Abstract
Geriatric depression consists of complex and heterogeneous behaviors unlikely to be caused by a single brain lesion. However, there is evidence that abnormalities in specific brain structures and their interconnections confer vulnerability to the development of late-life depression. Structural magnetic resonance imaging methods can be used to identify and quantify brain abnormalities predisposing to geriatric depression and in prediction of treatment response. This article reviews several techniques, including morphometric approaches, study of white matter hyperintensities, diffusion tensor imaging, magnetization transfer imaging, t2 relaxography, and spectroscopy, that have been used to examine these brain abnormalities with a focus on the type of information obtained by each method as well as each method’s limitations. The authors argue that the available methods provide complementary information and that, when combined judiciously, can increase the knowledge gained from neuroimaging findings and conceptually advance the field of geriatric depression. (Am J Geriatr Psychiatry 2006; 14:812–822)
Keywords: Depression, neuroimaging, white matter, gray matter
Geriatric depression is a heterogeneous syndrome that likely has multiple determinants. Among these, abnormalities in specific brain structures likely confer morbid vulnerability that increases the propensity for development and chronicity of late-life depression.1 Geriatric depression consists of complex and heterogeneous behaviors, however, that are unlikely to follow a simple lesion–syndrome relationship. The etiology of such brain abnormalities may be diverse and result from processes commonly occurring in older adults, including genetic influences and vascular, inflammatory, autoimmune, and endocrine processes.2 Although imaging studies thus far have identified group differences between normal subjects and those with late-life depression that are not robust or distinctive enough to be used for diagnosis of individuals, recent advances in magnetic resonance imaging (MRI) techniques allow the in vivo identification of the specific brain structure abnormalities that have the potential to further elucidate the role of brain structure abnormalities in the pathophysiology of geriatric depression and the mechanisms of treatment response. Because more than one brain abnormality may confer vulnerability to geriatric depression, structural neuroimaging studies need to have sharply focused hypotheses that take full advantage of the developing technology to clarify with greater specificity the location, type, and extent of abnormality. Effective use of such information can help constrain models of late-life depression.
We discuss the potential uses and limitations of both traditional and recently developed MRI approaches to the study of late-life depression. The review is limited to MRI studies because of: 1) the relative safety of MRI compared with computed tomography scans (i.e., no radiation is used in MRI scans); and 2) the optimization of new MRI techniques for examination of white matter (WM). Accordingly, we discuss morphometric volumetry studies as well as WM pathology and magnetic resonance spectroscopy (MRS) studies. These are summarized in Table 1. Representative data from each method discussed are presented in Figure 1. Because the terminology is evolving, we also provide a glossary (Table 2). The interested reader is referred to MR physics texts3,4 for more detailed information.
TABLE 1.
Comparison of Magnetic Resonance Imaging (MRI) Methods for Assessing Structural Deficits in Geriatric Depression
| Method | Strengths | Weaknesses | State of Development |
|---|---|---|---|
| MRI volumetry | Simple to implement | Time-consuming; laboratory-dependent | Commonly used |
| Voxelwise analyses | Highly automated | Depends highly on image registration quality | Relatively new |
| White matter hyperintensities | Simple to implement | Typically rated subjectively | Commonly used |
| DTI | Highly sensitive to pathology | Not specific as to pathology | Development of optimized sequences and analytic methods |
| MTR | Specific to macromolecules | Relatively low signal-to-noise ratio; not quantitative | Few studies |
| T2R | Specific to myelin | Time-consuming; does not yield whole brain information | Not applied to geriatric depression yet |
| MR spectroscopy | Chemically specific | Poor spatial and temporal resolution | Needs better multislice implementation |
FIGURE 1.
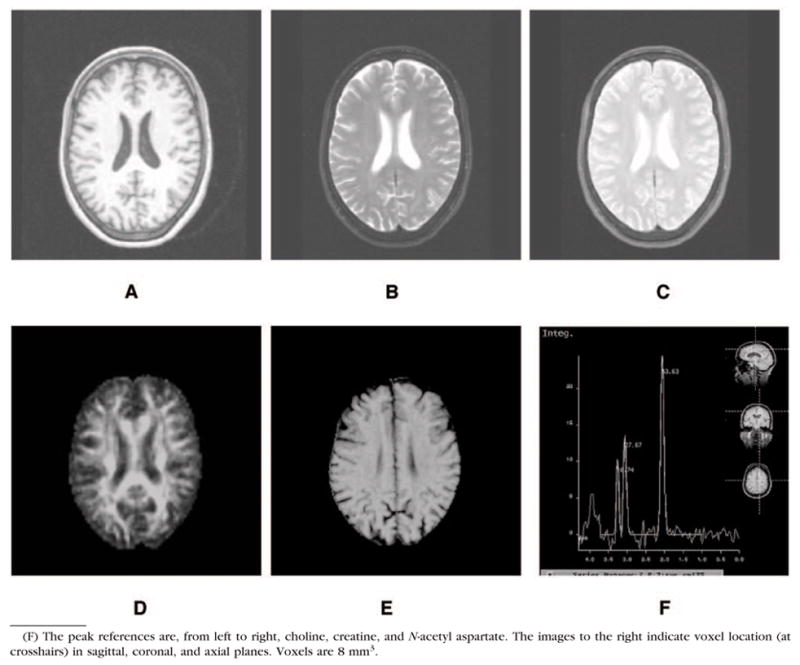
Images From (A) T1-Weighted, (B) T2-Weighted, (C) Proton Density-Weighted, (D) Fractional Anisotropy, (E) MTR, and (F) Spectroscopy Images
TABLE 2.
