Abstract
Thrombospondin 1, the prototypical protein of the thrombospondin protein family, is a potent endogenous inhibitor of angiogenesis. Although the effects of the thrombospondin 1 on neovascularization have been well studied, little is known about the anti-angiogenic potency of other proteins or peptide fragments derived from the proteins in this family. Here we identify a set of 18 novel, anti-angiogenic 17- to 20-amino acid peptides that are derived from proteins containing type I thrombospondin motifs. We have named these peptides adamtsostatin-4, adamtsostatin-16, adamtsostatin-18, cartilostatin-1, cartilostatin-2, fibulostatin-6.2, fibulostatin-6.3, papilostatin-1, papilostatin-2, properdistatin, scospondistatin, semastatin-5A.1, semastatin-5A.2, semastatin-5B, thrombostatin containing-1, thrombostatin contaning-3, thrombostatin contaning-6 and wispostatin-1 to reflect their origin. We further demonstrate that these peptides inhibit the proliferation and migration of human umbilical vein endothelial cells in vitro. The antiproliferative and antimigratory properties of the identified peptides may be important in maintaining angiogenic homeostasis in vivo and make these peptides suitable candidates for use as anti-angiogenic pharmaceutical agents in numerous therapeutic applications.
Introduction
The thrombospondin family of proteins consists of a group of five prototypical members (TSP-1, -2, -3, -4, -5) that contain a number of modules, among which are a globular amino terminal motif, a pro-collagen homology region, three type I thrombospondin repeats, three EGF or type 2 repeats, and a globular carboxy-terminal region [1]. Two of the five members of this family, thrombospondin 1 (TSP-1) and thrombospondin 2 (TSP-2), have the highest degree of similarity, both in terms of amino acid identity and structural organization.
The thrombospondins are known to have anti-angiogenic properties [2; 3; 4]. Thrombospondin 1, the first identified endogenous inhibitor of angiogenesis, has been shown to play a critical role in inhibiting neovascularization, and therefore tumor growth and metastasis. Thrombospondin 1 inhibits the proliferation and migration of endothelial cells both in vitro and in vivo [5].
A disintegrin and metalloproteinases (ADAMs) are a family of proteins with sequence similarity to the reprolysin family of snake venomases. These proteins share the metalloproteinase domain with matrix metalloproteinases (MMPs). They are structurally classified into two groups: the membrane-anchored ADAM group and the ADAM with thrombospondin motifs (ADAMTS) group. These molecules are involved in a variety of biological events, including cell adhesion, cell fusion, cell migration, membrane protein shedding and proteolysis [6]. Like the thrombospondins, the ADAMTS proteins are modular and contain a metalloproteinase catalytic domain, with a reprolysin-like zinc binding motif; a disintegrin-like domain; and a type I thrombospondin repeat. This type I TSP repeat is similar to the type I repeats found in TSP-1 and TSP-2.
Peptides from the type I thrombospondin domains of ADAMTS-1 (METH-I) and ADAMTS-8 (METH-II) have been shown to be anti-angiogenic [7; 8]. Both can inhibit vascular endothelial growth factor (VEGF)-induced angiogenesis in the chick chorioallantoic membrane assay as well as fibroblast growth factor (FGF-2)-induced neovascularization in the corneal micropocket assay [7; 8].
By using a bioinformatics algorithm, we have recently identified a set of 18 peptides of 17 to 20 amino acids that are similar to the type I thrombospondin domains of the aforementioned endogenous inhibitors of angiogenesis. These novel peptides are derived from the associated type I thrombospondin repeats of human endogenous proteins and share similarities to the known inhibitors. Several of the identified short peptides are derived from one of several ADAMTS proteins whose anti-angiogenic potency has not been identified, or from an extracellular matrix-residing protein, such as the cartilage intermediate layer protein (CILP), a fibulin, or papilin. We now present experimental evidence demonstrating that peptides derived from the type I thrombospondin repeats of these proteins are anti-angiogenic, inhibiting the proliferation and migration of human umbilical vein endothelial cells (HUVECs) in vitro.
