PVNmp are provided by NST and/or VLM neurons with collateralized inputs to the BNST [102]. Immunotoxic lesioning methods have demonstrated that these NA inputs are necessary for systemic yohimbine (a sympathomimetic adrenergic signal-enhancing drug) to activate neural expression of the immediate-early gene product, Fos, in CRF neurons within the PVNmp, and to increase plasma corticosterone levels [102]. Similar preliminary results have been obtained in toxin-lesioned rats subsequently treated with systemic lithium chloride (LiCl) (Figure 2). Conversely, inputs to magnocellular regions of the PVN and SON appear to arise from medullary NA neurons that do not also project to either the BNST or the PVNmp [102]. This finding complements previous evidence that magnocellular and parvocellular PVN subdivisions are differentially innervated by NA inputs arising from the VLM and NST, respectively [91]. In addition to its direct NA input, the PVNmp receives a dense projection from neurons within the same BNST region [35] that is heavily innervated by medullary NA neurons [103; 104]. Thus, NA-mediated viscerosensory modulation of neural function within the endocrine PVN includes direct inputs from medullary NA neurons, and indirect inputs that are relayed through the BNST.
Non-catecholaminergic viscerosensory inputs to the hypothalamus
Ascending NA projections to the hypothalamus and limbic forebrain are paralleled by projections arising from a separate and smaller population of non-NA neurons with widely arborizing axon terminals, whose cell bodies are located in the caudal NST and adjacent reticular formation. These neurons appear to coexpress immunoreactivity for multiple peptides, including glucagon-like peptide 1 (GLP-1) [63; 90; 105; 106; 107; 108]. The synaptic targets of GLP-1 neurons include CRF neurons in the PVNmp [109] and OT neurons in the magnocellular PVN and SON (Figure 3), where GLP-1 binding sites and receptor gene expression are localized [110; 111; 112; 113; 114]. Cells in the caudal NST and adjacent reticular formation provide the sole source of endogenous ligand for these GLP-1 receptors [90; 106], and ascending GLP-1 fiber projections appear to follow the VNAB, intermingled with NA fibers (Figure 1).
Figure 3.
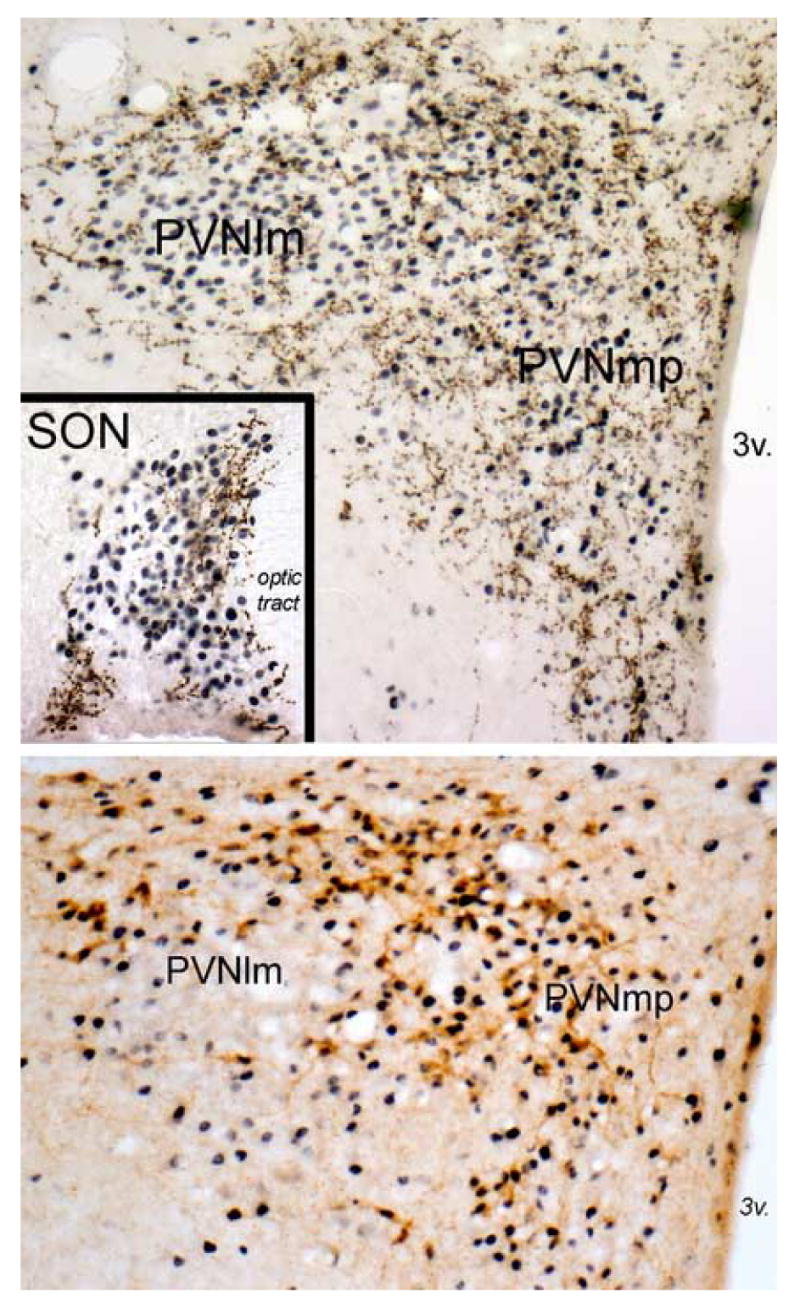
The top panel and inset depict immunoperoxidase labeling of GLP-1 immunopositive fibers (brown) within the PVN and SON (inset) in an adult rat after systemic administration of LiCl (0.15M, 2% BW, i.p.). Robust LiCl-induced neural Fos expression (blue-black nuclear label) is present throughout the PVN, including the PVNmp and PVNlm. GLP-1-positive fibers are largely absent within the core of the PVNlm, where AVP-positive neurons cluster in rats. Instead, GLP-1-positive fibers are distributed around the perimeter of the PVNlm, where magnocellular OT neurons predominate, and throughout the PVNmp, where parvocellular CRF and OT neurons predominate. LiCl-induced Fos expression also is prevalent throughout the SON (inset), where GLP-1-positive fibers cluster within the dorsal and medial SON where magnocellular OT neurons predominate. The lower panel depicts immunoperoxidase labeling of CRF-positive neurons (brown) within the PVN in an adult rat perfused 90 min after intracerebroventricular infusion of 1.0 μg of synthetic GLP-1-(7–36) amide. Fos expression is robust within the PVNmp, including activation of the majority of CRF-positive neurons. Conversely, Fos is largely absent within the core of the PVNlm, where magnocellular AVP neurons predominate. 3v, third ventricle. See abbreviation list.
