Abstract
The feasibility of using an osmotic pump in place of a syringe pump for microdialysis sampling in rat brain was investigated. The use of an osmotic pump permits the rat to be free from the constraints of the standard tethered system. The in vitro flow rates of a microdialysis syringe pump (set at 10.80 μl/h) and the osmotic pump (pump specifications were 11.35 μl/h) with no probe attached were compared, yielding results of 10.87 μl/h ± 1.7% and 10.95μl/h ± 8.0%, respectively. The average of four flow rate experiments in vivo yielded RSDs less than 10% and an average flow rate of 11.1μl/h. Following the flow rate studies, in vivo sampling of neurotransmitters was accomplished with the osmotic pump coupled to a microdialysis probe implanted in the brain. Finally, after determination of basal levels of DOPAC, HVA, and 5-HIAA in the rats, the rats were dosed with benserazide followed by L-DOPA. The results from the dosing study showed at least a 10-fold increase in compounds in the L-DOPA metabolic pathway (DOPAC and HVA) and a slight or no increase in 5-HIAA (serotonin metabolic pathway.) These results indicate that the osmotic pump is a viable alternative to the syringe pump for use in microdialysis sampling.
Introduction
Microdialysis sampling is a well-established technique for in vivo monitoring of neurotransmitters in awake, freely moving animals (Justice et al., 1993; Zhou et al., 1995; Mas et al., 1996; Herrera-Marschitz et al., 1997; Everett et al., 2000; McKenzie et al., 2002; Fillenz, 2005; Watson et al. 2006). However, microdialysis in awake, freely moving animals requires tethering the animal to the syringe pump using at least one meter of narrow bore tubing (Martin et al., 2000). In addition, the animal is generally confined to a bowl to ensure that the connecting tubing does not become tangled or broken. This situation can be inconvenient or even unacceptable for many types of studies, such as behavioral studies, exercise physiology experiments, and protocols using specialized training cages or mazes. In these situations, osmotic pumps may provide a worthwhile alternative to syringe pumps.
Osmotic pumps have been used extensively in drug delivery studies (ALZET Pump Bibliography, 2006). Pumps are normally filled with a solution of the drug of interest and implanted subcutaneously in the animal. Following an equilibration time, the drug is delivered at a constant rate to the site of interest due to osmotic pressure generated inside the pump. There have been many studies in which an osmotic pump has been utilized to deliver a drug while microdialysis sampling with a syringe pump has been used separately to collect samples (Srinivasan et al., 1991; Beagles et al., 1998; Newman et al., 2000; Javitt et al., 2004; Alzet Pump Bibliography, 2006). Microdialysis probes have also been connected to osmotic pumps to facilitate the delivery of drugs to specific regions of the brain (Bazzett et al., 1991). However, to the best of our knowledge, microdialysis sampling with an osmotic pump for recovery of analyte from brain tissue has not been evaluated.
There are many potential advantages to using an osmotic pump as part of the microdialysis system. One is the portability and the lack of constraints on the animal, which would permit untethered experiments in any environment, including mazes and training cages. Sample collection vials or miniaturized sensors can be mounted directly on the head of the animal to allow it to move freely. In this case, only a small amount of tubing is required to connect the probe to the collection system, which results in a shorter delay time and less back pressure. The osmotic pumps are also very light compared to other portable syringe pumps. The largest osmotic pump, the ALZET 2ML series pump (Durect, Cupertino, CA) weighs approximately 7 g when filled with fluid. Commercially available small, battery-driven pumps usually weigh more than 100 g, making them too heavy for use with small animals such as rats or mice. Lastly, although the osmotic pumps are not reusable, they are, fortunately, not very expensive. The pumps used in this study cost approximately $20 each.
Osmotic pumps are available in three different fill volumes: 100 μl, 200 μl, and 2 ml. The available flow rates range from 0.25 to 10 μl/h. The flow rates obtained with these pumps are compatible with high recovery microdialysis experiments (Menacherry et al., 1992; Lada and Kennedy, 1995; Stenken et al., 2001; Cano-Cebrian et al., 2005). Depending on the pump that is chosen and the flow rate, the lifetime of the pump ranges from 1 day to 4 weeks. In this paper, the feasibility of using the ALZET 2ML1 osmotic pump (Durect) combined with an in situ collection system for neurochemical studies is evaluated. This study focused on construction and characterization of the portable system. This included determination of reproducibility of recovery and delivery using these pumps, development of an optimal collection system, and demonstration of the system for the in vivo sampling of neurotransmitter concentrations following i.p. administration of L-DOPA.