Glossary of Magnetic Resonance Terms
Magnetic resonance image (MRI) sequences work by the application of radiofrequency (RF) pulses in the presence of a magnetic field. In proton MRI, the magnetic field serves to align a very small proportion of the protons (typically water) in the brain along the direction of that magnetic field. In the basic MR experiment, an RF pulse of an appropriate frequency perturbs the protons so that they are no longer aligned with the magnetic field. A typical RF pulse might perturb the protons perpendicular to the magnetic field. This is referred to as a 90° pulse. After the RF pulse(s) is applied, the protons return (“relax”) to their original orientation, releasing energy in the process. The signal from this relaxation process serves as the basis of MRI.
|
VOLUMETRIC STUDIES
Manual Morphometry
The most common method to examine differences in brain morphometry in vivo is the use of T1-weighted images. Most morphometric studies have depended on manual measurements of regions of interest (ROI). These studies provide measures of the volume of specific brain areas and have the advantage that the boundaries used are typically guided by relevant neuroanatomic parameters. The reliability of such measures tends to be high, at least within a particular laboratory or among a small set of raters. ROI studies of geriatric depression suggest marked volume reductions in the brains of older depressives in multiple frontal, limbic, and subcortical regions, including the anterior cingulate gyrus, prefrontal cortices, the neostriatum, and the hippocampus.5–9
A number of methodological limitations must be considered when interpreting morphometry studies. First, bearing in mind that loss of neuronal bodies, decrease in neuronal size, and decreases in dendritic arborization are all potential sources of volume reductions on T1-weighted images, the specific neurohistologic processes that account for the observed volume reductions cannot be distinguished. Second, larger or smaller volumes of the same brain structure may be associated with different mechanisms related to late-life depression. For example, enlarged amygdala volume has been observed during the first depressive episode10 and interpreted as a predisposing factor to depression. In contrast, reduced amygdala volumes in recurrent depression11 may be a result of impaired neurogenesis during previous depressive episodes. Third, antidepressant medication itself may modulate regional brain volumes. For example, Lavretsky et al.12 found that orbitofrontal gray matter volumes were larger in patients with geriatric depression treated with antidepressant medication compared with drug-naive patients, although these volumes were smaller than in age-matched controls.
A significant limitation of manual ROI morphometry is the lack of standard guidelines used for delineation of ROIs across laboratories. Thus, differences between studies may be influenced by differences between research groups in the neuroanatomic boundaries used to identify ROIs. Manual ROI measurements also are time-consuming. The ROI often is drawn on every slice of an MR image, and the time necessary to establish inter- and intrarater reliability can be prohibitive. For these reasons, some investigators have begun to champion an atlas-based approach in which an ROI is derived in standard space and warped back to the individual’s brain space. An advantage of this method is that only one ROI needs to be drawn for the entire sample. This method is ideal for hypothesis-driven approaches in which the region(s) of interest are chosen with regard to the literature but is not applicable in cases in which hypothesis generation is the goal.
Voxel-Based Morphometry
Voxel-based morphometry (VBM)13 is a newer analytic method in which anatomic (typically T1-weighted) scan data are coregistered into a standard space, for example, Talairach space, so that comparisons can be made at homologous locations across images. The images typically are segmented into gray matter, WM, and cerebrospinal fluid components before analysis. The findings from these studies are generally interpreted as differences in tissue density. The advantage of VBM is the ease with which it can be carried out as well as its ability to interrogate the entire brain at once, meaning that novel findings can be more easily detected.
One disadvantage of VBM is its relative insensitivity to complex relatively nonlocalized differences in brain structure.14 Thus, VBM has been shown to be biased toward detection of very local differences in brain structure but biased against detecting more distributed differences across brain regions.
The issue of false-positive findings is nontrivial in VBM and all voxelwise measures. As the number of statistical tests increase, the chance of a false-positive (type I error) increases. Because the tests, computed at each voxel, are not statistically independent, Bonferroni correction (simple division of the nominal p value by the number of tests) is not appropriate. Several alternative strategies have emerged. Most of these involve setting a magnitude threshold, whereby a group difference must be significant at a specified p level. In addition, it is common to set an extent threshold, whereby a certain number of contiguous voxels (cluster) have to be significant at a given p value. Within this framework, Friston et al. have developed the Gaussian random fields15 method to correct for multiple comparisons. Others suggest using the false discovery rate16 that corrects for, at minimum, false-positives. More recently, Baudewig et al.17 have advocated using staged p values. In this approach, a conservative seed p value is computed and a minimum cluster size is set. All other voxels in the cluster need meet only a conventional threshold.