Materials and Methods
Cell Culture
HUVECs from a single donor were obtained from Cambrex (Walkersville, MD). The cells were propagated in EGM-2 medium, consisting of a basal cell medium with 2% FBS, growth factors (hbFGF and VEGF) and antibiotics (gentamicin/amphotericin B). All the cells used were from passage 3 to 6.
Peptide Synthesis and Handling
The peptides were produced using a solid-phase synthesis technique by a commercial provider (Abgent, San Diego, CA). HPLC and mass spectroscopy analyses of each peptide were performed. For each of the peptides the synthetic procedure yielded 10 mg of >95% purity. The peptides were provided in solid form and solubilized in water before use. The molecular weight of each peptide was confirmed by mass spectrometry. In the case of highly hydrophobic peptides, dimethyl sulfoxide (DMSO) at a maximum concentration of 0.1% (v/v) was used as a solvent. We experimentally verified that at this concentration the solvent had no effect on the experimental results.
In Vitro Cell Viability Assay
We assessed the effects of our anti-angiogenic agents on the proliferation of endothelial by measuring the metabolic activity of the live cells using the colorimetric cell proliferation reagent WST-1 (Roche, Indianapolis, IN) [9]: Approximately 2×103 cells/well were seeded in a 96-well microplate without any extracellular matrix substrate and exposed for 3 days to different peptide concentrations: 0.01, 0.1, 1 and 10 μg/ml and 20, 30 and 40 μg/ml. The molecular weight of each of the peptides is approximately 2 kDa; thus, the aforementioned concentrations are equivalent to 5 nM, 50 nM, 500 nM, 5 μM, 10 μM, 15 μM and 20 μM. Each of the concentrations was tested simultaneously in quadruplicate, and each of the experiments was repeated three times. As a positive control we applied 100 ng/ml (0.22 μM) TNP-470 [10]. As a negative control (normal viability) the cells were cultured without any agent in full medium containing growth factors and serum. A more detailed description of the relevant methods is provided in [11].
In Vitro Cell Migration Assay
A modified Boyden chamber migration assay (BD Biosciences, San Jose, CA) was used to examine endothelial cell migration in the presence of an activator and the peptide solution; in our case, we used 20 ng/ml VEGF (Invitrogen, Carlsbad, CA) and 1, 10, and 30 μg/ml of the tested peptide solution. A serum- and growth factor-free medium was used as a negative control, and 20 ng/ml VEGF was used as a positive control. The chambers were then incubated for 20 h at 37°C. The cells that had migrated into the lower chamber were stained with calcein (Invitrogen, Molecular Probes, Carlsbad, CA) for 90 min prior to termination of the experiment and counted.
Computational Analysis
Statistical significance was assessed using Student's t-test, with p-values < 0.001 defined as significant.
Results and Discussion
Using a bioinformatics analysis, we have identified a set of 18 peptides derived from type I thrombospondin repeats of different proteins that show similarity to known angiogenesis inhibitors derived from these repeats, such as METH-I (ADAMTS-1) and METH-II (ADAMTS-8) and the thrombospondin repeats of the thrombospondin 1 protein (Table 1). Here we first describe the parent proteins and then present the results of our proliferation and migration experiments. We then summarize the data demonstrating that all the identified peptides can inhibit the proliferation and migration of human umbilical endothelial cells in vitro. We refer to these peptides as anti-angiogenic or exhibiting anti-angiogenic activity, with a realization that subsequent in vivo validation of these properties in different tissues is necessary.
Table 1.
The amino acid sequences of the short anti-angiogenic peptides derived from the type I thrombospondin repeat-containing proteins.