Materials and Methods
Chemicals
Xylazine was purchased from Bayer (Shawnee Mission, KS) and ketamine was purchased from Fort Dodge Animal Health (Fort Dodge, IA). Disodium-ethylenediaminetetraacetic acid (EDTA), diethylamine HCl, 1-octanesulfonic acid (sodium salt), N,N dimethylacetamide, caffeine, theophylline, cysteine, homovanillic acid (HVA), 3,4-dihydroxyphenylacetic acid (DOPAC), 5-hydroxyindole-3-acetic acid (5-HIAA), benserazide, dihydroxybenzylamine hydrobromide (DHBA) and L-3,4-dihydroxyphenylalanine (L-DOPA) were all obtained from Sigma (St. Louis, MO). Lactated Ringer's solution was purchased from Baxter (Deerfield, IL). Acetonitrile, methanol (ACS grade), ammonium hydroxide, phosphoric acid, sodium dihydrogen phosphate·H2O, and sodium citrate were obtained from Fisher (Fair Lawn, NJ). HClO4 (perchloric acid) was obtained from Spectrum Quality Products, (Gardena, Ca).
Surgical procedures
All surgical procedures were approved by the IACUC committee at the University of Kansas. Male Sprague-Dawley rats (350–450 g) were anesthetized by i.m. injection using xylazine and ketamine (0.011 and 0.2 mg/kg, respectively), and additional doses of anesthesia (ketamine only) were administered as needed to maintain anesthesia for the duration of the surgery. A brain cannula (the cannula for a CMA/12 microdialysis probe, CMA, Stockholm, Sweden) was implanted into the right striatum (A/P: +0.7; Lat: −2.7; Vert: −3.4) with respect to the bregma/midline intersection. An ALZET 2ML1 (10 μl/h flow rate, 2 ml fill volume, and 1 week pump duration) osmotic pump filled with lactated Ringer's solution was implanted subcutaneously into the rat along the left side of its back. In these studies, the two surgical procedures were performed at the same time so that the rat was anesthesized only once. However, it would be possible to perform the brain surgery first and then subsequently implant the pump 24–48 h later if a longer recovery time was desired following implantation of the brain cannula. The osmotic pump was connected to a 15-cm piece of FEP tubing (0.65 mm OD × 0.12 mm ID, Bioanalytical Systems (BAS), West Lafayette, IN) using a tubing connector (BAS) to connect the flow moderator of the osmotic pump to the FEP tubing. The tubing connector was UV glued to both the flow moderator and FEP tubing to secure it in place. Then 10 cm of protector medical catheter tubing (0.095 OD × 0.066 ID, Braintree Scientific, Braintree, MA) was placed over the FEP tubing and was also UV glued to the flow moderator. The protective sheath was used to help prevent crimps and kinks in the FEP tubing. The other end of the FEP tubing was connected to the inlet port of the microdialysis brain probe (CMA/12; shaft length 14 mm, 0.5 × 3.0 mm; polycarbonate (PC) membrane, 20,000 Da molecular weight cut-off; CMA, Stockholm, Sweden). After connecting the microdialysis probe to the osmotic pump, the microdialysis probe was inserted into the brain cannula (see Fig. 1).
Figure 1.
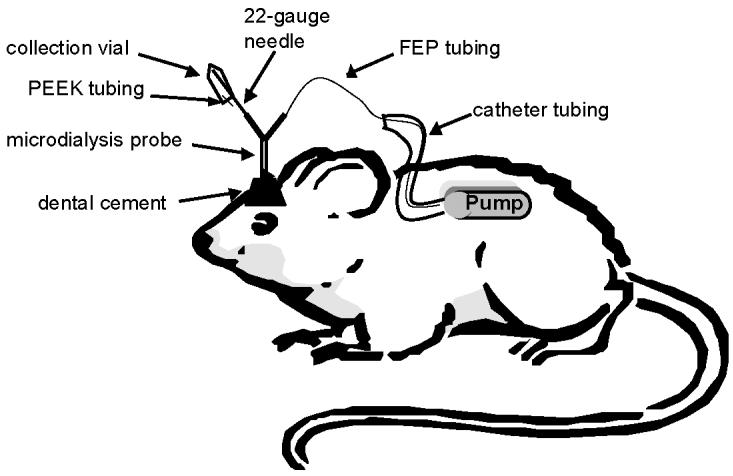
Schematic of the on-animal collection system with the microdialysis probe and osmotic pump
In vitro characterization of pump performance
The in vitro performance of the osmotic pump for microdialysis sampling was evaluated by (a) comparing the flow rate of the osmotic pump to a standard syringe pump with and without a microdialysis brain probe attached and (b) measuring extraction efficiency (i.e., recovery and delivery) of the model compounds caffeine and theophylline through a brain probe (CMA/12 described previously) using the osmotic pump to perfuse the probe.