The quality of intersubject image registration can significantly influence results obtained using VBM.18 To empirically examine some of the methods of intrasubject image registration, we quantitatively compared our nonlinear registration, automated registration toolbox (ART19), with both SPM99 (available at www.fil.ion.ucl.ac.uk) and automated intersubject registration (AIR20), two commonly used intrasubject registration algorithms. We measured the influence of registration methods on both the initial and final location of 20 different landmarks. For almost every landmark, ART gave a smaller intersubject dispersion than either SPM99 or AIR.21 This high-quality intersubject registration significantly influenced functional MRI results.22 Given age-related increases in the variability of the shape of various structures (e.g., ventricles), it is important to use age-appropriate templates.
Finally, WM hyperintensities, which are prevalent in geriatric depression, may cause tissue to be misclassified during the segmentation process, leading to inaccuracies in VBM results.
Despite the aforementioned caveats, recent studies indicate that VBM is an effective tool for the detection of structural anomalies in geriatric depression. For example, in a VBM study, hippocampal and prefrontal volumes were smaller in depressed than nondepressed elderly subjects.23 Furthermore, smaller hippocampal–entorhinal cortex volumes were associated with number of years since the first episode of major depression.
WHITE MATTER PATHOLOGY
White Matter Hyperintensities
The most common method to study WM neuropathology on MRI has been the examination of WM hyperintensities (WMH). These hyperintensities are typically observed in T2-weighted or fluid-attenuated inversion recovery sequences and are taken to indicate WM damage. WMH are more prevalent and severe in older depressed individuals than age-matched control subjects and mainly occur in subcortical regions and frontal WM projections.24 Subcortical hyperintensities have been found to be associated with executive dysfunction.25,26 Both executive dysfunction and subcortical hyperintensities have been shown to predict poor outcomes of geriatric depression.27–30
The methods of quantifying WMH are beset with some of the same limitations as morphometry studies. First, there are several WMH rating scales and these scales vary in scope, sensitivity, and reliability.31 Second, postmortem studies of the histology of WMH suggest that such WM abnormalities reflect a number of pathologic processes; however, the methodology prevents a reliable discrimination among such mechanisms. Third, analysis of WMH does not allow identification of specific affected WM tracts. One hopes that the introduction of new MRI approaches (e.g., diffusion tensor, magnetization transfer) will help to achieve not only better differentiation between true WM lesions and spurious unidentified bright objects, but will also help to clarify the underlying pathologic causes of the WMH.
Diffusion Tensor Imaging
Diffusion tensor imaging (DTI) offers a promising new technique for the identification of cerebral networks critical to geriatric depression.32 This method measures the self-diffusion of water. When no barriers to such diffusion are present, it occurs equally in all directions (i.e., it is isotropic). However, when barriers are present, diffusion tends to follow the long axis of those barriers (i.e., diffusion is anisotropic). DTI involves the acquisition of at least six images that are sensitive to diffusion in different directions as well as one reference image with no diffusion weighting. Diffusion anisotropy can be quantified using DTI by a number of different scalar metrics, including fractional anisotropy (FA). FA values range from 0 (perfectly isotropic diffusion) to 1 (the hypothetical case of an infinitely long, infinitesimally narrow tube).
The basis for anisotropic diffusion in brain appears to depend on cell membrane integrity and is modulated by myelin.33,34 DTI is sensitive to WM pathology in a number of disorders, including multiple sclerosis,35 schizophrenia,36–38 and dyslexia.39 It is also sensitive to the effects of aging.40,41 Moreover, relationships between FA and function have been shown in a number of domains, including impulsivity,42,43 cognitive function,44–46 and psychopathologic symptomatology.47
Depressed elderly patients exhibit compromised integrity (reduced FA) of the right superior frontal WM.48 Furthermore, in a preliminary DTI study, reduced FA of the WM lateral to the anterior cingulate gyrus was associated with poor response to citalopram in depressed older patients.49 In a study of 51 patients, we found significant associations between FA and the executive aspects of the Stroop in multiple frontostriatal–limbic regions, including WM lateral to the anterior and posterior cingulate cortex, and WM in prefrontal, insular, and parahippocampal regions. Moreover, systolic blood pressure is inversely associated with FA at the WM of the anterior cingulate and basal ganglia, especially in patients with executive dysfunction (based on performance on the Stroop Color-Word task). These correlations were less extensive in patients without such executive dysfunction.50 The association of systolic blood pressure with reduced FA in corticostriatal–limbic regions is consistent with the vascular depression model.51,52
It has been suggested that diffusivity perpendicular to (radial diffusivity [DRA]) and parallel to (axial diffusivity [DAX]) the direction of principal diffusion may be sensitive to different neural pathology. For example, increased DRA has been attributed to compromised myelin. The knockout mouse shiverer, which lacks myelin basic protein, shows reduced diffusion anisotropy and increased DRA with no change in DAX.53 Decreased DAX has been associated with axonal degeneration. Mice with retinal ischemia show axonal degeneration followed by demyelination. DTI scans of these animals showed decreased DAX followed by increased DRA, consistent with axonal loss followed by demyelination in the same animals.54 These measures might prove helpful in clarifying the relative roles of myelin and axonal integrity in WM changes in geriatric depression.
