Abstract
A decrease in D2 dopamine receptor subtype (D2R) binding in the striatum has been reported in obese individuals and drug addicts. We examined D2R density in the striatum of food-restricted rats that had contingent access to food with different incentive values. Results showed that animals receiving limited access to 0.3 M sucrose paired 2 h with a chow meal for 7 days had a significantly lower D2R binding in nucleus accumbens shell and dorsolateral striatum compared with animals that had limited access to chow. There was no differential binding, however, in the accumbens core in any of the groups. These findings indicate that feeding conditions and sucrose intake influence D2R density specifically in subregions of the striatum.
Keywords: D2-receptor autoradiography, Dopamine, Homeostasis, Ingestive behavior, Nucleus accumbens, Striatum, Sucrose licking
INTRODUCTION
Alterations in dopamine (DA) neurotransmission have been implicated in the pathology of obesity and eating disorders [1]. In particular, it has been shown that striatal D2 dopamine receptor subtype (D2R) availability is significantly decreased in severely obese individuals [2]. Interestingly, combined treatment with dopamine D1 and D2 receptor agonists (e.g. SKF3893 and bromocryptine) in genetically obese mice (i.e., ob/ob) reduced the hyperglycemia, hyperphagia, hyperinsulinemia and elevated body weight associated with this strain [3] suggesting an overall hyposensitivity of the dopaminergic system associated with obesity.
The mesoaccumbens DA system (MADS) appears to play a role in regulation of ingestive behaviors including feeding and drug use [4–6]. Specifically, it has been shown that direct application of a D2R antagonist (i.e., sulpiride) in the rat nucleus accumbens (NAcc) increases DA levels and sucrose intake [5], whereas an infusion of the D2R agonist (i.e. quinpirole) into the NAcc shell increases aversive oro-facial behaviors to the intra-oral infusion of quinine [6]. Additionally, low D2R binding in the MADS is predictive of higher rates of cocaine self-administration in non-human primates [7], and a lower D2R density has also been observed in drug-dependent humans [8,9]. Suggesting that in instances where there is chronic excitation of the MADS, a reactive down-regulation of D2R occurs. In addition to an influence of D2R on the magnitude or frequency of ingestive behaviors (i.e. natural and non-natural), an increasing body of evidence indicates that D2R manipulation alters feeding patterns [4,10,11]. The reverse relationship, the influence of feeding patterns on D2R density in the striatum, however, has not been addressed.
Feeding behavior can be manipulated by food restriction [12,13], or limited access [14], as well as by offering foods with different preference values [13,14]. Food restriction has been demonstrated to affect DA transmission in the NAcc [15]. Moreover, highly preferred foods, including sucrose solutions, reliably elicit an increase in extracellular DA in the MADS [5] even when it is without caloric consequences (i.e. saccharin [16] or sham-fed sucrose [17]). Therefore, repeated access to sucrose under food restricted conditions should elicit a down-regulation of D2R in the MADS. On this basis, we hypothesized that rats with limited access to sucrose, that is, contingently presented with a less preferred chow meal, will have lower D2R binding in the NAcc compared with rats that are presented chow either non-contingently with sucrose or receive exclusively chow in contingent sessions. Therefore, repeated access to sucrose under food restricted conditions should elicit a down-regulation of D2R in the MADS. In the present experiment, we used a 7 day food-restriction with limited access to a preferred 0.3M sucrose solution or regular laboratory chow presented in a scheduled (i.e., contingent) or random, non-predictive (i.e., non-contingent) fashion and examined D2R density in the dorsolateral striatum and the NAcc (i.e., shell and core). We hypothesized that rats with access to sucrose that is contingently (i.e., 2 h paired) presented with a chow meal will have lower D2R binding in the NAcc, the terminal region of the MADS.
MATERIALS AND METHODS
Animals
Twenty-four male Sprague–Dawley rats (Charles River, Wilmington, MA), initially weighing 350–400 g were individually housed and placed on a 12:12 h light:dark schedule (lights on 07.00 h). During an initial acclimatization period (~1 week), all rats had ad libitum access to regular laboratory chow pellets (Rodent Diet-W 8604, Harlan Teklad, Madison, WI) and water. The entire experiment was conducted in the animals’ home cages. All experimental protocols were approved by the Institutional Animal Care and Use Committee and were in accordance with NIH guidelines.