Central infusions of GLP-1 or GLP-1 receptor agonists activate PVN neurons (Figure 3) and increase plasma levels of ACTH and OT [115; 116; 117; 118; 119; 120; 121; 122]. Several different interoceptive stressors (e.g., systemic CCK, LiCl, lipopolysaccharide) activate Fos expression by medullary GLP-1 neurons, including those that project to the PVN [62]. Other studies have demonstrated that central GLP-1 receptor signaling pathways contribute to the ability of these stressors to recruit the HPA axis [121; 123; 124]. Thus, “stressful” viscerosensory stimuli appear to recruit ascending GLP-1-containing neural pathways in a manner similar to the recruitment of ascending NA pathways. Interestingly, although medullary NA neurons are activated in rats after food intake [77], voluntary ingestion of even a very large meal does not activate GLP-1 neurons [62]. Mechanical gastric distension can activate Fos expression in GLP-1 neurons [125], evidence that they do respond to gastric sensory input if stimulation is robust enough. Recruitment of hindbrain GLP-1 neurons, including those that innervate the hypothalamus, may depend on the intensity or modality of visceral stimulation, and may even differentiate stressful from non-stressful visceral stimuli [62].
Postnatal development of viscerosensory inputs to the endocrine hypothalamus
The natural process of neural circuit development offers special opportunities to probe the functional organization of ascending viscerosensory pathways. Developmental research in this area has been fairly limited, but the results are intriguing. The first few postnatal weeks in rats correspond to a so-called “stress hyporesponsive period” (SHRP) characterized by reduced or absent HPA axis responsiveness to certain types of stressors [126; 127; 128], including interoceptive stressors [47; 129; 130]. The causal factors underlying the SHRP remain controversial, and are likely to be multiple and complex [129; 131; 132; 133]. It appears that the SHRP is not simply due to an inability of hypothalamic, anterior pituitary, and/or adrenal components of the HPA axis to respond to input, because certain stimuli can increase hypothalamic Fos expression, activate the HPA axis, and increase plasma levels of ACTH, cortisol, OT and AVP in rat pups during the SHRP [128; 129; 134]. We have proposed that the SHRP is at least partially due to structural and/or functional immaturity of ascending NA (and perhaps GLP-1-containing) neural pathways that carry visceral sensory inputs from the caudal medulla to the PVN.
Early catecholamine histofluorescence studies suggested that NA inputs to the hypothalamus and limbic forebrain are essentially absent in rats at birth [135; 136]. More sensitive immunocytochemical techniques and tract-tracing later demonstrated that NA projections from the NST and VLM to the PVN actually are already present in newborn rats [130]. However, the density of NA terminals in the PVN increases markedly during the first three weeks of postnatal development [130], in concert with increasing levels of hypothalamic NA [137; 138]. Additional evidence for delayed maturation of ascending NA inputs is offered by analysis of fiber immunolabeling for prolactin releasing peptide (PrRP), which is co-expressed by a subset of NA neurons within the NST [66; 139]. The NST is the only source of PrRP-positive fibers within the CNS; thus, immunohistochemical detection of PrRP-positive fibers provides a useful and discrete marker for ascending NST projections during development. PrRP-positive fibers are first visible within the ventral lateral BNST on postnatal day (P) 3 [140]. By P6, PrRP fibers are present within the PVN and other forebrain targets, although their density is not as great at this early time point as in adult rats [140].
The apparent structural immaturity of ascending NA inputs to the PVN in neonatal rats predicts that neurons within the developing hypothalamus are less sensitive to viscerosensory signals in neonatal vs. adult rats. Indeed, although systemic CCK activates neurons within the caudal brainstem [47] and suppresses independent ingestion in neonatal rats [141; 142; 143], CCK does not activate Fos expression in the hypothalamus or other forebrain regions, and does not stimulate pituitary hormone release in neonatal rats [47]. The ability of exogenous CCK to engage medullary NST neurons and to suppress food intake in 2-day-old rats means that CCK-1 receptor-mediated activation of vagal afferent inputs to the NST and subsequent processes for recruitment of brainstem circuits that underlie CCK-induced hypophagia already are functional. This is not surprising, because anatomical tracing studies revealed a precocious development of vagal viscerosensory inputs to the NST during embryonic development [144]. In newborn rat pups, exogenous CCK causes a significant increase in the excitability of NST neurons that are synaptically activated by electrical stimulation of the subdiaphragmatic vagus nerve, and the increased sensitivity is blocked by a specific CCK-1 receptor antagonist [145]. A similar pattern of NST activation occurs in neonatal rats after more naturalistic feeding-induced vagal stimulation [146], although that study did not investigate hypothalamic activation after feeding in neonates.