In the flow rate comparison experiment, a 0.5 ml syringe filled with 200 μM theophylline in lactated Ringer's was placed into a CMA syringe pump and set at a flow rate of 0.18 μl/min. The 0.18 μl/min corresponds to 10.8 μl/h, which is similar to the ∼10 μl/h value listed for the osmotic pump. The osmotic pump was filled with 2 ml of the same perfusate solution and placed in a beaker containing a solution of 0.9% NaCl at 37 °C as per the technical information manual from ALZET. The pumps were tested by flowing through standard lengths of microdialysis tubing (FEP, 0.65 mm OD × 0.12 mm ID, BAS) with and without the brain probe attached. For testing without the probe, the pumps were connected directly to a Honeycomb fraction collector (BAS) using 1 m of tubing. To test the flow rate with the microdialysis brain probe, 30 cm of FEP tubing connected the pump to the probe and 1 m of the same tubing connected the probe outlet to the fraction collector. The probe was placed in a 37 °C thermostated block (Dri-Bath,Thermodyne, Dubuque, IA) with a beaker containing the recovery solution, which was 200 μM caffeine in lactated Ringer's. The fraction collector contained preweighed 250 μl capped glass vials, and samples were collected at selected time intervals (90, 60, 40, 30, 20 and 10 min) for gravimetric determination of the flow rate.
The extraction efficiency experiments with caffeine and theophylline were set up identically to the flow rate comparison experiments, using the brain probe, solutions, and conditions described above with two exceptions. Instead of using the fraction collector, samples were collected on-line to an HPLC injection valve using 1 m of tubing, and concentrations of analytes were determined by an established HPLC/UV method for caffeine and theophylline described later in this section. The sampling rate was 60 min for this experiment.
In vitro characterization of neurotransmitter recovery using the osmotic pump
In vitro microdialysis recovery studies of neurotransmitters were performed using DOPAC, 5-HIAA, and HVA. The osmotic pump was filled with a perfusate solution containing lactated Ringer's and placed in a covered 20 ml vial containing 0.9% NaCl in a thermostated block at 37 °C. A 15-cm length of FEP tubing was connected to the pump at one end and to a brain probe at the other end. The outlet of the probe was connected to a Honeycomb fraction collector by 30 cm of FEP tubing. The probe was then placed into a 20-ml vial containing 2 μM of each of the following in lactated Ringer's: DOPAC, 5-HIAA, and HVA. The 20-ml vial was placed in the 37 °C Dri-Bath, and samples were collected in capped glass vials containing 5 μl of a solution of 2 μM DHBA (internal standard), 0.25 M HClO4, and 0.1% cysteine. Perchloric acid and cysteine were added for sample stability. Samples were collected every 60 min and analyzed by microbore HPLC with electrochemical detection as described later in this section.
In vivo gravimetric sampling
With the in vivo microdialysis sampling experiments there was a 16-h delay prior to sampling for osmotic pump equilibration and animal recovery. For the in vivo flow rate gravimetric study using the osmotic pump, the outlet port of the brain probe was attached to a 4 °C refrigerated fraction collector by 1 m of FEP tubing and the inlet port of the brain probe was attached as described earlier. The rats were tethered in a Raturn awake animal system (BAS). Samples were initially collected every 90 min into preweighed vials for gravimetric flow rate analysis.