Experimental groups and feeding protocols
On the last day of the acclimatization period, rats were divided into two main feeding conditions. One group was put on a restricted-fed regimen, whereas another group of rats, not subject to any deprivation (naive, n = 6), was added for histological controls. The feeding protocols were introduced in a staggered fashion for pairs of animals in each group (two animals/group/day for each condition on 3 consecutive days). This method made euthanasia of all the animals possible within the best matching time period across all conditions.
With respect to stimulus properties and contingencies, further groups were formed within the restricted-fed condition. Two separate groups of restricted-fed rats were presented daily for 7 days with either a sucrose solution (0.3 M) or chow at 10.00–10.20 h. Two hours later both groups (each n = 6), had a 20 min access to chow (12.20–12.40 h; contingent sucrose food (CSF), and contingent food-food (CFF) groups, respectively). A third restricted-fed group (n = 6) served as a non-contingent control receiving sucrose, food, or no stimulus at the two 20 min access sessions (2 h inter-access period interval) in a random, non-predictive fashion (non-contingent sucrose food (NCSF) group). All restricted-fed groups (CSF, CFF, and NCSF) had ad libitum access to chow and water for 2 h each afternoon (14.00–16.00 h). All access periods consisted of placing a filled graduated bottle (i.e., sucrose or water) on the front of the animal’s cage or in the case of chow, placing pellets in the animal’s cage for the allotted time (20 min or 2 h).
Histology
On day 7, all rats were decapitated between 11.45 and 12.20 h. After decapitation brains were removed and immediately immersed in −40°C isopentane (2-methyl-butane) and stored at −70°C. The brains were sectioned on a cryostat in the coronal plane at 20 μm and thaw-mounted on poly-lysine-coated slides. In this particular experiment, the brain regions examined were from the dorsal and ventral striatum (1.7–1.1 mm from Bregma; inclusive of the NAcc and dorsal striatum) [18]. Four sections from each brain region were serially mounted on a slide. Approximately eight slides were taken from each brain region (representing an approximate distance of 160 μm between sections). One slide from each brain region was subject to cresyl Lecht violet staining to accurately determine anterior-posterior anatomical coordinates for proper analytical comparisons. The remaining sectioned tissue was kept at −70°C until assayed.
Autoradiography
We followed a modified published protocol for [125I]iodosulpride ligand binding for dopamine D2 receptor subtype (with Kd of 0.6 nM and 1.2 nM for D2 and D3, respectively [19]; Amersham, sp. ac. 2000 Ci/mmol) [20]. Slide-mounted, matched sections were removed from −70°C freezer storage and brain sections were allowed to thaw for at least 3 min at room temperature. Briefly, slide mounted sections were air dried for 3 min, pre-incubated for 15 min in 50 mM Tris–HCl (pH 7.4), 120 mM NaCl at room temperature and followed by an incubation for 30 min at room temperature in 50 mM Tris–HCl (pH 7.4), 120 mM NaCl, 5 mM KCl, 2 mM CaCl, 1 mM MgCl2, and 0.6 nM [125I]iodosulpride. This concentration of iodosulpride was determined by previously running a series of test sections at stepped concentrations bracketing a suggested concentration of 0.3 nM. Slides were rinsed twice for 5 min each in ice-cold 50 mM Tris–HCl (pH 7.4) buffer and placed before a stream of air at room temperature to dry for several hours. Non-specific binding was determined on a set of test sections incubated in a parallel manner as described above with the addition of 50 μM s (+)-apomorphine to the [125I]iodosulpride incubation solution. Subsequently, the slides were placed in a cassette with [125I] microscale standards (Amersham, Arlington Heights, IL) and apposed Kodak X-OMAT AR film (Eastman Kodak, Rochester, NY) for 18 h.