The hindbrain distribution of neural Fos expression is virtually identical in 2-day-old and adult rats after CCK treatment, with activated neurons located in specific subregions of the NST that receive gastric vagal sensory input [47]. Conversely, the lack of hypothalamic activation in neonatal rats after CCK treatment is consistent with other evidence for delayed postnatal maturation of ascending NA projections from the NST and VLM [147] that transmit viscerosensory information from hindbrain to hypothalamus in adult rats [19; 26; 46; 148]. Another recent study investigated the postnatal maturation of central neural Fos responses to LiCl, a malaise-inducing agent [149]. Rat pups were injected i.p. with 0.15M LiCl (2% BW) or control solution (0.15M NaCl) at multiple time points between the day of birth (P0) and P28. Compared to Fos activation after control saline treatment, LiCl did not increase Fos in the PVN or other forebrain regions on P0, but did so on P7 and later. Maximal PVN Fos responses to LiCl were observed on P14, whereas LiCl-induced BNST activation continued to increase through P28. Comparable results have been obtained by others examining central Fos responses to an acute lipopolysaccharide challenge in developing rats [150]. These findings provide additional evidence that central interoceptive circuits in rats are not fully functional at birth, but instead show age-dependent increases in neural recruitment following viscerosensory stimulation.
Given the apparent inability of systemic CCK, LiCl, or lipopolysaccharide to activate hypothalamic neurons in neonatal rats, one might hypothesize that the endocrine hypothalamus is refractory to all excitatory inputs early in development. This turns out not to be the case. For example, acute osmotic dehydration robustly activates hypothalamic Fos expression and increases pituitary hormone levels in neonatal rats [134]. The important difference seems to be the ability of osmotic dehydration to activate PVN and SON neurons without requiring ascending viscerosensory inputs from the caudal brainstem [151]. Thus, the lack of neonatal hypothalamic responsiveness to CCK, LiCl, and other visceral sensory stimuli is most likely due to functional immaturity of ascending viscerosensory inputs from the NST and VLM to the hypothalamus.
Conclusion
Visceral sensory information reaches the endocrine hypothalamus via central neural pathways that are primarily, but not exclusively, noradrenergic. NA and complementary peptidergic (e.g., GLP-1) inputs to magnocellular and parvocellular endocrine neurons within the SON and PVN arise directly from medullary NST and VLM neurons, with additional viscerosensory inputs relayed through the pontine PBN and other central sites. The functional importance of these pathways for modulating and driving HPA axis and other endocrine responses to interoceptive stimuli has been demonstrated by experiments involving pathway lesioning and pharmacological blockade in adult rats, and through the natural process of neural development in neonatal rats.
Abbreviations
- ACTH
adrenocorticotropic hormone
- AP
area postrema
- AVP
arginine vasopressin
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdale
- CCK
cholecystokinin octapeptide
- CNS
central nervous system
- CRF
corticotropin-releasing factor (hormone)
- DbH
dopamine beta hydroxylase
- GLP-1
glucagon-like peptide 1
- LiCl
lithium chloride
- NA
noradrenergic
- NST
nucleus of the solitary tract
- OT
oxytocin
- P
postnatal day
- PBN
parabrachial nucleus
- PrRP
prolactin releasing peptide
- PVN
paraventricular nucleus of the hypothalamus
- PVNlm
lateral magnocellular PVN
- PVNmp
medial parvocellular PVN
- SHRP
stress hyporesponsive period
- SON
supraoptic nucleus of the hypothalamus
- VLM
ventrolateral medulla
- VNAB
ventral noradrenergic ascending bundle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Green T, Dockray GJ. Calcitonin gene-related peptide and substance P in afferents to the upper gastrointestinal tract in the rat. Neuroscience Letters. 1987;76:151–156. doi: 10.1016/0304-3940(87)90707-5. [DOI] [PubMed] [Google Scholar]
- 2.Willis JWD, Westlund KN. The role of the dorsal column pathway in visceral nociception. Current Pain and Headache Reports. 2001;5:20–26. doi: 10.1007/s11916-001-0006-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang CC, Willis WD, Westlund KN. Ascending projections from the area around the spinal cord central canal: a Phaseolus vulgaris leucoagglutinin study in rats. The Journal of Comparative Neurology. 1999;415:341–367. doi: 10.1002/(sici)1096-9861(19991220)415:3<341::aid-cne3>3.0.co;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cliffer KD, Burstein R, Giesler JGJ. Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. The Journal of Neuroscience. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menetrey D, dePommery J. Origins of spinal ascending pathways that reach central areas involved in visceroception and visceronociception in the rat. European Journal of Neuroscience. 1991;3:249–259. doi: 10.1111/j.1460-9568.1991.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 6.Potts JT, Lee SM, Anguelov PI. Tracing of projection neurons from the cervical dorsal horn to the medulla with the anterograde tracer biotinylated dextran amine. Autonomic Neuroscience: Basic and Clinical. 2002;98:64–69. doi: 10.1016/s1566-0702(02)00034-6. [DOI] [PubMed] [Google Scholar]
- 7.Menetrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. The Journal of Comparative Neurology. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 8.Berkley KJ, Scofield SL. Relays from the spinal cord and solitary nucleus through the parabrachial nucleus to the forebrain in the cat. Brain Research. 1990;529:333–338. doi: 10.1016/0006-8993(90)90847-5. [DOI] [PubMed] [Google Scholar]
- 9.Palkovits M. Interconnections between the neuroendocrine hypothalamus and the central autonomic system. Frontiers in Neuroendocrinology. 1999;20:270–295. doi: 10.1006/frne.1999.0186. [DOI] [PubMed] [Google Scholar]