On-animal collection system
For the on-animal in vivo studies, the outlet end of the brain probe was connected to a 1-in piece of a 22-gauge stainless steel needle, which was filed blunt on both ends, by a tubing connector that was UV glued to one end of the 22-gauge needle. A 75-μl septum-capped plastic vial was placed on the remaining end of the 22-gauge blunt needle; a 1-cm piece of standard PEEK tubing was placed through the septum for a pressure bleed (see Fig. 1 for a diagram of the on-animal system). A 5 μl aliquot of a solution of 2 μM DHBA (internal standard), 0.25 M HClO4, and 0.1% cysteine was added after sample collection to samples collected from the on-animal system. For the dosing study, the osmotic pump was filled with lactated Ringer's and was implanted as specified earlier. After a 24–48-h recovery/equilibration time and collection of blanks, the rat was dosed i.p. with 50 mg/kg benserazide followed 20 min later with 50 mg/kg of L-DOPA (de Souza Silva et al., 1997). Samples were collected every hour following dosing by holding on to the 22-gauge needle, removing the sampling vial, and placing a new vial onto the needle. This process took approximately 5–10 s and could be easily accomplished without picking up the animal. The animals did not show any sign of agitation during this process.
Separation and detection methods
The caffeine and theophylline samples were analyzed by HPLC with UV detection. A PM-80 HPLC pump (BAS), a SPD-6AV UV-VIS detector (Shimadzu, Kyoto, Japan) and a 100 × 3.2 mm, 3-micron phase II ODS column (BAS) were employed for the analyses. The mobile phase for the separation of caffeine and theophylline consisted of 10% ACN, 15% MeOH, and 75% 50 mM ammonium phosphate buffer prepared from ammonium hydroxide and phosphoric acid at pH 2.5. The flow rate was 0.8 ml/min. Injections were performed using one of two 6.4 μl loops on a Valco 8-port injection valve (Valco Instruments, Houston, TX). Detection was performed at 273 nm and data collection was accomplished using a DA-5 Chromgraph interface (BAS) connected to a Gateway 486 DX-66 computer (Gateway, Poway, CA).
The system used to monitor the analytes in the neurotransmitter study (i.e., DOPAC, HVA, 5-HIAA, L-DOPA, and benserazide) was composed of a LC-6A (Shimadzu) HPLC pump and an electrochemical detector. A Unijet 5 micron, C-18, 150 × 1 mm microbore column (BAS) was used for the separation. The mobile phase was composed of 95:5% aqueous phase:organic phase (Cheng et al., 1992). The aqueous phase consisted of 25 mM sodium dihydrogen phosphate·H2O, 50 mM sodium citrate, 27 μM disodium-EDTA, 10 mM diethylamine.HCl, and 2.2 mM 1-octanesulfonic acid sodium salt, adjusted to pH 3.2 with phosphoric acid. The organic phase was 58% methanol and 42% N,N-dimethylacetamide. Electrochemical detection was accomplished using a Unijet 3-mm glassy carbon electrode (BAS) with a detection potential of +750 mV vs. Ag/AgCl and the current was monitored by a LC-4C potentiostat (BAS). Injections were performed using a 10.5 μl loop (overfill) on a Valco 6-port injection valve (Valco Instruments), and data collection was accomplished with a DA-5 Chromgraph interface (BAS) connected to a Gateway 486 DX-66 computer (Gateway).
Results and discussion
In vitro studies
Osmotic pump and syringe pump flow rate comparison
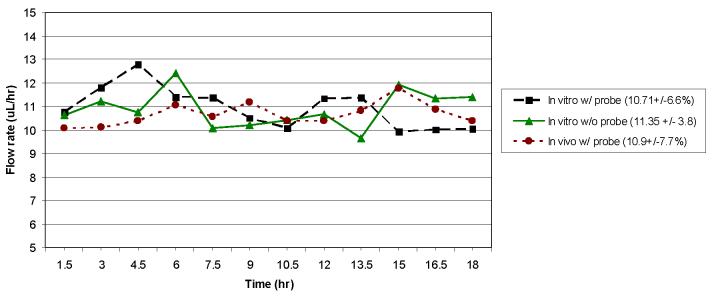
The primary goal of the in vitro studies was to compare the osmotic pump to the syringe pump for accuracy and precision of flow rate. The 90-min gravimetric analysis of the syringe pump without a probe attached yielded a flow rate of 10.87 μl/h ± 1.7% compared to the syringe pump set value of 10.8 μl/h (0.18 μl/min). The %RSD for the syringe pump through the probe was also below 2%, indicating that the probe does not have a significant effect on the precision of the syringe pump flow rate. A 90-min gravimetric analysis of the osmotic pump without a probe attached produced a flow rate of 10.95 μl/h ± 8.0% compared to the theoretical value of 11.35 μl/h ± 3.8% listed for that specific lot of 2ML1 osmotic pumps. The osmotic pump connected to a brain probe produced an average flow rate of 10.71 μl/h ± 6.6%. The precision of the osmotic pump was not as good as that observed for the syringe pump, but the values were relatively close. Also, the addition of the microdialysis probe did not significantly alter the flow rate or precision of the osmotic pump. The data for the in vitro osmotic pump flow rate study, with and without the probe, over an 18-h period for a collection of gravimetric samples is shown in Figure 2.