A potentially promising application of DTI is fiber tractography,55,56 in which directional information from the diffusion tensor can be used to map out putative fiber tracts. This application should ultimately provide information on regional connectivity and has been suggested as a method to generate regions of interest. Only recently have quantitative methods been developed to evaluate tractography.57,58 It would be useful to examine the extent to which tracts differ in individuals who are predisposed to geriatric depression relative to those who are not.
There are a number of limitations to DTI. For instance, a number of studies have used small samples and methods for analysis of the data are not well standardized. In addition, most, but not all,59 DTI scanning sequences use a rapid imaging technique called echoplanar imaging. This technique is affected by magnetic susceptibility effects that occur, for example, at barriers between tissue and air or between tissue and bone, e.g., near the nasal sinuses. These susceptibility effects worsen at higher magnetic fields and can lead to distortion of images or, in extreme cases, loss of information.
A number of interpretation issues also remain. Many authors have characterized DTI as a measure of WM, whereas in reality, it is only a method to measure anisotropic diffusion. Some34 have argued that DTI primarily measures axonal membrane integrity with myelin playing a modulatory role. A consideration of tensor components such as DRA and DAX may prove informative in this regard. Another important issue is the influence on DTI measurements of regions in which fibers with different orientations cross. A related issue is that most DTI studies have used low spatial resolutions, which can lead to partial volume effects in which fibers of varying orientations will be included in a given image voxel that could be separated with better spatial resolution. Some have proposed using high angular diffusion imaging to address this problem,60 but it is unclear whether such methods can unambiguously separate crossing fibers at the voxel level. Low spatial resolution also plagues tractography because the resolution can be several orders of magnitude coarser than the fiber maps produced by tractography programs.
Intersubject image registration is an important concern for voxelwise DTI analysis for two reasons: first, coregistration of images of different modalities (e.g., T2-weighted to T1-weighted) is of questionable validity on the fine anatomic level. Second, the directional aspects of the tensor need to be registered individually.61,62 Note that this second problem is not relevant if only scalar measures such as FA are of concern.
The optimal solution to the first problem is to register images of the same modality. For example, in our studies,38,43,63 a T1-weighted image is registered to a T1-weighted template using a nonlinear registration algorithm. Then, the T2-weighted image is linearly registered to the registered T1-weighted image. Finally, the unweighted diffusion tensor image (which is T2-weighted) is nonlinearly matched to the T2-weighted image. This last step corrects for susceptibility-induced distortion. These three steps are combined into one transformation step to minimize interpolation errors. This transformation can then be applied to FA maps as well as coregistered maps of other modalities. The result is highly accurate and precise intrasubject registrations of the image maps, which affords a greater ability to integrate results across imaging modalities.
The second problem arises because the transformation described in the preceding paragraph only deals with scalar measures. The approach described here can be applied to each directional component of the diffusion tensor, thereby yielding high-quality transformations of directional aspects of DTI.
Magnetization Transfer Imaging
Magnetization transfer imaging (MTI64) offers another method to assess the role of WM in geriatric depression. MTI provides contrast based on the interaction of the normally observed tissue water signal with protons contained in large macromolecules (including myelin) in tissue. The latter are invisible in standard MR sequences because of their very short T2 relaxation properties, but nevertheless can have an indirect effect on free water signal if the magnetization of water bound to the macromolecules is prepared by a special saturation pulse with large-frequency offset. In this imaging modality, two sequences are used, one of which is essentially proton density-weighted. In the other sequence, an off-resonance pulse is prepended to the original sequence, which eliminates the signal due to the protons associated with macromolecules, thereby lowering image contrast. The contrast difference between the two image sets is usually given by the magnetization transfer ratio (MTR). In a study comparing DTI with MT in multiple sclerosis,65 normal-appearing periplaque regions (PWM) were compared with normal-appearing WM remote from the plaques. FA in the PWM regions was significantly lower than in remote regions. However, magnetization transfer ratio did not detect a significant difference between the two regions. The authors concluded that MT is sensitive to demyelination and axonal degeneration, which is present in and near lesions, but less sensitive to inflammation and edema, which is present in less affected regions, whereas FA is more sensitive to these processes. This result suggests that MTR and FA can provide complementary information in WM. Recently, however, Kubicki et al.66 found substantial correlations between MT and FA in patients with schizophrenia. Thus, the nature of the relationship between FA and MT remains unclear.
Kumar and colleagues67 demonstrated the usefulness of MT in the study of WM abnormalities in geriatric depression. In a preliminary study, they examined MT in eight patients with late-life depression and eight nondepressed comparison subjects. They found that, relative to age-matched control subjects, older depressed patients have lower MTRs in the genu and splenium of the corpus callosum, the neostriatum, and the occipital WM.
MTR is not a quantitative measure because the reduction in signal intensity on the scan with the saturated pulse is measured relative to the reference scan. The MTR gives rise to a single value and has been shown to be sensitive to scanner and sequence differences. Recently, quantitative magnetic transfer imaging has been implemented in humans.68,69 Although these methods, which involves acquisition of numerous scans with varying saturation pulse frequencies and strengths, are time-consuming, they do yield quantitative estimates of magnetization transfer effects. Their value in geriatric depression has yet, to our knowledge, to be demonstrated, although the implementation of this method represents a potentially important research direction.