| Peptide Name | Peptide Origin | Accession Number | Peptide Sequence |
|---|---|---|---|
| Adamtsostatin-4 | ADAMTS-4 | AAD41494(527-545) | GPWGDCSRTCGGGVQFSSR |
| Adamtsostatin-16 | ADAMTS-16 | Q8TE57(1133-1149) | SPWSQCTASCGGGVQTR |
| Adamtsostatin-18 | ADAMTS-18 | CAC83612.1(595-613) | SKWSECSRTCGGGVKFQER |
| Cartilostatin-1 | CILP-1 | O75339(156-175) | SPWSKCSAACGQTGVQTRTR |
| Cartilostatin-2 | CILP-2 | Q8IUL8(153-171) | GPWGPCSGSCGPGRRLRRR |
| Fibulostatin-6.2 | Fibulin-6 | CAC37630.1(1745-1763) | ASWSACSVSCGGGARQRTR |
| Fibulostatin-6.3 | Fibulin-6 | CAC37630.1(1688-1706) | QPWGTCSESCGKGTQTRAR |
| Papilostatin-1 | Papilin | EAW81098.1(391-408) | GPWAPCSASCGGGSQSRS |
| Papilostatin-2 | Papilin | EAW81098.1(33-51) | SQWSPCSRTCGGGVSFRER |
| Properdistatin | Properdin | AAB63280.1(143-161) | GPWEPCSVTCSKGTRTRRR |
| Scospondistatin | SCO-spondin | CAJ43920.1(3652-3670) | GPWEDCSVSCGGGEQLRSR |
| Semastatin-5A.1 | Sema 5A | Q13591(660-678) | GPWERCTAQCGGGIQARRR |
| Semastatin-5A.2 | Sema 5A | Q13591(848-866) | SPWTKCSATCGGGHYMRTR |
| Semastatin-5B | Sema 5B | AAQ88491.1(916-934) | TSWSPCSASCGGGHYQRTR |
| Thrombostatin cont-1 | TSRC-1 | Q9NS62(347-365) | QPWSQCSATCGDGVRERRR |
| Thrombostatin cont-3 | TSRC-3 | AAI01021.1(333-351) | SPWSPCSGNCSTGKQQRTR |
| Thrombostatin cont-6 | TSRC-6 | NP_998769(44-60) | WTRCSSSCGRGVSVRSR |
| Wispostatin-1 | WISP-1 | O95388(221-238) | SPWSPCSTSCGLGVSTRI |
As already mentioned, the ADAMTS family of proteins has been previously shown to contain two members, ADAMTS-1 and ADAMTS-8, with anti-angiogenic potency. Here we have identified three novel peptides derived from different ADAMTS proteins: one from ADAMTS-4, which we called adamtsostatin-4; one from ADAMTS-16, which we called adamtsostatin-16; and one from ADAMTS-18, which we called adamtsostatin-18. Cartilage intermediate layer proteins (CILPs) are glycoproteins that are found in the interterritorial matrix of the deeper layers of cartilage and are more prevalent in older tissues. Here we have identified two small peptide fragments derived from CILP-1 and CILP-2, which we have named cartilostatin-1 and cartilostatin-2, respectively.
Fibulins are extracellular matrix secreted glycoproteins that have been shown to modulate cell morphology, growth, adhesion and motility during tumor progression. We have identified two anti-angiogenic peptides derived from the TSP-1 repeat of fibulin-6. We have called these peptides fibulostatin-6.2 and fibulostatin-6.3. Papilin is a proteoglycan-like sulfated glycoprotein. Here we have identified two anti-angiogenic peptides from papilin, named papilostatin-1 and papilostatin-2, which are derived from its TSP-1 repeat. Properdin (factor P) is a plasma protein that is active in the alternative complement pathway of the innate immune system. The anti-angiogenic peptide derived from properdin has been named properdistatin.
Different members of the semaphorin protein family induce neural guidance cues and also participate in developmental and postnatal vessel formation and patterning. Here we have identified three peptides derived from the type I thrombospondin repeats of semaphorin 5A, named semastatin-5A.1 and semastatin-5A.2, and from semaphorin 5B, named semastatin-5B, with anti-angiogenic properties. SCO-spondin is a large glycoprotein involved in axonal pathfinding. Here we show that a peptide derived from a TSP-1 repeat of SCO-spondin, which we call scospondistatin, also inhibits the proliferation and migration of endothelial cells.