Figure 2.
Flow rate profiles for the 2ML1 osmotic pump with and without brain probe attached (in vitro and in vivo). Collection intervals were 90 minutes.
After comparing the flow rate profiles for a 90-min collection, a study to determine the effect of frequency of sampling on precision was performed. A series of sampling frequencies starting with 90 min and decreasing to 10 min using an osmotic pump (same type, ALZET 2ML1) was analyzed gravimetrically for flow rate profiles as shown in Table 1. It can be seen that the flow rate is less consistent for the shorter gravimetric collection times with the osmotic pump. It appears that the loss in precision with a decrease in sampling time is due to a pulsing effect. A vial that has a larger volume of fluid is almost always either preceded by or followed by one of less volume. For longer sampling times, the pulsing seems to average out. The largest pulsing effects were seen in the 10-min sampling and had an RSD of 18%. For more consistent flow rates (RSDs less than 10%), a collection time of at least 30 min must be employed.
Table 1.
Comparison of time sampling frequency in relation to the reproducibility of the osmotic pump flow rate.
| Collection duration |
90 min | 90 min | 40 min | 30 min | 20 min | 10 min |
|---|---|---|---|---|---|---|
| Probe | no | yes | yes | yes | yes | yes |
| Avg. flow rate (μl/h) |
10.95 | 10.71 | 9.70 | 10.27 | 10.03 | 9.59 |
| % RSD of flow rate |
8.03 | 6.59 | 10.97 | 8.87 | 14.29 | 17.97 |
In vitro extraction efficiency of model compounds and neurotransmitters
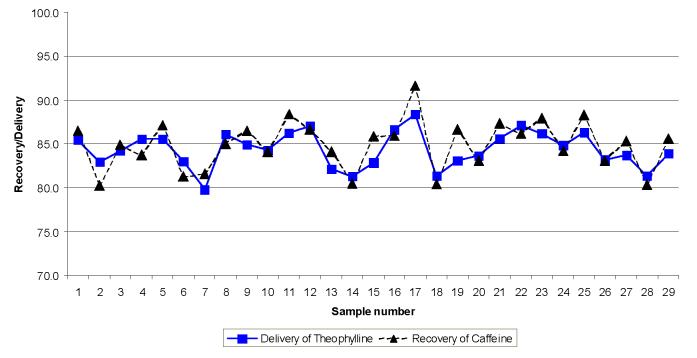
Next, the effect of the osmotic pump on microdialysis recovery and delivery was investigated in vitro. Theophylline and caffeine were chosen as model compounds since their behaviour under microdialysis conditions is well characterized and they are easy to separate and detect using liquid chromatography with UV detection (Zhao et al., 1995; Heppert and Davies, 1999). The extraction efficiency results with the osmotic pump were 84.9 ± 2.1% for caffeine recovery and a 84.4 ± 2.8% for theophylline delivery. Figure 3 shows the plot of the recovery and delivery values as a function of sample number. Samples were collected every 60 minutes for these studies. For the in vitro neurotransmitter recovery study using the osmotic pump, following 8 hours of sampling, steady-state recovery values were between 84 and 90 percent for DOPAC, HVA, and 5-HIAA.
Figure 3.
Recovery and delivery data obtained for caffeine and theophylline using a using the 2ML1 osmotic pump. Flow rate was 10.8 μL/hr. Collection intervals were 60 minutes.
In vivo studies
The results from the 90-min gravimetric in vivo flow rate study can be seen in Fig. 2 along with the in vitro results described previously. As seen in this plot, the flow rate is fairly consistent over time, and only a few samples contribute to the majority of the error. Also, the flow rate and reproducibility observed in the in vivo studies (10.9 μl/h ± 7.7%) are similar to those reported for the in vitro experiments (10.71 μl/h ± 6.6%). The in vivo flow rate experiment was repeated 3 more times using 3 different osmotic pumps. The results for each of the pumps are as follows: 10.9 μl/h ± 7.7% (shown in Fig. 2), 11.9 μl/h ± 5.8%, 11.2 μl/h ± 6.0%, and 10.3 μl/h ± 9.5%.