T2 Relaxography
More recently, T2 relaxography (T2R70) has been used to examine WM function. In this imaging modality, T2 signal characteristics are determined at multiple echo times. The T2 relaxation times (typically less than 50 msec) are attributed to water in myelin layers. Intermediate relaxation times (typically 50–200 msec) are attributed to intracellular water, whereas the longest relaxation times (typically greater than 200 msec) are attributed to extracellular water. In T2R, a myelin fraction (MF) is computed as the signal at the shorter echo times (TE <50 ms) divided by the total signal at all echo times. Flynn et al.71 found reduced MF in patients with schizophrenia. This method could be useful in geriatric depression such that decreased MF could be taken to suggest a reduction in myelin. A drawback of this method is that until recently, only a single imaging slice could be acquired in a reasonable amount of imaging time as a result of pulse sequence limitations. Vidarsson and colleagues72 have suggested modifications to the T2R protocol to allow for the collection of multiple image slices in a reasonable scanning time. To our knowledge, this is the only published study using this promising new method; however, it is likely that its use will increase in the near future.
Magnetic Resonance Spectroscopy
MRS provides important information on the neurochemical environment. Different molecules have unique MR spectra that can be quantified by taking the area under the signal curve. In most cases, the values are not absolute, so it is customary to take ratios of the measure of interest to some standard metabolite, for example, choline.
Most spectroscopy studies thus far have used single voxel acquisitions with a high number of signal repetitions to get adequate signal-to-noise ratio. Other studies use chemical shift imaging to obtain spectroscopy data on an image slice or set of slices.
One of the primary metabolites of interest, N-acetyl aspartate (NAA), is present only in neurons73 and has been viewed as a marker for neuronal integrity. Kumar and colleagues74 used MRS to examine biochemical abnormalities in left frontal WM and bilateral anterior cingulate gray matter of elderly depressed patients. They observed higher choline to creatine as well as myoinositol to creatine ratios in the white, but not gray, matter of patients relative to age-matched comparison subjects. Results from a follow-up MRS study suggested that cognitive performance was associated with levels of metabolites as measured by 2–D MRS in healthy control subjects but not in patients with geriatric depression.75 These authors interpreted their results as indicating that cognitive performance in geriatric depression may be associated with biochemical changes in frontostriatal circuitry.
An issue that needs to be addressed in spectroscopic studies of pathologic populations is that the T2 signal, from which spectra are derived, can differ across groups. This makes it difficult to distinguish whether observed effects reflect differences in metabolite concentration or differences in the metabolite’s microenvironment. One solution to this problem is to acquire full T2 spectra of the metabolite. This process would increase scan time by an order of magnitude or more, however. Recently, some have advocated for J-coupled spectroscopy, which examines the Fourier transform of the T2 spectrum.76–78 An alternative is to use modified sequences, although these are more difficult to implement than conventional sequences.
Another issue concerns the effect of antidepressants on metabolite levels. For example, occipital GABA levels increase after treatment with serotonin specific reuptake inhibitors in depressed patients.79 These issues need to be systematically studied in patients on or off antidepressant medication, although medication washout in patients raises ethical concerns. In some ways, the sensitivity of spectroscopic measurements makes it an ideal method with which to examine medication effects on brain metabolism, leading to the possibility of clinical trials of the brain metabolic efficacy of antidepressant agents in geriatric depression.
Synthetic Approaches
MT, DTI, T2R, and MRS scans have been analyzed using ROI approaches. In this case, ROIs are either drawn or placed in relevant regions. The ROIs assess relatively circumscribed areas within anatomically meaningful structures, but they typically are measured on relatively low-resolution images. As a result, the anatomic specificity of such ROIs is limited. Moreover, the ROIs do not provide an assessment of changes throughout the brain. Thus, it is difficult to know from ROI-based methods how specific the findings are. As mentioned previously, a potentially useful approach, particularly with large data sets, is the use of atlas-based ROIs.
Another approach to such measurements is to use voxelwise analyses, which provide the advantage that the entire brain is sampled. However, because of large individual differences in brain structure, the validity of this method on a fine anatomic level is unclear. Nonetheless, many voxelwise studies using DTI have yielded results consistent with those found in ROI methods.37,38 Important issues to consider include how much the data are smoothed to meet assumptions of statistical normality80 as well as quality of intersubject image registration.21,22
A particular limitation across studies is the lack of consistent data acquisition and analytic strategies. In part, this occurs because of the rapid progress being made in neuroimaging. New imaging techniques are developed constantly, and different groups are working in different modalities. Nonetheless, this problem makes it difficult to compare results from different laboratories. There is a need for standardization of these strategies. This could drive multicenter studies that could address issues of 1) statistical power, 2) sample and disease heterogeneity, and 3) the reliability of methods across sites. Standardized methods also would permit the implementation of large clinical trials. The Biomedical Informatics Research Network (BIRN; www.nbirn.net) is an example of a group that is attempting such standardizations. They are currently in the process of creating DTI calibration data sets. Other data sets of this type will be welcome.