Quantitative analysis
Immediately after the exposure, the films were developed using standard photography procedure. Receptor density was determined semi-quantitatively by a densitometry procedure using the microscales to generate a standard curves. Film analysis was done with the PC compatible AIS (Analytical Imaging Station; Imaging Research Inc. St. Catherines, Ontario) software. Binding was assessed for target region unilaterally in all tissue. Both background density (as sampled from corpus callosum) and nonspecific binding density were subtracted from all assayed tissue.
Statistical analysis
Binding of [125I]iodosulpride for each restricted-fed group was expressed as percentage of binding of the ad lib-fed naive group. Absolute binding levels for each group and striatal structure is represented in fmol/mg of tissue equivalent and shown in Table 1. Results from collapsed data of the binding assays across sections from each brain region (NAcc core and shell, and dorsolateral striatum) were analyzed by two-way ANOVA (treatment × structure) and post hoc LSD tests were made where applicable (Statistica 5.0, Tulsa, OK) and statistical significance was regarded as p<0.05.
Table 1.
Absolute binding of [125I]iodosulpride in the striatum of restricted-fed and ad libitum-fed rats.
| Feeding conditions | Striatal regions | ||
|---|---|---|---|
| Restricted feeding | NAcc core | NAcc shell | Dorsolateral striatum |
| Contingent sucrose-food (CSF; n= 6) | 5.80 ± 0.25 | 6.54 ± 0.36 | 9.52 ± 0.37 |
| Non-contingent sucrose-food (NCSF; n= 6) | 5.99 ± 0.19 | 6.82 ± 0.17 | 11.70 ± 0.28* |
| Contingent food-food (CFF; n= 6) | 6.08 ± 0.21 | 7.40 ± 0.50* | 10.78 ± 0.34 |
| Ad libitum feeding | |||
| Naive (n= 6) | 5.80 ± 0.26 | 5.90 ± 0.36 | 10.21 ± 0.23 |
Binding is expressed in fmol/mg tissue equivalent ± SEM. Autoradiography binding was performed on striatal sections (AP:1.7–1.1mm from Bregma) on day 7 of the feeding regimen (see Materials and methods for details). Statistical comparisons of restricted fed groups to ad libitum fed groups are indicated
p<0.01 and p<0.05. For binding differences between groups within striatal structures see Fig. 1.
RESULTS
Nucleus accumbens
Overall analysis of D2R-binding in the NAcc core (core) did not reveal any group effect or difference from naive animals (Fig. 1). In the medial aspect of the NAcc (Shell), D2R binding analysis showed a significant effect across all experimental groups (group: F(3,40) = 3.72, p<0.01). Post hoc test revealed that binding in all restricted-fed groups was significantly elevated from the naive controls (CSF and NCSF, p<0.05; CFF p<0.01; Fig. 1). In the shell, binding in the restricted-feeding condition was the lowest in the CSF group, whereas the highest amount of binding was observed in CFF animals (110 ± 2.4% and 126 ± 4.9%, respectively), with the difference between these groups being statistically significant (p<0.05). Although there was a greater mean binding in the NCSF group (115 ± 0.3%) than in the CSF group, this difference did not yield statistical significance.
Fig. 1.
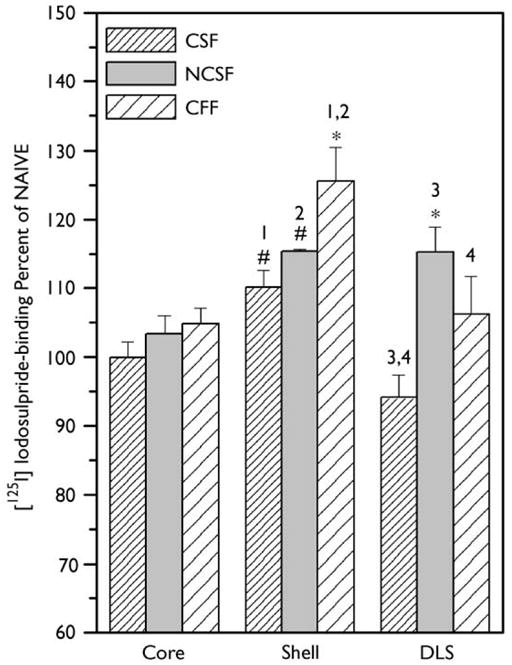
[125I]iodosulpride binding in the striatum of the restricted fed groups expressed as a percentage of naive controls. The striatal regions examined were the medial shell of the NAcc (shell), core of the NAcc (core), and dorsolateral striatum (DLS). Values indicate mean binding within of each group and error bars indicate SEM. Statistical comparisons shown in this figure reflect difference from naive *p<0.01, p<0.05; with restricted-fed group differences (see results for details) and post-hoc analysis between groups; 1: p<0.05, 2: p<0.05, 3: p<0.01, 4: p<0.01.