While the in vivo gravimetric flow rate results were promising for the osmotic pump; the rats were still tethered. The primary advantage of the osmotic pump is the increase in mobility; therefore, studies using the osmotic pump with an untethered rat were conducted. As seen in Fig. 1, a collection device was placed on a rat's head following implantation of the probe and pump. This collection device was a 75-μl plastic vial with a rubber NMR tube stopper on the top for a seal. A 1-cm piece of PEEK tubing was placed through the septum to serve as a pressure relief point. Samples were then collected every 60 min from the awake and freely moving rat to determine the feasibility of changing the collection vial on an awake animal. To determine the in vivo concentration of the neurotransmitters using the osmotic pump, microdialysis samples were collected overnight and every hour for two hours prior to the dosing study. The basal levels (uncorrected for recovery) varied slightly from animal to animal, but were in the low micromolar range for DOPAC, HVA, and 5-HIAA .
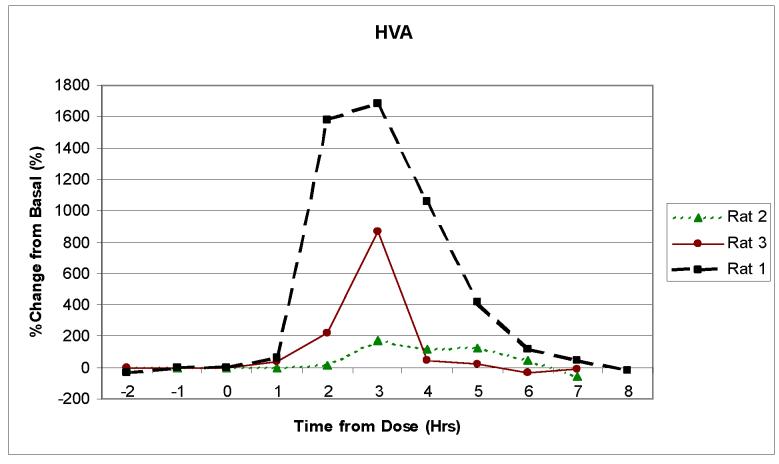
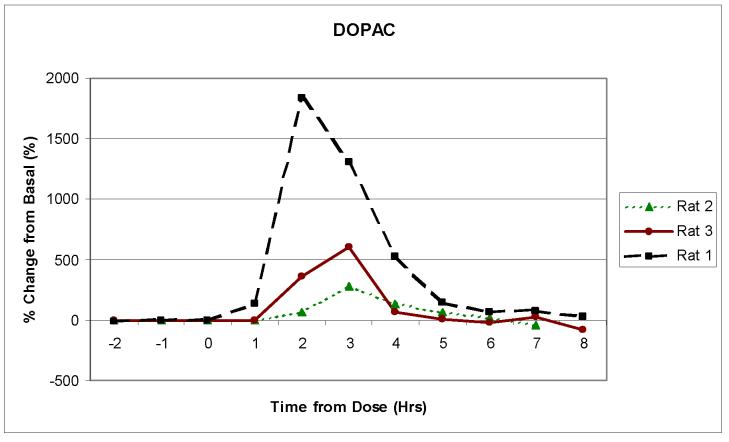
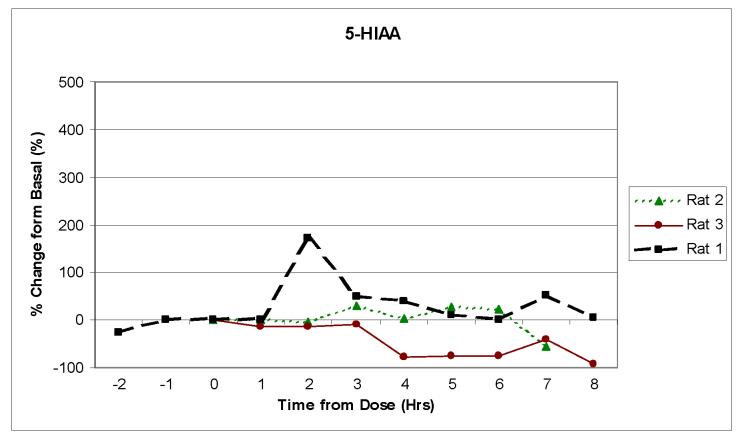
Three separate animals were then dosed with 50mg/kg of benserazide followed 20 min later by 50 mg/kg of L-DOPA. Benserazide was used to prolong the elevated concentration of the neurotransmitters in the brain (de Souza Silva et al., 1997). Figures 4a, b and c show the change over time in the concentrations of DOPAC, HVA, and 5-HIAA following dosing for three separate rats. Although the peak concentration values for each of the neurotransmitters varied for each rat, the general pharmacokinetic profiles were similar. All of the rats exhibited a significant increase in DOPAC and HVA concentrations. This increase was expected because they are in the L-DOPA metabolic pathway. The 5-HIAA, however, stayed near basal level. This was also expected, because 5-HIAA is generated through the serotonin metabolic pathway and not the L-DOPA pathway. The results seen with the increase in DOPAC and HVA show similar time profiles to what was previously reported by de Souza Silva et al., but the level of increase in concentration was much larger in our experiments.