CONCLUSION
Although the etiology of geriatric depression is unknown, there is consensus that diverse processes (e.g., vascular lesions, impaired neurogenesis, inflammation, genetics), alone or in synergy, disrupt certain brain structures and confer vulnerability to depression.2 Frontostriatal structures, the hippocampus, and the amygdala are structures whose functional disruption is thought to predispose to geriatric depression. WM pathology may interfere with functional communication among these structures and create vulnerability to geriatric depression. This model postulates that vulnerability to geriatric depression is not created by a single lesion or a failing of a single brain structure but by a state of disruption that can be created by diverse brain abnormalities. Accordingly, several models of predisposition to geriatric depression have been advanced, and each may account for a significant percentage of vulnerable patients.1
Central to testing hypotheses related to structurally based vulnerability to geriatric depression is the availability of neuroimaging techniques. The diversity of extant structural neuroimaging modalities allows assessment of various aspects of brain structures and WM regions implicated in geriatric depression. From the clinical point of view, structural neuroimaging has begun to identify subgroups of depressed elders resistant to traditional treatments. Understanding the underlying mechanisms can guide the development of novel therapeutic approaches.2 The advances in technology, however, do not lessen the importance of rigorous study design. Hypothesis-specific decisions must be made about appropriate comparison groups or regions and must also consider how individual variation in lesion location, for example, might be obscured by averaging methods.
Each MR imaging approach is best suited to provide information about some aspects of brain structures but not others. However, the information derived from different imaging approaches can be complementary and lead to a coherent understanding of brain dysfunction contributing to depression. For example, volumetric assessment demonstrated reduced caudate9 and putamen81 volumes in depression as well as components of frontolimbic pathways.6 A recent study offered preliminary evidence of reduced magnetization transfer in the neostriatum of depressed elders suggesting demyelination and axonal degeneration.67 T2 maps have shown a larger volume of WM hyperintensities in geriatric depressives compared with normal elders.82 DTI studies documented microstructural abnormalities in WM of frontolimbic pathways. Both WM hyperintensities and microstructural abnormalities have been associated with executive dysfunction, and all three predict poor antidepressant response.2,27–30,49,83–85 Therefore, although each approach offers limited information, converging findings support the hypothesis that frontolimbic integrity is critical for antidepressant response.
Clear hypotheses that consider the technical and interpretative strengths and limitations of the proposed methods can leverage the recent advances in structural imaging techniques despite existing technical and interpretive shortcomings. The richness of current structural neuroimaging methods allows the advancement of hypotheses that could not be conceived before these technical developments. Therefore, clear understanding of what structural neuroimaging can and cannot measure reliably, and the strategic use of such information, is central for the conceptual advancement of the field of geriatric depression.
Footnotes
This study is supported by grant RO1 MH65653 (Alexopoulos), P30 MH68638 (Alexopoulos), by grant RO1 MH60662 (KOL), by K23 MH074818 (FGD), K23 MH067702 (CFM), R01 MH64783 (MJH), and by the Sanchez Foundation.
Dr. Alexopoulos has received research grants by Forest Pharmaceuticals, Inc., and Cephalon and participated in scientific advisory board meetings of Forest Pharmaceuticals. He has given lectures supported by Forest, Cephalon, Bristol Meyers, Janssen, Pfizer, and Lilly and has received support by Comprehensive Neuroscience, Inc. for the development of treatment guidelines in late-life psychiatric disorders.
References
- 1.Alexopoulos GS, Schultz SK, Lebowitz BD. Late-life depression: a model for classification. Biol Psychiatry. 2005;58:283–289. doi: 10.1016/j.biopsych.2005.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexopoulos GS. Depression in the elderly. Lancet. 2005;365:1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 3.Hornak JP. The basics of MRI. [Accessed March 17, 2006];2006 Available at: www.cis.rit.edu/htbooks/mri.