We have also identified three peptides from thrombospondin type I domain-containing proteins -1, -3 and -6 (TSRC1 or ADAMTS-like 4, TSRC3 and TSRC6). These are transmembrane proteins that contain TSP-1 repeats. The three identified peptides, which we have named thrombostatin containing-1, -3 and -6, also inhibit the proliferation and migration of endothelial cells. The last identified peptide is derived from the type I thrombospondin repeat of WNT1 inducible signaling pathway protein 1 (WISP-1). WISP-1 is the fourth member of the CCN family (CCN-4). Wispostatin-1, the peptide we have identified within the type I thrombospondin domain of WISP-1, also exhibits anti-angiogenic activity.
The identified peptides inhibit the proliferation of HUVECs in vitro
A significant determinant of the peptides' ability to inhibit angiogenesis is their activity in suppressing the proliferation of endothelial cells. Therefore, we tested the ability of the peptides to suppress the proliferation of HUVECs in vitro. The results from the proliferation assay were scaled to allow us to express the peptide activity relative to the positive control, 100 ng/ml of TNP-470, and are presented in Figure 1.
Figure 1.
Effect of the 18 peptides derived from the type I thrombospondin repeat-containing proteins on the proliferation of HUVECs. After 3 days of incubation with the test peptides, the endothelial cells were detected using a WST-1 colorimetric assay and counted. The results are scaled so that 0% represents the optical signal from the negative control (endothelial cells incubated with medium containing growth factor and serum, data not shown), and 100% represents the signal from the positive control (cells incubated with 100 ng/ml TNP-470, data not shown). Vertical bars indicate the standard error. All values are significantly different from 0% at p<0.001, except those marked by NS (non-significant). In all cases, the standard error for the controls was <3% (n=8).
From the results in the proliferation assay for the 18 tested peptides we identified two patterns of behavior. A group of peptides, composed of cartilostatin-2, fibulostatin-6.3, properdistatin and scospondistatin, showed a monotonic dose response, with the antiproliferative activity increasing with increasing the peptide concentration. In this case, saturation was reached with increasing the peptide concentration. Within this group of peptides, we identified a subset of peptides that exhibited a monotonic dose response, but their activity had already reached saturation at the minimum tested concentration of 0.01 μg/ml. In this subset were adamtsostatin-16, adamtsostatin-16 and semastatin-5B. A second group of peptides, adamtsostatin-4, cartilostatin-1, fibulostatin-6.2, papilostatin-2, semastatin-5A.1, semastatin-5A.2, thrombostatin containing-3 and wispostatin-1, was characterized by a biphasic dose response. The peptide activity was non-monotonic, increasing with increasing peptide concentration, reaching a maximum, and then declining with increasing the peptide concentration. This non-monotonic activity is typical of endogenous anti-angiogenic peptides that have been previously described. Examples of such biphasic responses include the activity exhibited in proliferation experiments by full-length endostatin [12; 13] and its small-fragment derivatives [14]. Such biphasic dose-response activity is also exemplified by peptides derived from previously discovered anti-angiogenic fragments of thrombospondins: for example, the anti-angiogenic fragments derived from the TSP-1 [15] and TSP-2 [16] proteins and anti-angiogenic fragments derived from SPARC [17; 18] and urokinase [19].
In order to exclude false-positive results that could be attributed to the nature of the peptides tested we repeated the proliferation experiments using two scrambled peptides, where the amino acid sequences of the peptides were randomly permuted. The results obtained in the proliferation experiments using the scrambled peptides were not statistically different than those for the negative control (data not shown).