- 10.Guyenet PG. The sympathetic control of blood pressure. Nature Reviews - Neuroscience. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 11.Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. Journal of Comparative Neurology. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 12.Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104:502–509. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. Journal of Comparative Neurology. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- 14.Saper CB. Central Autonomic System. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; San Diego: 2004. pp. 761–796. [Google Scholar]
- 15.Horst GJT, Boer PD, Luiten PGM, Willigen JDV. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- 16.Bailey TW, Hermes SM, Andresen MC, Aicher AA. Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns. The Journal of Neuroscience. 2006;26:11893–11902. doi: 10.1523/JNEUROSCI.2044-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Experimental Neurology. 2006;198:539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Kawano H, Masuko S. Neurons in the caudal ventrolateral medulla projecting to the paraventricular hypothalamic nucleus receive synaptic inputs from the nucleus of the solitary tract: a light and electron microscopic double-labeling study in the rat. Neuroscience Letters. 1996;218:33–36. doi: 10.1016/0304-3940(96)13115-3. [DOI] [PubMed] [Google Scholar]
- 19.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Research Reviews. 1982;4:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 20.Chan RKW, Peto CA, Sawchenko PE. A1 catecholamine cell group: fine structure and synaptic input from the nucleus of the solitary tract. Journal of Comparative Neurology. 1995;351:62–80. doi: 10.1002/cne.903510107. [DOI] [PubMed] [Google Scholar]
- 21.Kawai Y, Takagi H, Yanai K, Tohyama M. Adrenergic projection from the caudal part of the nucleus of the tractus solitarius to the parabrachial nucleus in the rat: immunocytochemical study combined with a retrograde tracing method. Brain Research. 1988;459:369–372. doi: 10.1016/0006-8993(88)90654-3. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. The Journal of Comparative Neurology. 1985;234:344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham ET, Jr, Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience. 1994;58:635–648. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 24.Milner TA, Joh TH, Pickel VM. Tyrosine hydroxylase in the rat parabrachial region: ultrastructural localization and extrinsic sources of immunoreactivity. The Journal of Neuroscience. 1986;6:2585–2603. doi: 10.1523/JNEUROSCI.06-09-02585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanca AJ, Dvd Kooy. A serotonin-containing pathway from the area postrema to the parabrachial nucleus in the rat. Neuroscience. 1985;14:1117–1126. doi: 10.1016/0306-4522(85)90281-7. [DOI] [PubMed] [Google Scholar]
- 26.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- 27.Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. The Journal of Neuroscience. 2003;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Research. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 29.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. The Journal of Comparative Neurology. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita H, Kannan H, Ueta Y. Involvement of caudal ventrolateral medulla neurons in mediating visceroreceptive information to the hypothalamic paraventricular nucleus. Progress in Brain Research. 1989;81:293–302. doi: 10.1016/s0079-6123(08)62018-x. [DOI] [PubMed] [Google Scholar]
- 31.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Research Reviews. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 32.Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Research Bulletin. 1993;30:163–172. doi: 10.1016/0361-9230(93)90054-f. [DOI] [PubMed] [Google Scholar]
- 33.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Annals of the New York Academy of Sciences. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- 34.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Hormones and Behavior. 2000;37:261–283. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 35.Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 36.Dyball REJ. Stimuli for the release of neurohypophysial hormones. British Journal of Pharmacology and Chemotherapy. 1968;33 doi: 10.1111/j.1476-5381.1968.tb00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang HC, Chia KF, Huang JJ, Lim RKS. Vagus-post-pituitary reflex; antidiuretic effect. Clinical Journal of Physiology. 1939;14:161–172. [Google Scholar]
- 38.Mills E, Wang SC. Liberation of antidiuretic hormone: location of ascending pathways. American Journal of Physiology. 1964;207:1399–1404. doi: 10.1152/ajplegacy.1964.207.6.1399. [DOI] [PubMed] [Google Scholar]
- 39.Mills E, Wang SC. Liberation of antidiuretic hormone: pharmacologic blockade of ascending pathways. American Journal of Physiology. 1964;207:1405–1410. doi: 10.1152/ajplegacy.1964.207.6.1405. [DOI] [PubMed] [Google Scholar]
- 40.Thieblot L, Duchene-Marullaz P, Berthelay J. Nouvelle preuve de la libération de principes posthypophysaires par l'excitation centripète du vague. Annales D'endocrinologie. 1957;18:651–653. [PubMed] [Google Scholar]
- 41.Ueta Y, Kannan H, Yamashita H. Gastric afferents to the paraventricular nucleus in the rat. Experimental Brain Research. 1991;84:487–494. doi: 10.1007/BF00230960. [DOI] [PubMed] [Google Scholar]
- 42.Jin Y, Ueta Y, Kannan H, Yamashita H. Synaptic inputs from the stomach to tuberoinfundibular neurons in the paraventricular nucleus of the hypothalamus in rats. Brain Research. 1993;617:151–154. doi: 10.1016/0006-8993(93)90627-y. [DOI] [PubMed] [Google Scholar]
- 43.Ueta Y, Kannan H, Higuchi T, Negoro H, Yamashita H. CCK-8 excites oxytocin-secreting neurons in the paraventricular nucleus in rats - possible involvement of noradrenergic pathway. Brain Research Bulletin. 1993;32:453–459. doi: 10.1016/0361-9230(93)90290-r. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz GJ, Moran TH. Integrative gastrointestinal actions of the brain-gut peptide cholecystokinin in satiety. Progress in Psychobiology and Physiological Psychology. 1998;17:1–34. [Google Scholar]