- 4.Haacke EM, Brown RW, Thompson MR, et al. Magnetic Resonance Imaging: Physical Principles and Sequence Design. New York: John Wiley & Sons-Liss; 1999. [Google Scholar]
- 5.Ballmaier M, Toga AW, Blanton RE, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Bilker W, Jin Z, et al. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life major depression. Neuropsychopharmacology. 2000;22:264–274. doi: 10.1016/S0893-133X(99)00124-4. [DOI] [PubMed] [Google Scholar]
- 7.Lai T, Payne ME, Byrum CE, et al. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- 8.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan KR, McDonald WM, Doraiswamy PM, et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Clin Neurosci. 1993;243:41–46. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- 10.Frodl T, Meisenzahl E, Zetzsche T, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- 11.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 12.Lavretsky H, Roybal DJ, Ballmaier M, et al. Antidepressant exposure may protect against decrement in frontal gray matter volumes in geriatric depression. J Clin Psychiatry. 2005;66:964–967. doi: 10.4088/jcp.v66n0801. [DOI] [PubMed] [Google Scholar]
- 13.Ashburner J, Friston KJ. Voxel based morphometry—the methods. Hum Brain Mapp. 2000;7:254–266. doi: 10.1002/1097-0193(200011)11:3<223::AID-HBM80>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Friston KJ, Holmes A, Poline JB, et al. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini Y, Drai D, Elmer G, et al. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 17.Baudewig J, Dechent P, Merboldt KD, et al. Thresholding in correlational analyses of magnetic resonance functional neuroimaging. Magn Reson Imaging. 2003;21:1121–1130. doi: 10.1016/j.mri.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Bookstein FL. ‘Voxel-based morphometry’ should not be used with imperfectly registered images. Neuroimage. 2001;14:1452–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- 19.Ardekani BA, Braun M, Hutton BF, et al. A fully automatic multimodality image registration algorithm. J Comput Assist Tomogr. 1995;19:615–623. doi: 10.1097/00004728-199507000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Woods RP, Grafton ST, Holmes CJ, et al. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 21.Ardekani BA, Guckemus S, Bachman A, et al. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;142:67–76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Ardekani BA, Bachman AH, Strother SC, et al. Impact of inter-subject image registration on group analysis of fMRI data. International Congress Series. 2004;1265:49–59. [Google Scholar]
- 23.Bell-McGinty S, Butters MA, Meltzer CC, et al. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 24.Coffey CE, Figiel GS, Djang WT, et al. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- 25.Boone KB, Miller BL, Lesser IM, et al. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect Arch Neurol. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- 26.Aizenstein HJ, Nebes RD, Meltzer CC, et al. The relation of white matter hyperintensities to implicit learning in healthy older adults. Int J Geriatr Psychiatry. 2002;17:664–669. doi: 10.1002/gps.685. [DOI] [PubMed] [Google Scholar]
- 27.Hickie I, Scott E, Mitchell P, et al. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien J, Ames D, Chiu E, et al. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. BMJ. 1998;317:982–984. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson S, Baldwin RC, Jackson A, et al. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28:1015–1026. doi: 10.1017/s003329179800693x. [DOI] [PubMed] [Google Scholar]
- 30.Steffens DC, Conway CR, Dombeck CB, et al. Severity of subcortical gray matter hyperintensity predicts ECT response in geriatric depression. J ECT. 2001;17:45–49. doi: 10.1097/00124509-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Mantyla R, Erkinjuntti T, Salonen O, et al. Variable agreement between visual rating scales for white matter hyperintensities on MRI.Comparison of 13 rating scales in a poststroke cohort. Stroke. 1997;28:1614–1623. doi: 10.1161/01.str.28.8.1614. [DOI] [PubMed] [Google Scholar]
- 32.Basser P, Mattiello J, LeBihan D, et al. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaulieu C, Allen PS. Water diffusion in the giant axon of the squid: implications for diffusion-weighted MR imaging of the human brain. Magn Reson Med. 1994;32:579–583. doi: 10.1002/mrm.1910320506. [DOI] [PubMed] [Google Scholar]
- 34.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 35.Werring DJ, Clark CA, Barker GJ, et al. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- 36.Lim KO, Hedehus M, Moseley M, et al. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 37.Buchsbaum MS, Tang CY, Peled S, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- 38.Ardekani BA, Nierenberg J, Hoptman MJ, et al. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- 39.Klingberg T, Vaidya CJ, Gabrieli JDE, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;20:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 40.Pfefferbaum A, Sullivan EV, Hedehus M, et al. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Salat DH, Tuch DS, Greve DN, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Hoptman MJ, Volavka J, Johnson G, et al. Frontal white matter, aggression and impulsivity in men with schizophrenia. Biol Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- 43.Hoptman MJ, Ardekani BA, Butler PD, et al. DTI and impulsivity in schizophrenia: a first voxelwise correlational study. Neuroreport. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy CF, Gunning-Dixon F, Hoptman MJ, et al. White matter integrity predicts Stroop performance in patients with geriatric depression. Biol Psychiatry. doi: 10.1016/j.biopsych.2006.07.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nestor PG, Kubicki M, Gurrera RJ, et al. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18:629–637. doi: 10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim KO, Ardekani BA, Nierenberg J, et al. Neurocognitive correlates of white matter integrity in schizophrenia. Am J Psychiatry. in press. [Google Scholar]