Among the ADAMTS-derived peptides, adamtsostatin-4 exhibited a biphasic response, with a maximum activity of 30% at 10 μg/ml, while adamtsostatin-16 and adamtsostatin-18 had already reached saturation at the lowest concentration tested (0.01 μg/ml), with 15% and 30% activity, respectively. Cartilostatin-1 showed a biphasic response, with a maximum activity of 30% at 0.1 μg/ml, while cartilostatin-2 was monotonic, with a maximum activity 50% at the highest concentration tested. Fibulostatin-6.2 had only a non-significant level of activity in the proliferation assay, with a maximum activity of 10%, while fibulostatin-6.3 was monotonic, with a maximum activity of 40%. Papilostatin-1 had only minimal activity in the proliferation assay, but papilostatin-2 was biphasic, with a maximum activity of 40% at 0.1 μg/ml. Properdistatin reached saturation at 20% activity at 30 μg/ml, and similarly scospondistatin also showed a maximum activity of 20% at 30 μg/ml. Of the semaphorin-5 proteins, the peptides derived from semaphorin-5A were biphasic, and both reached maximal activity (40% for semastatin-5A.1 and 30% for semastatin-5A.2) at 0.1 μg/ml. The activity of semastatin-5B reached saturation at 25% activity at the lowest concentration tested. Among the thrombospondin type I domain-containing proteins, thrombostatin containing-1 and thrombostatin containing-6 exhibited non-significant activity in the proliferation assay, while thrombostatin containing-3 was biphasic, with a maximum activity of 30% at the lowest concentration tested and declining with increasing concentrations above 20 μg/ml. Finally, wispostatin-1 was also biphasic, with a maximum activity of 30% at 1 μg/ml and decreasing activity with increasing peptide concentration.
The identified peptides inhibit the migration of HUVECs in the presence of VEGF
A critical aspect of the angiogenic process is the migration of endothelial cells in the presence of an activator or a chemoattractant such as VEGF. In order to assess the ability of the short peptides to inhibit the migration of endothelial cells, we used a modified Boyden chamber assay to measure the suppression of VEGF-stimulated migration of HUVECs through a porous membrane covered by laminin (Figure 2). As a positive control (causing decreased motility), we incubated the cells in serum- and growth factor (VEGF)-free cell medium. As a negative control, we incubated the cells with VEGF alone, and no peptide.
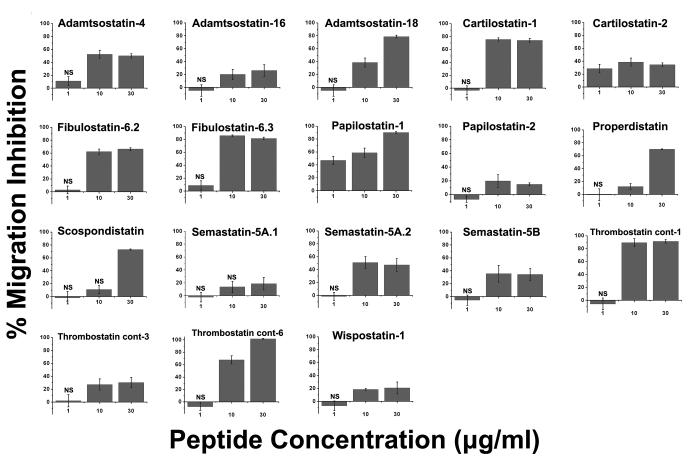
Figure 2.
Effect of the peptides on the migration of HUVECs in a modified Boyden chamber migration assay. Endothelial cells were allowed to migrate for 20 h in the presence of 20 ng/ml VEGF and 30 μg/ml peptide solution, then stained with calcein and counted. The fluorescent signal was initially scaled so that 0% represents the negative control (endothelial cells in serum- and growth factor-free medium) and 100% the positive control (migration in the presence of 20 ng/ml VEGF). Tthe percentage migration inhibition, shown in the figure, was calculated. Vertical bars indicate the standard error. All values are significantly different from 0% at p<0.001, except those marked by NS (non-significant). In all cases, the standard error for the controls was <5% (n=8).
Of the 18 tested peptides, 10 showed significant activity, above 70%, in suppressing the ability of the HUVECs to increase their motility and migrate in the presence of VEGF. Among the ADAMTS-derived peptides, adamtsostatin-18 strongly inhibited the migration of the endothelial cells, with a linear dose-response pattern. It showed 80% inhibition at 30 μg/ml, 40% at 10 μg/ml, and non-significant inhibition at 1 μg/ml. Adamtsostatin-4 exhibited an intermediate level of inhibition, with its activity reaching a maximum of 50% at 10 μg/ml. Carilostatin-1 was also a significant inhibitor of endothelial cell migration, with a maximum activity of 80% that was also saturated at 10 μg/ml. The peptides derived from fibulin-6 were both extremely potent inhibitors of cell migration, with activities of 60% and 80%, respectively, that were saturated at 10 μg/ml. Of the two peptides derived from papilin, only papilostatin-1 was active; it suppressed the migration of the HUVECs by 90% at 30 μg/ml. The peptides derived from properdin and SCO-spondin both inhibited the migration of the HUVECs by 70% at 30 μg/ml. Of the thrombospondin type I domain-containing proteins, thrombostatin containing-1 and -6 were potent inhibitors, completely suppressing the migration of the endothelial cells at 30 μg/ml. Thrombostatin containing-1 activity was actually saturated at 10 μg/ml, while at the same concentration thrombostatin containing-6 exhibited 70% inhibition of migration (Figure 2).