Figure 4.
Monitoring in vivo release of neurotransmitters using the 2ML1 osmotic pump and the on-rat collection device. Rats (n = 3) were dosed with 50 mg/kg of benserazide and L-DOPA. Samples were collected every 60 minutes. The pump flow rate was approximately 11 μL/hr. Analytes in Figure 4 are: (a) HVA, (b) DOPAC and (c) 5-HIAA.
It should also be noted that each of the samples was an integrated value over the previous 60-min sampling period. One of the disadvantages of the osmotic pump approach is that it is not useful for studying very fast processes occurring in vivo. For analytes where long sampling times might be a problem due to photooxidation or degradation, antioxidants or other reagents (including an internal standard) can be added to the sample collection vial to stabilize the sample and correct for any volume errors.
Conclusions and future applications
The osmotic pump offers the possibility of portability that was previously not available, and the pump is shown to be compatible with microdialysis sampling. Most importantly, on-animal collection was shown to be feasible, enabling microdialysis sampling in non-constrained environments. Such an apparatus would benefit researchers employing training cages, exercise, mazes, etc. where animal mobility is important. Due to the low flow rates employed, the osmotic pumps are particularly attractive for long-term studies in which the desired temporal resolution is on the order of hours or days as opposed to minutes. Future applications for on-rat studies can be utilized where sampling time points of 30 min or greater are useful or where using the conventional system is a burden or impossible. The low flow rates of these pumps are advantageous for microdialysis experiments investigating analytes that occur at low concentrations in vivo. The osmotic pump, due to its limitations and lower precision, will not replace the syringe pump in all studies; however, in certain situations, the osmotic pump can be an invaluable improvement over the syringe pump.
Acknowledgments
This research was supported by research grants from the National Institutes of Health R01 (NS042929-04) and from the National Science Foundation (CHE-0111618). The authors would also like to thank Nancy Harmony for her assistance in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALZET Pump Bibliography 2006 http://www.alzet.com/bibliography/agent.php.
- Bazzett T, Becker JB, Albin RL. Chronic intrastriatal administration of high and low dose quinolinic acid using a novel device for dialytic delivery. J Neurosci Methods. 1991;40:1–8. doi: 10.1016/0165-0270(91)90111-c. [DOI] [PubMed] [Google Scholar]
- Beagles KE, Morrison PF, Heyes MP. Quinolinic acid in vivo synthesis rates, extracellular concentrations, and intercompartmental distributions in normal and immune-activated brain as determined by multiple-isotope microdialysis. J Neurochem. 1998;70:281–91. doi: 10.1046/j.1471-4159.1998.70010281.x. [DOI] [PubMed] [Google Scholar]
- Cano-Cebrian MJ, Zornoza T, Polache A, Granero L. Quantitative microdialysis in pharmacokinetic studies: some reminders. Curr. Drug. Metab. 2005;6:83–90. doi: 10.2174/1389200053586109. [DOI] [PubMed] [Google Scholar]
- Cheng FC, Yang LL, Chang FM, Chia LG, Kuo JS. Simultaneous measurement of serotonin, catecholamines and their metabolites. J Chromatog. 1992;582:19–27. doi: 10.1016/0378-4347(92)80297-4. [DOI] [PubMed] [Google Scholar]
- de Souza Silva MA, Mattern C, Hacker R, Tomaz C, Huston JP, Schwarting RKW. Increased neostriatal dopamine activity after intraperitoneal or intranasal administration of L-DOPA: on the role of benserazide pretreatment. Synapse. 1997;27:294–302. doi: 10.1002/(SICI)1098-2396(199712)27:4<294::AID-SYN3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Everett WR, Bohs C, Davies MI. Use of a new interface cell for off-column CE-EC determination of catecholamine neurotransmitters. Curr Sep. 2000;19:25–8. [Google Scholar]