- 47.Wolkin A, Choi SJ, Szilagyi S, et al. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003;160:572–574. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- 48.Taylor WD, MacFall JR, Payne ME, et al. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- 49.Alexopoulos GS, Kiosses D, Choi S, et al. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–1931. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 50.Hoptman MJ, Murphy CF, Gunning-Dixon F, et al. Cardiovascular risk factors and white matter integrity in geriatric depression [Abstract] Biol Psychiatry. 2005;57:30S. [Google Scholar]
- 51.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 52.Alexopoulos GS, Meyers BS, Young RC, et al. ’Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 53.Song S-K, Sun S-W, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 54.Song S-K, Sun S-W, Ju W-K, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Wieshmann UC, Symms MR, Clark CA, et al. Wallerian degeneration in the optic radiation after temporal lobectomy demonstrated in vivo with diffusion tensor imaging. Epilepsia. 1999;40:1155–1158. doi: 10.1111/j.1528-1157.1999.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 56.Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 57.Courage I, Gouttard S, Gerig G. Towards a shape model of white matter fiber bundles using diffusion tensor MRI. Piscataway, NJ: International Symposium on Biomedical Imaging (ISBI’04); 2004. pp. 344–347. [Google Scholar]
- 58.Jones DK, Travis AR, Eden G, et al. PASTA: pointwise assessment of streamline tractography attributes. Magn Reson Med. 2005;53:1462–1467. doi: 10.1002/mrm.20484. [DOI] [PubMed] [Google Scholar]
- 59.Kubicki M, Westin C-F, Maier SE, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frank LR. Anisotropy in high angular diffusion-weighted MRI. Magn Reson Med. 2001;45:935–939. doi: 10.1002/mrm.1125. [DOI] [PubMed] [Google Scholar]
- 61.Jones DK, Griffin LD, Alexander D, et al. Spatial normalization and averaging of diffusion tensor MRI data sets. Neuroimage. 2002;17:592–617. [PubMed] [Google Scholar]
- 62.Alexander DC, Pierpaoli C, Basser PJ, et al. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- 63.Ardekani BA, Bappal A, D’Angelo D, et al. Brain morphometry using diffusion-weighted magnetic resonance imaging: application to schizophrenia. Neuroreport. 2005;16:1455–1459. doi: 10.1097/01.wnr.0000177001.27569.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed. 2001;14:57–64. doi: 10.1002/nbm.683. [DOI] [PubMed] [Google Scholar]
- 65.Guo AC, Jewells VL, Provenzale JM. Analysis of normal-appearing white matter in multiple sclerosis: comparison of diffusion tensor MR imaging and magnetization transfer imaging. AJNR Am J Neuroradiol. 2001;22:1893–1900. [PMC free article] [PubMed] [Google Scholar]
- 66.Kubicki M, Park H, Westin CF, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar A, Gupta RC, Albert TM, et al. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res. 2004;130:131–140. doi: 10.1016/j.pscychresns.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Sled JG, Pike B. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magn Reson Med. 2001;46:923–931. doi: 10.1002/mrm.1278. [DOI] [PubMed] [Google Scholar]
- 69.Tozer D, Ramani A, Barker GJ, et al. Quantitative magnetization transfer mapping of bound protons in multiple sclerosis. Magn Reson Med. 2003;50:83–91. doi: 10.1002/mrm.10514. [DOI] [PubMed] [Google Scholar]
- 70.Whittall KP, MacKay AL, Graeb DA, et al. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med. 1997;37:34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- 71.Flynn SW, Lang DJ, MacKay AL, et al. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- 72.Vidarsson L, Conolly SM, Lim KO, et al. Echo time optimization for linear combination myelin imaging. Magn Reson Med. 2005;53:398–407. doi: 10.1002/mrm.20360. [DOI] [PubMed] [Google Scholar]
- 73.Urenjak J, Williams SR, Gadian DG, et al. Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem. 1992;59:55–61. doi: 10.1111/j.1471-4159.1992.tb08875.x. [DOI] [PubMed] [Google Scholar]
- 74.Kumar A, Thomas A, Lavretsky H, et al. Frontal white matter biochemical abnormalities in late-life major depression detected with proton magnetic resonance spectroscopy. Am J Psychiatry. 2002;159:630–636. doi: 10.1176/appi.ajp.159.4.630. [DOI] [PubMed] [Google Scholar]
- 75.Elderkin-Thompson V, Thomas MA, Binesh N, et al. Brain metabolites and cognitive function among older depressed and healthy individuals using 2D MR spectroscopy. Neuropsychopharmacology. 2004;29:2251–2257. doi: 10.1038/sj.npp.1300553. [DOI] [PubMed] [Google Scholar]
- 76.Wilman AH, Astridge M, Snyder RE, et al. Same-scan acquisition of both edited J-coupled multiplets and singlet resonances of uncoupled spins for proton MRS. J Magn Reson. 1995;109:202–205. doi: 10.1006/jmrb.1995.0010. [DOI] [PubMed] [Google Scholar]
- 77.Shen J. Slice-selective J-coupled coherence transfer using symmetric linear phase pulses: applications to localized GABA spectroscopy. J Magn Reson. 2003;163:73–80. doi: 10.1016/s1090-7807(03)00112-5. [DOI] [PubMed] [Google Scholar]
- 78.Schulte RF, Trabesinger AH, Boesiger P. Chemical-shift-selective filter for the in vivo detection of J-coupled metabolites at 3T. Magn Reson Med. 2005;53:275–281. doi: 10.1002/mrm.20362. [DOI] [PubMed] [Google Scholar]
- 79.Sanacora G, Mason GF, Rothman DL, et al. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 80.Jones DK, Symms MR, Cercignani M, et al. The effect of filter size on VBM analysis of DT-MRI data. Neuroimage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Husain MM, McDonald WM, Doraiswamy PM, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–99. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 82.Taylor WD, MacFall JR, Payne ME, et al. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 83.Potter GG, Kittinger JD, Wagner HR, et al. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29:2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- 84.Simpson SW, Baldwin RC, Burns A, et al. Regional cerebral volume measurements in late-life depression: relationship to clinical correlates, neuropsychological impairment and response to treatment. Int J Geriatr Psychiatry. 2001;16:469–476. doi: 10.1002/gps.364. [DOI] [PubMed] [Google Scholar]
- 85.Alexopoulos GS, Kiosses DN, Murphy C, et al. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]