In summary, in the present study we have identified a set of 18 novel peptides of 17 to 20 amino acids that are derived from proteins containing type I thrombospondin repeats, and we provide evidence that these 18 peptides have anti-angiogenic activity, inhibiting the proliferation and migration of HUVECs in vitro. In our proliferation assay, we identified two distinct populations of peptides on the basis of their activity profiles: a set that exhibited a monotonic dose response, showing increasing activity with increasing peptide concentration, and a set that exhibited a non-monotonic, biphasic dose response. In the second set, the peptide activity reached a maximum and then decreased with increasing its concentration.
Recent studies concerning the mechanisms of the anti-angiogenic activity of TSP-1, the prototype type I thrombospondin repeat-containing protein, have implicated CD36 as the cell-surface receptor that mediates its effects on endothelial cells [20]. CD36 is an 88-kDa transmembrane glycoprotein expressed on endothelial cells and a collagen-binding molecule. Analyses using affinity chromatography have demonstrated that various motifs of the type I thrombospondin repeats of the TSP-1 protein can bind to CD36 [21]. Recently, β1 integrins were identified as critical components of the thrombospondin repeats' anti-migratory effects on endothelial cells [22; 23]. The epitope that is responsible for the α3β1 integrin binding maps within the amino-terminus of the thrombospondin-1 protein [24]. Future studies, including mutagenesis studies, need to be performed with the identified peptides in order to determine whether this receptor interaction can explain any or all of the data we present here.
Angiogenesis is regulated by the orchestrated expression of endogenous regulatory elements [25; 26; 27; 28; 29], which include an array of growth factors that stimulate and control the proliferation and migration of endothelial cells. There is also a growing population of endogenous regulators that work competitively by inhibiting both of these processes, thus suppressing angiogenesis. The fine control of these two opposing elements, referred to as the angiogenic balance [30], is vital for the homeostasis of a physiologic tissue and is disrupted during pathologic conditions, such as cancer. Thus, the identification of novel endogenous anti-angiogenic peptides that may play a role in both physiological and pathological conditions, such as the peptides described here, have the potential to increase our understanding of angiogenesis in health and disease.
Acknowledgments
The authors thank Zaver Bhujwalla and Roberto Pili for useful discussions; David Noren, Venu Raman, Kristine Glunde, Noriko Mori, Paul Winnard and David Qian for their valuable advice on the experimental assays and Deborah McClellan for editorial assistance. The work was supported in part by NIH grants NHLBI R01 HL079653 and NCI P50 CA103175.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iruela-Arispe ML, Luque A, Lee N. Thrombospondin modules and angiogenesis. Int J Biochem Cell Biol. 2004;36:1070–8. doi: 10.1016/j.biocel.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 2.Carpizo D, Iruela-Arispe ML. Endogenous regulators of angiogenesis--emphasis on proteins with thrombospondin--type I motifs. Cancer Metastasis Rev. 2000;19:159–65. doi: 10.1023/a:1026570331022. [DOI] [PubMed] [Google Scholar]
- 3.Ren B, Yee KO, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765:178–88. doi: 10.1016/j.bbcan.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–42. [PubMed] [Google Scholar]
- 5.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349–57. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- 8.Iruela-Arispe ML, Vazquez F, Ortega MA. Antiangiogenic domains shared by thrombospondins and metallospondins, a new family of angiogenic inhibitors. Ann N Y Acad Sci. 1999;886:58–66. doi: 10.1111/j.1749-6632.1999.tb09400.x. [DOI] [PubMed] [Google Scholar]