- 45.Day HEW, McKnight AT, Poat JA, Hughes J. Evidence that cholecystokinin induces immediate early gene expression in the brainstem, hypothalamus and amygdala of the rat by a CCKA receptor mechanism. Neuropharmacology. 1994;33:719–727. doi: 10.1016/0028-3908(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 46.Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. The Journal of Comparative Neurology. 1995;360:246–56. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- 47.Rinaman L, Hoffman GE, Stricker EM, Verbalis JG. Exogenous cholecystokinin activates cFos expression in medullary but not hypothalamic neurons in neonatal rats. Developmental Brain Research. 1994;77:140–145. doi: 10.1016/0165-3806(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 48.Rinaman L, Verbalis JG, Stricker EM, Hoffman GE. Distribution and neurochemical phenotypes of caudal medullary neurons activated to express cFos following peripheral administration of cholecystokinin. J Comp Neurol. 1993;338:475–90. doi: 10.1002/cne.903380402. [DOI] [PubMed] [Google Scholar]
- 49.Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–1419. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- 50.McCann MJ, Verbalis JG, Stricker EM. Capsaicin pretreatment attenuates multiple responses to cholecystokinin in rats. Journal of the Autonomic Nervous System. 1988;23:265–272. doi: 10.1016/0165-1838(88)90101-4. [DOI] [PubMed] [Google Scholar]
- 51.Renaud LP, Tang M, McCann MJ, Stricker EM, Verbalis JG. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. American Journal of Physiology. 1987;253:R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- 52.Peuranen E, Vasar E, Koks S, Volke V, Lang A, Rauhala P, Mannisto PT. Further studies on the role of cholecystokinin-A and B receptors in secretion of anterior pituitary hormones in male rats. Neuropeptides. 1995;28:1–11. doi: 10.1016/0143-4179(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 53.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 54.Millington WR, Mueller GP, Lavigne GJ. Cholecystokinin type A and type B receptor antagonists produce opposing effects on cholecystokinin-stimulated beta-endorphin secretion from the rat pituitary. The Journal of Pharmacology and Experimental Therapeutics. 1992;261:454–461. [PubMed] [Google Scholar]
- 55.Miaskiewicz SL, Stricker EM, Verbalis JG. Neurohypophyseal secretion in response to cholecystokinin but not meal-induced gastric distension in humans. Journal of Clinical Endocrinology and Metabolism. 1989;68:837–843. doi: 10.1210/jcem-68-4-837. [DOI] [PubMed] [Google Scholar]
- 56.Billig I, Yates BJ, Rinaman L. Plasma hormone levels and central cFos expression in ferrets after systemic administration of cholecystokinin. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2001;281:R1243–R1255. doi: 10.1152/ajpregu.2001.281.4.R1243. [DOI] [PubMed] [Google Scholar]
- 57.Schreihofer DA, Golden GA, Cameron JL. Cholecystokinin (CCK)-induced stimulation of luteinizing hormone (LH) secretion in adult male rhesus monkeys: examination of the role of CCK in nutritional regulation of LH secretion. Endocrinology. 1993;132:1553–1560. doi: 10.1210/endo.132.4.8462453. [DOI] [PubMed] [Google Scholar]
- 58.Verbalis JG, Richardson DW, Stricker EM. Vasopressin release in response to nausea-producing agents and cholecystokinin in monkeys. American Journal of Physiology. 1987;252:R749–R753. doi: 10.1152/ajpregu.1987.252.4.R749. [DOI] [PubMed] [Google Scholar]
- 59.Perera AD, Verbalis JG, Mikuma N, Majumdar SS, Plant TM. Cholecystokinin stimulates gonadotropin-releasing hormone release in the monkey (Macaca mulatta) Endocrinology. 1993;132:1723–1728. doi: 10.1210/endo.132.4.8462472. [DOI] [PubMed] [Google Scholar]
- 60.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Frontiers in Neuroendocrinology. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 61.Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. American Journal of Physiology. 1988;253:R853–R856. doi: 10.1152/ajpregu.1988.254.6.R853. [DOI] [PubMed] [Google Scholar]
- 62.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. American Journal of Physiology. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 63.Sawchenko PE, Benoit R, Brown MR. Somatostatin 28-immunoreactive inputs to the paraventricular and supraoptic nuclei: principal origin from non-aminergic neurons in the nucleus of the solitary tract. Journal of Chemical Neuroanatomy. 1988;1:81–94. [PubMed] [Google Scholar]
- 64.Sawchenko PE, Swanson LW, Grzanna R, Howe PRC, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nculeus of the hypothalamus. The Journal of Comparative Neurology. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 65.Khanna S, Sibbald JR, Day TA. Neuropeptide Y modulation of A1 noradrenergic input to supraoptic vasopressin cells. Neuroscience letters. 1993;161:60–64. doi: 10.1016/0304-3940(93)90140-g. [DOI] [PubMed] [Google Scholar]
- 66.Chen CT, Dun SL, Dun NJ, Chang JK. Prolactin-releasing peptide-immunoreactivity in A1 and A2 noradrenergic neurons of the rat medulla. Brain Research. 1999;822:276–279. doi: 10.1016/s0006-8993(99)01153-1. [DOI] [PubMed] [Google Scholar]
- 67.Cunningham JET, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. Journal of Comparative Neurology. 1990;292:651–667. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- 68.Miyahara S, Oomura Y. Inhibitory action of the ventral noradrenergic bundle on the lateral hypothalamic neurons through alpha-noradrenergic mechanisms in the rat. Brain Research. 1982;234:459–463. doi: 10.1016/0006-8993(82)90887-3. [DOI] [PubMed] [Google Scholar]
- 69.Plotsky P. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987;121:924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- 70.Day T, Ferguson AV, Renaud L. Noradrenergic afferents facilitate the activity of tuberoinfundibular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1985;41:17. doi: 10.1159/000124148. [DOI] [PubMed] [Google Scholar]