Dorsolateral striatum
Binding in the dorsolateral striatum (DLS), in the identical anterior–posterior coronal plane of analysis as the NAcc, also showed a significant treatment effect (F(3,38) = 4.02, p<0.002; Fig. 1). The NCSF group had the highest level of binding (115 ± 3.6%) and this was the only group that was different from the ad lib-fed naive animals (p<0.001). The CFF group had somewhat less binding (106 ± 5.5%), which was not significant from the NCSF group. As was the case in the NAcc shell, the CSF group had the lowest binding (94.1 ± 3.2%) in DLS, which was statistically significant compared with the NCSF (p<0.01) and CFF groups (p<0.01).
DISCUSSION
In the present experiment, adult rats were exposed to a short (i.e. 7 day) restricted-feeding regimen with limited access to sucrose and food to investigate density of dopamine D2R binding in the striatum. To our knowledge, only two studies have examined the effects of food restriction on D2R density. One assessed the basal telencephalon of developing chickens [21], and the other in the rat striatum but without limited access feeding [15], and neither reported a change in D2R density. In contrast, we observed an overall increase in D2R-binding in the NAcc shell of rats in response to food restriction with limited access feeding in comparison with the ad libitum-fed animals. Binding, however, was the highest in the experimental group that was subjected to the CFF regimen. Since the CFF group did not have access to sucrose, this suggests that ingestion of palatable sucrose results in lower D2R binding as observed in the CSF and NCSF groups. Hence, differential D2R density in the shell may reflect food-stimulus properties (i.e. sweet vs not sweet) rather than feeding contingencies. We also observed an increased D2R binding in the DLS in the NCSF group in comparison with ad libitum-fed controls. This finding may be due, at least in part, to the non-contingent presentation of the sucrose and food or to an increase in exploratory behavior (not measured in the present experiment) associated with stimulus expectancy [22]. Moreover, lower D2R density in DLS reflects feeding contingencies (lower in CSF and CFF), but also may be influenced by stimulus properties of the food (i.e., CSF<CFF).
Smith [23] has speculated that D2R antagonism (via intra-accumbens or intraperitoneal injection of raclopride) decreases the positive feedback associated with sucrose intake in rats. Furthermore, Hagan and Moss [13] demonstrated that food restriction in rats with bouts of re-feeding with highly palatable foods leads to persistent rebound hyperphagia. These results suggest that changes in dopaminergic tone may be associated with ingestion of palatable foods. Thus, alterations in D2R density in the NAcc and the DLS as a result of feeding patterns could potentially lead to a dysregulation of homeostatic feedback (allostasis) [24] contributing to compensatory DA-related changes, and may also lead to behavioral manifestations like binge eating. Similar dysregulation in accumbal dopaminergic systems have been reported in experiments with mineralocorticoid-induced salt-appetite [25]. Since a decrease in D2R availability has been observed in obese individuals [2], alteration of D2R as consequence of feeding patterns could also be implicated in certain types of human obesity leading to a cycle of aberrant eating patterns.
CONCLUSION
Despite considerable evidence to indicate that central and peripheral administration of D2R antagonist can alter feeding behavior, this study was the first to examine the influences of feeding patterns on D2R density in restricted-fed rats. The results showed that D2R density is altered in striatal regions, which are associated with incentive-motivational and motor-related behaviors, in response to feeding patterns. This study also suggests that dopaminergic mechanisms can be differentially altered as a function of food restriction and incentive value of the food.