- Fillenz M. In vivo neurochemical monitoring and the study of behaviour. Neurosci. and Behav. Rev. 2005;29:949–62. doi: 10.1016/j.neubiorev.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Heppert KE, Davies MI. Simultaneous determination of caffeine from blood, brain and muscle using microdialysis in an awake rat and the effect of caffeine on rat activity. Curr Sep. 1999;18:3–7. [Google Scholar]
- Herrera-Marschitz M, Goiny M, You ZB, Meana JJ, Pettersson E, Rodriguez-Puertas R, Xu ZQ, Terenius L, Hoekfelt T, Ungerstedt U. On the release of glutamate and aspartate in the basal ganglia of the rat: interactions with monoamines and neuropeptides. Neurosci Biobehav Rev. 1997;21:489–95. doi: 10.1016/s0149-7634(96)00033-4. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine-site agonists. Neuropsychopharmacology. 2004;29:300–7. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- Justice JB., Jr Quantitative microdialysis of neurotransmitters. J Neurosci Methods. 1993;48:263–76. doi: 10.1016/0165-0270(93)90097-b. [DOI] [PubMed] [Google Scholar]
- Lada MW, Kennedy RT. Quantitative in vivo measurements using microdialysis online with capillary zone electrophoresis. J Neurosci Methods. 1995;63:147–52. doi: 10.1016/0165-0270(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Martin JC, Wu Q, Stanisz LA, Martyn S, Kissinger C, Hampsch J, Garris PA. Contingent and non-contingent intracranial electrical stimulation using the raturn. Curr Sep. 2000;18:133–7. [Google Scholar]
- Mas M, Gonzalez-Mora JL, Hernandez L. In vivo monitoring of brain neurotransmitter release for the assessment of neuroendocrine interactions. Cell Mol Neurobiol. 1996;16:383–96. doi: 10.1007/BF02088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie JAM, Watson CJ, Rostand RD, German I, Witowski SR, Kennedy RT. Automated capillary liquid chromatography for simultaneous determination of neuroactive amines and amino acids. J Chromatog A. 2002;962:105–15. doi: 10.1016/s0021-9673(02)00533-2. [DOI] [PubMed] [Google Scholar]
- Menacherry S, Hubert W, Justice JB., Jr In vivo calibration of microdialysis probes for exogenous compounds. Anal Chem. 1992;64:577–83. doi: 10.1021/ac00030a003. [DOI] [PubMed] [Google Scholar]
- Newman ME, Gur E, Dremencov E, Garcia F, Lerer B, Van de Kar LD. Chronic clomipramine alters presynaptic 5-HT1B and postsynaptic 5-HT1A receptor sensitivity in rat hypothalamus and hippocampus, respectively. Neuropharmacol. 2000;39:2309–17. doi: 10.1016/s0028-3908(00)00077-0. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Yamamoto Y, Brodin E, Persson H. Chronic treatment with SCH-23390, a selective dopamine D1 receptor blocker decreases preprotachykinin-A mRNA levels in nucleus tractus solitarii of the rabbit: role in respiratory control. Brain Res Mol Brain Res. 1991;9:233–8. doi: 10.1016/0169-328x(91)90006-j. [DOI] [PubMed] [Google Scholar]
- Stenken JA, Chen R, Yuan X. Influence of geometry and equilibrium chemistry on relative recovery during enhanced microdialysis. Anal Chim Acta. 2001;436:21–9. [Google Scholar]
- Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Anal. Chem. 2006;78(5):1391–99. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Liang X, Lunte CE. Comparison of recovery and delivery in vitro for calibration of microdialysis probes. Anal Chim Acta. 1995;316:403–10. [Google Scholar]
- Zhou SY, Zuo H, Stobaugh JF, Lunte CE, Lunte SM. Continuous in vivo monitoring of amino acid neurotransmitters by microdialysis sampling with online derivatization and capillary electrophoresis separation. Anal Chem. 1995;67:594–9. doi: 10.1021/ac00099a017. [DOI] [PubMed] [Google Scholar]