- 71.Kannan H, Osaka T, Kasai M, Okuya S, Yamashita H. Electrophysiological properties of neurons in the caudal ventrolateral medulla projecting to the paraventricular nucleus of the hypothalamus in rats. Brain Research. 1986;376:342. doi: 10.1016/0006-8993(86)90197-6. [DOI] [PubMed] [Google Scholar]
- 72.Schreihofer DA, Cameron JL, Verbalis JG, Rinaman L. Cholecystokinin induces Fos expression in catecholaminergic neurons of the macaque monkey caudal medulla. Brain Res. 1997;770:37–44. doi: 10.1016/s0006-8993(97)00732-4. [DOI] [PubMed] [Google Scholar]
- 73.Day TA, Ferguson AV, Renaud LP. Facilitatory influence of noradrenergic afferents on the excitability of rat paraventricular nucleus neurosecretory cells. Journal of Physiology. 1984;355:237–249. doi: 10.1113/jphysiol.1984.sp015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kendrick K, Leng G, Higuchi T. Noradrenaline, dopamine and serotonin release in the paraventricular and supraoptic nuclei of the rat in response to intravenous cholecystokinin injections. Journal of Neuroendocrinology. 1991;3:139–144. doi: 10.1111/j.1365-2826.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 75.Verbalis JG, Stricker EM, Robinson AG, Hoffman GE. Cholecystokinin activates cFos expression in hypothalamic oxytocin and corticotropin-releasing hormone neurons. Journal of Neuroendocrinology. 1991;3:205–213. doi: 10.1111/j.1365-2826.1991.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 76.Kaneyuki T, Morimasa T, Shohmori T. Action of peripherally administered cholecystokinin on monoaminergic and GABAergic neurons in the rat brain. Acta Med Okayama. 1989;43:153–159. doi: 10.18926/AMO/30887. [DOI] [PubMed] [Google Scholar]
- 77.Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. American Journal of Physiology. 1998;275:R262–R268. doi: 10.1152/ajpregu.1998.275.1.R262. [DOI] [PubMed] [Google Scholar]
- 78.Michaloudi HC, Majdoubi ME, Poulain DA, Papadopoulos GC, Theodosis DT. The noradrenergic innervation of identified hypothalamic magnocellular somata and its contribution to lactation-induced synaptic plasticity. Journal of Neuroendocrinology. 1997;9:17–23. doi: 10.1046/j.1365-2826.1997.00583.x. [DOI] [PubMed] [Google Scholar]
- 79.Alonso G. Differential organization of synapses immunoreactive to phenylethanolamine-n-methyltransferase or neuropeptide Y in the parvicellular compartments of the hypothalamic paraventricular nucleus of the rat. Journal of Chemical Neuroanatomy. 1993;6:55–67. doi: 10.1016/0891-0618(93)90028-3. [DOI] [PubMed] [Google Scholar]
- 80.Horie S, Shioda S, Nakai Y. Catecholaminergic innervation of oxytocin neurons in the paraventricular nucleus of the rat hypothalamus as revealed by double-labeling immunoelectron microscopy. Acta Anatomica. 1993;147:184–192. doi: 10.1159/000147502. [DOI] [PubMed] [Google Scholar]
- 81.Ochiai H, Nakai Y. Ultrastructural demonstration of dopamine-b-hydroxylase immunoreactive nerve terminals on vasopressin neurons in the paraventricular nuclues of the rat by double-labeling immunocytochemistry. Neuroscience Letters. 1990;120:87–90. doi: 10.1016/0304-3940(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 82.Decavel C, Geffard M, Calas A. Comparative study of dopamine- and noradrenaline-immunoreactive terminals in the paraventricular and supraoptic nuclei of the rat. Neuroscience Letters. 1987;77:149–154. doi: 10.1016/0304-3940(87)90577-5. [DOI] [PubMed] [Google Scholar]
- 83.Kitazawa S, Shioda S, Nakai Y. Catecholaminergic innervation of neurons containing corticotropin-releasing factor in the paraventricular nucleus of the rat hypothalamus. Acta Anatomica (Basel) 1987;129:337. [PubMed] [Google Scholar]
- 84.Liposits Z, Paull WK, Wu P, Jackson IMD, Lechan RM. Hypophysiotrophic thyrotropin releasing hormone (TRH) synthesizing neurons. Histochemistry. 1987;88:1–10. doi: 10.1007/BF00490159. [DOI] [PubMed] [Google Scholar]
- 85.Shioda S, Nakai Y, Sato A, Sunayama S, Shimoda Y. Electron-microscopic cytochemistry of the catecholaminergic innervation of TRH neurons in the rat hypothalamus. Cell and Tissue Research. 1986;245:247–252. doi: 10.1007/BF00213928. [DOI] [PubMed] [Google Scholar]
- 86.Nakada H, Nakai Y. Electron microscopic examination of the catecholaminergic innervation of neurophysin- or vasopressin-containing neurons in the rat hypothalamus. Brain Research. 1985;361:247–257. doi: 10.1016/0006-8993(85)91296-x. [DOI] [PubMed] [Google Scholar]
- 87.Oldfield BJ, Hou-Yu A, Silverman AJ. A combined electron microscopic HRP and immunocytochemical study of the limbic projections to rat hypothalamic nuclei containing vasopressin and oxytocin neurons. The Journal of Comparative Neurology. 1985;231:221–231. doi: 10.1002/cne.902310209. [DOI] [PubMed] [Google Scholar]
- 88.Liposits Z, Phelix C, Paull WK. Electron microscopic analysis of tyrosine hydroxylase, dopamine-beta-hydroxylase and phenylethanolamine-N-methyltransferasae immonoreactive innervation of the hypothalamic paraventricular nucleus in the rat. Histochemistry. 1986;84:105–120. doi: 10.1007/BF00499821. [DOI] [PubMed] [Google Scholar]
- 89.Olschowka JA, Molliver ME, Grzanna R, Rice FL, Coyle JJ. Ultrastructural demonstration of noradrenergic synapses in the rat central neuron system by dopamine-beta-hydroxylase immunocytochemistry. Journal of Histochemistry and Cytochemistry. 1981;29:271–289. doi: 10.1177/29.2.7019303. [DOI] [PubMed] [Google Scholar]
- 90.Sawchenko PE, Plotsky PM, Pfeiffer SW, Cunningham JET, Vaughan J, Rivier J, Vale W. Inhibin B in central neural pathways involved in the control of oxytocin secretion. Nature. 1988;335:615–617. doi: 10.1038/334615a0. [DOI] [PubMed] [Google Scholar]
- 91.Cunningham JET, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. Journal of Comparative Neurology. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 92.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 93.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 94.Watts AG. The impact of physiological stimuli on the expression of corticotropin-releasing hormone (CRH) and other neuropeptide genes. Frontiers in Neuroendocrinology. 1996;17:281–326. doi: 10.1006/frne.1996.0008. [DOI] [PubMed] [Google Scholar]