Acknowledgments
The authors thank Mr. N. Acharya for assistance with administration of the feeding regimen and Ms. Z. Celen technical assistance with the D2R autoradiography. This research was supported by NIH grants DC 04751, DC 00240, MH43787.
Contributor Information
Nicholas T. Bello, Department of Behavioral Science and Neuroscience Graduate Program, The Pennsylvania State University, College of Medicine, Hershey, PA17033
Louis R. Lucas, Laboratory of Neuroendocrinology, The Rockefeller University, New York, NY 10021, USA
Andras Hajnal, Department of Behavioral Science and Neuroscience Graduate Program, The Pennsylvania State University, College of Medicine, Hershey, PA17033.
References
- 1.Leibowitz SF, Hoebel B. Behavioral neuroscience and obesity. In: Bray GA, Bouchard C, James PT, editors. The Handbook of Obesity. New York: Marcel Dekker; 1998. pp. 313–359. [Google Scholar]
- 2.Wang GJ, Volkow ND, Logan J, et al. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 3.Cincotta AH, Tozzo E, Scislowski PW. Life Sci. 1997;61:951–956. doi: 10.1016/s0024-3205(97)00599-7. [DOI] [PubMed] [Google Scholar]
- 4.Ikemoto S, Panksepp J. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 5.Hajnal A, Norgren R. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 6.Sederholm F, Johnson E, Brodin U, et al. Psychopharmacology (Berl) 2002;160:161–169. doi: 10.1007/s00213-001-0966-1. [DOI] [PubMed] [Google Scholar]
- 7.Morgan D, Grant KA, Gage HD, et al. Nature Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 8.Volkow ND, Wang GJ, Fowler JS, et al. J Pharmacol Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- 9.Hietala J, West C, Syvalahti E, et al. Psychopharmacology (Berl) 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- 10.Nowend KL, Arizzi M, Carlson BB, et al. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- 11.Swanson CJ, Heath S, Stratford TR, et al. Pharmacol Biochem Behav. 1997;58:933–945. doi: 10.1016/s0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 12.Carr KD. Neurochem Res. 1996;21:1455–1467. doi: 10.1007/BF02532386. [DOI] [PubMed] [Google Scholar]
- 13.Hagan MM, Moss DE. Int J Eat Disord. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Corwin RL, Wojnicki FH, Fisher JO, et al. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 15.Pothos EN, Creese I, Hoebel BG. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mark GP, Blander DS, Hoebel BG. Brain Res. 1991;551:308–310. doi: 10.1016/0006-8993(91)90946-s. [DOI] [PubMed] [Google Scholar]
- 17.Hajnal A, Smith GP, Norgren R. SSIB, Appetite. 2001;37:141–142. [Google Scholar]
- 18.Paxinos G, Watson C. Compact. 3. Sydney: Academic Press; 1997. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 19.Sokoloff P, Giros B, Martres MP, et al. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 20.Bouthenet ML, Martres MP, Sales N, et al. Neuroscience. 1987;20:117–155. doi: 10.1016/0306-4522(87)90008-x. [DOI] [PubMed] [Google Scholar]
- 21.Kostal L, Vyboh P, Savory CJ, et al. Neuroscience. 1999;94:323–328. doi: 10.1016/s0306-4522(99)00255-9. [DOI] [PubMed] [Google Scholar]
- 22.van Oosten RV, Cools AR. Exp Neurol. 2002;173:245–255. doi: 10.1006/exnr.2001.7816. [DOI] [PubMed] [Google Scholar]
- 23.Smith GP. Prog Psychobiol Physiol Psychol. 1995;16:83–144. [PubMed] [Google Scholar]
- 24.Koob GF, Le Moal M. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 25.Lucas LR, Pompei P, McEwen BS. Neuroendocrinology. 2000;71:386–395. doi: 10.1159/000054559. [DOI] [PubMed] [Google Scholar]