- 9.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518–20. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- 10.Farinelle S, Malonne H, Chaboteaux C, Decaestecker C, Dedecker R, Gras T, Darro F, Fontaine J, Atassi G, Kiss R. Characterization of TNP-470-induced modifications to cell functions in HUVEC and cancer cells. J Pharmacol Toxicol Methods. 2000;43:15–24. doi: 10.1016/s1056-8719(00)00080-0. [DOI] [PubMed] [Google Scholar]
- 11.Karagiannis ED, Popel AS. Identification of novel short peptides derived from the α4, α5 and α6 fibrils of type IV collagen with anti-angiogenic properties. Biochem Biophys Res Commun. 2007 doi: 10.1016/j.bbrc.2006.12.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 13.Celik I, Surucu O, Dietz C, Heymach JV, Force J, Hoschele I, Becker CM, Folkman J, Kisker O. Therapeutic efficacy of endostatin exhibits a biphasic dose-response curve. Cancer Res. 2005;65:11044–50. doi: 10.1158/0008-5472.CAN-05-2617. [DOI] [PubMed] [Google Scholar]
- 14.Tjin Tham Sjin RM, Satchi-Fainaro R, Birsner AE, Ramanujam VM, Folkman J, Javaherian K. A 27-amino-acid synthetic peptide corresponding to the NH2-terminal zinc-binding domain of endostatin is responsible for its antitumor activity. Cancer Res. 2005;65:3656–63. doi: 10.1158/0008-5472.CAN-04-1833. [DOI] [PubMed] [Google Scholar]
- 15.Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopes N, Gregg D, Vasudevan S, Hassanain H, Goldschmidt-Clermont P, Kovacic H. Thrombospondin 2 regulates cell proliferation induced by Rac1 redox-dependent signaling. Mol Cell Biol. 2003;23:5401–8. doi: 10.1128/MCB.23.15.5401-5408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sage EH, Reed M, Funk SE, Truong T, Steadele M, Puolakkainen P, Maurice DH, Bassuk JA. Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J Biol Chem. 2003;278:37849–57. doi: 10.1074/jbc.M302946200. [DOI] [PubMed] [Google Scholar]
- 18.Chlenski A, Liu S, Baker LJ, Yang Q, Tian Y, Salwen HR, Cohn SL. Neuroblastoma angiogenesis is inhibited with a folded synthetic molecule corresponding to the epidermal growth factor-like module of the follistatin domain of SPARC. Cancer Res. 2004;64:7420–5. doi: 10.1158/0008-5472.CAN-04-2141. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Mazar AP, Lebrun JJ, Rabbani SA. An antiangiogenic urokinase-derived peptide combined with tamoxifen decreases tumor growth and metastasis in a syngeneic model of breast cancer. Cancer Res. 2002;62:4678–84. [PubMed] [Google Scholar]
- 20.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–17. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwalt DE, Lipsky RH, Ockenhouse CF, Ikeda H, Tandon NN, Jamieson GA. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992;80:1105–15. [PubMed] [Google Scholar]
- 22.Short SM, Derrien A, Narsimhan RP, Lawler J, Ingber DE, Zetter BR. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol. 2005;168:643–53. doi: 10.1083/jcb.200407060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, Mosher DF, Roberts DD. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem. 2004;279:41734–43. doi: 10.1074/jbc.M406267200. [DOI] [PubMed] [Google Scholar]
- 24.Krutzsch HC, Choe BJ, Sipes JM, Guo N, Roberts DD. Identification of an alpha(3)beta(1) integrin recognition sequence in thrombospondin-1. J Biol Chem. 1999;274:24080–6. doi: 10.1074/jbc.274.34.24080. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 26.Folkman J. Endogenous inhibitors of angiogenesis. Harvey Lect. 1996;92:65–82. [PubMed] [Google Scholar]
- 27.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 28.Folkman J. Endogenous angiogenesis inhibitors. Apmis. 2004;112:496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- 29.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–79. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 30.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]