- 95.Al-Damluji S. Adrenergic mechanisms in the control of corticotropin secretion. Journal of Endocrinology. 1988;119:5–14. doi: 10.1677/joe.0.1190005. [DOI] [PubMed] [Google Scholar]
- 96.Rivier CL, Plotsky PM. Mediation by corticotropin-releasing factor of adenohypophysial hormone secretion. Annual Review of Physiology. 1986;48:475–494. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- 97.Plotsky PM, Cunningham JET, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocrine Reviews. 1989;10:437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- 98.Gaillet S, Lachuer J, Malaval F, Assenmacher I, Szafarczyk A. The involvement of noradrenergic ascending pathways in the stress-induced activation of ACTH and corticosterone secretions is dependent on the nature of the stressors. Experimental Brain Research. 1991;87:173–180. doi: 10.1007/BF00228518. [DOI] [PubMed] [Google Scholar]
- 99.Liposits Z, Phelix C, Paull WK. Adrenergic innervation of corticotropin releasing factor (CRF) - synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. Histochemistry. 1986;84:201–205. doi: 10.1007/BF00495783. [DOI] [PubMed] [Google Scholar]
- 100.Kiss A, Aguilera G. Participation of alpha1-adrenergic receptors in the secretion of hypothalamic corticotropin-releasing hormone during stress. Neuroendocrinology. 1992;56:153–160. doi: 10.1159/000126223. [DOI] [PubMed] [Google Scholar]
- 101.Alonso G, Szafarczyk A, Balmefrezol M, Assenmacher I. Immunocytochemical evidence of stimulatory control by the ventral noradrenergic bundle of parvicellular neurons of the paraventricular nucleus secreting corticotropin-releasing hormone and vasopressin in rats. Brain Research. 1986;397:297–307. doi: 10.1016/0006-8993(86)90631-1. [DOI] [PubMed] [Google Scholar]
- 102.Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria teminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Annals of the New York Academy of Sciences. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- 104.Brownstein MJ, Palkovits M. Catecholamines, serotonin, acetylcholine, and g-aminobutyric acid in the rat brain: Biochemical studies. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Elsevier: Amsterdam; 1984. pp. 23–54. [Google Scholar]
- 105.Sawchenko PE, Arias C, Bittencourt JC. Inhibin B, somatostatin, and enkephalin immunoreactivities coexist in caudal medullary neurons that project to the paraventricular nucleus of the hypothalamus. Journal of Comparative Neurology. 1990;291:269–280. doi: 10.1002/cne.902910209. [DOI] [PubMed] [Google Scholar]
- 106.Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. Journal of Comparative Neurology. 1988;271:519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- 107.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in rat hypothalamus and brainstem. Neuroscience. 1997;77:257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 108.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. Journal of Comparative Neurology. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 109.Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7–36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Research. 2003;985:163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- 110.Zueco JA, Esquifino AI, Chowen JA, Alvarez E, Castrillon PO, Blazquez E. Coexpression of glucagon-like peptide-1 (GLP-1) receptor, vasopressin, and oxytocin mRNAs in neurons of the rat hypothalamic supraoptic and paraventricular nuclei: effect of GLP-1(7–36)amide on vasopressin and oxytocin release. Journal of Neurochemistry. 1999;72:10–16. doi: 10.1046/j.1471-4159.1999.0720010.x. [DOI] [PubMed] [Google Scholar]
- 111.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. European Journal of Neuroscience. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 112.Uttenthal LO, Toledano A, Blazquez E. Autoradiographic localization of receptors for glucagon-like peptide-1 (7–36) amide in rat brain. Neuropeptides. 1992;21:143–146. doi: 10.1016/0143-4179(92)90036-v. [DOI] [PubMed] [Google Scholar]
- 113.Alvarez E, Roncero I, Chowen JA, Thorens B, Blazquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. Journal of Neurochemistry. 1996;66:920–927. doi: 10.1046/j.1471-4159.1996.66030920.x. [DOI] [PubMed] [Google Scholar]
- 114.Navarro M, Fonseca FRd, Alvarez E, Chowen JA, Zueco JA, Gomez R, Eng J, Blazquez E. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. Journal of Neurochemistry. 1996;67:1982–1991. doi: 10.1046/j.1471-4159.1996.67051982.x. [DOI] [PubMed] [Google Scholar]
- 115.Kinzig KP, D'Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueredo HF, Murphy EK, Seeley RJ. CNS Glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. The Journal of Neuroscience. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- 117.Thiele TE, Van-Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. American Journal of Physiology. 1997;272:R726–R730. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- 118.Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. American Journal of Physiology. 1997;273:G920–G927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 119.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 120.Seeley RJ, Dijk Gv, Blake J, Thiele TH. Brainstem Mechanisms of Ingestion and Gastrointestinal Physiology. Lumley Castle; County Durham, UK: 1997. The role of glucagon-like-peptide-1-(7–36) amide (GLP-1) in the control of food intake and mediating visceral illness in the CNS; p. 34. [Google Scholar]
- 121.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D'Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. The Journal of Neuroscience. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. American Journal of Physiology. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 123.Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. American Journal of Physiology. 1999;277:R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. [DOI] [PubMed] [Google Scholar]
- 124.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. The Journal of Neuroscience. 2002;22:10470–10475. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2003;285:R470–R478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 126.Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB. Maternal separation in neonatal rats elicits activation of the hypothalamic-pituitary-adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology. 1993;18:485–493. doi: 10.1016/0306-4530(93)90042-j. [DOI] [PubMed] [Google Scholar]
- 127.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;4:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 128.Walker CD, Scribner VA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time dependent and stress-specific fashion. Endocrinology. 1991;128:1385–1396. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 129.Walker SJ, Vrana KE. Pituitary corticotroph function during the stress hyporesponsive period in neonatal rats. Neuroendocrinology. 1993;57:1003–1010. doi: 10.1159/000126464. [DOI] [PubMed] [Google Scholar]
- 130.Rinaman L. Postnatal development of catecholamine inputs to the paraventricular nucleus of the hypothalamus in rats. The Journal of Comparative Neurology. 2001;438:411–422. doi: 10.1002/cne.1324. [DOI] [PubMed] [Google Scholar]
- 131.Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neuroscience and Biobehavioral Reviews. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- 132.Chautard T, Boudouresque F, Guillaume V, Oliver C. Effect of excitatory amino acid on the hypothalamo-pituitary-adrenal axis in the rat during the stress-hyporesponsive period. Neuroendocrinology. 1993;57:70–78. doi: 10.1159/000126344. [DOI] [PubMed] [Google Scholar]
- 133.Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- 134.Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience. 1997;79:1165–1175. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- 135.Loizou LA. The postnatal ontogeny of monoamine-containing neurones in the central nervous system of the albino rat. Brain Research. 1972;40:395–418. doi: 10.1016/0006-8993(72)90142-4. [DOI] [PubMed] [Google Scholar]
- 136.Khachaturian H, Sladek JR. Simultaneous monoaminehistofluorescence and neuropeptide immunocytochemistry: III. Ontogeny of catecholamine varicosities and neurophysin neurons in the rat supraoptic and paraventricular nuclei. Peptides. 1980;1:77–95. doi: 10.1016/0196-9781(80)90040-6. [DOI] [PubMed] [Google Scholar]
- 137.Coyle JT, Axelrod J. Development of the uptake and storage of L-[3H]norepinephrine in the rat brain. Journal of Neurochemistry. 1971;18:2061–2075. doi: 10.1111/j.1471-4159.1971.tb05065.x. [DOI] [PubMed] [Google Scholar]
- 138.Coyle JT, Axelrod J. Dopamine-b-hydroxylase in the rat brain: developmental characteristics. Journal of Neurochemistry. 1972;19:449–459. doi: 10.1111/j.1471-4159.1972.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 139.Morales T, Hinuma S, Sawchenko PE. Prolactin-releasing peptide is expressed in afferents to the endocrine hypothalamus, but not in neurosecretory neurones. Journal of Neuroendocrinology. 2000;12:131–140. doi: 10.1046/j.1365-2826.2000.00428.x. [DOI] [PubMed] [Google Scholar]
- 140.Yano T, Iijima N, Kataoka Y, Hinuma S, Tanaka M, Ibata Y. Developmental expression of prolactin releasing peptide in the rat brain: localization of messenger ribonucleic acid and immunoreactive neurons. Developmental Brain Research. 2001;128:101–111. doi: 10.1016/s0165-3806(01)00148-1. [DOI] [PubMed] [Google Scholar]
- 141.Weller A, Smith GP, Gibbs J. Endogenous cholecystokinin reduces feeding in young rats. Science. 1990;247:1589–1591. doi: 10.1126/science.2321020. [DOI] [PubMed] [Google Scholar]
- 142.Robinson PH, Moran TH, McHugh PR. Cholecystokinin inhibits independent ingestion in neonatal rats. American Journal of Physiology. 1988;255:R14–R20. doi: 10.1152/ajpregu.1988.255.1.R14. [DOI] [PubMed] [Google Scholar]
- 143.Robinson PH, Moran TH, McHugh PR. Gastric cholecystokinin receptors and the effect of cholecystokinin on feeding and gastric emptying in the neonatal rat. Annals of the New York Academy of Science. 1985;448:627–629. [Google Scholar]
- 144.Rinaman L, Levitt P. Establishment of vagal sensorimotor circuits during fetal development in rats. J Neurobiol. 1993;24:641–59. doi: 10.1002/neu.480240509. [DOI] [PubMed] [Google Scholar]
- 145.Barber WD, Yuan CS, Burks TF, Feldman JL, Greer JJ. In vitro brainstem-gastric preparation with intact vagi for study of primary visceral afferent input to dorsal vagal complex in caudal medulla. Journal of the Autonomic Nervous System. 1995;51:181–189. doi: 10.1016/0165-1838(94)00129-8. [DOI] [PubMed] [Google Scholar]
- 146.Hironaka S, Shirakawa T, Toki S, Kinoshita K, Oguchi H. Feeding-induced c-fos expression in the nucleus of the solitary tract and dorsal medullary reticular formation in neonatal rats. Neuroscience Letters. 2000;293:175–178. doi: 10.1016/s0304-3940(00)01515-9. [DOI] [PubMed] [Google Scholar]
- 147.Rinaman L, Levitt P, Card JP. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. The Journal of Neuroscience. 2000;20:2731–2741. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. The Journal of Neuroscience. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koehnle T, Rinaman L. Progressive postnatal increases in Fos immunoreactivity in the forebrain and brainstem of rats after viscerosensory stimulation with lithium chloride. American Journal of Physiology Regulatory Integrative Comparative Physiology. 2007 doi: 10.1152/ajpregu.00666.2006. In Press. [DOI] [PubMed] [Google Scholar]
- 150.Oladehin A, Blatteis CM. Lipopolysaccharide-induced fos expression in hypothalamic nuclei of neonatal rats. Neuroimmunomodulation. 1995;2:282–289. doi: 10.1159/000097207. [DOI] [PubMed] [Google Scholar]
- 151.Kovacs KJ, Sawchenko PE. Mediation of osmoregulatory influences on neuroendocrine corticotropin-releasing factor expression by the ventral lamina terminalis. Proceedings of the National Academy of Sciences USA. 1993;90:7681–7685. doi: 10.1073/pnas.90.16.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]


