Abstract
Batrachotoxin (BTX) is a steroidal alkaloid that causes Na+ channels to open persistently. This toxin has been used widely as a tool for studying Na+ channel gating processes and for estimating Na+ channel density. In this report we used point mutations to identify critical residues involved in BTX binding and to examine if such mutations affect channel gating. We show that a single asparagine → lysine substitution of the rat muscle Na+ channel α-subunit, μ1-N434K, renders the channel completely insensitive to 5 μM BTX when expressed in mammalian cells. This mutant channel nonetheless displays normal current kinetics with minimal changes in gating properties. Another substitution, μ1-N434A, yields a partial BTX-sensitive mutant. Unlike wild-type currents, the BTX-modified μ1-N434A currents continue to undergo fast and slow inactivation as if the inactivation processes remain functional. This finding implies that the μ1-N434 residue upon binding with BTX is critical for subsequent changes on gating; alanine at the μ1–434 position apparently diminishes the efficacy of BTX on eliminating Na+ channel inactivation. Mutants of two adjacent residues, μ1-I433K and μ1-L437K, also were found to exhibit the identical BTX-resistant phenotype. We propose that the μ1-I433, μ1-N434, and μ1-L437 residues in transmembrane segment I-S6 probably form a part of the BTX receptor.
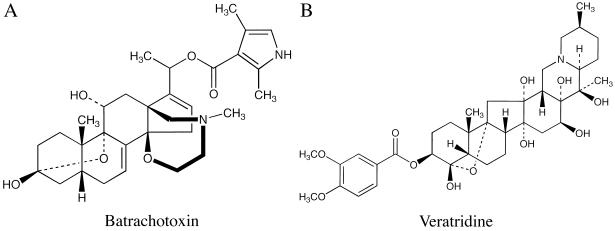
Batrachotoxin (BTX, Diagram D1, pKa ≈7.5) is one of the most toxic small molecules known and is surprisingly abundant in the skin of South American frogs Phyllobates terribilis (1–3). These frogs were used by native Indians of Colombia to prepare poison blowdarts. The primary target of BTX is known to be voltage-gated Na+ channels in excitable membranes. For self-preservation, the Na+ channels of muscle and nerve from such neotropical Phyllobates frogs are insensitive to 5 μM BTX (3). Daly et al. (3) suggested that this BTX-resistant phenotype is “genetically controlled” and that the site of this mutation has minimal effects on Na+ channel function. This hypothesis seems counterintuitive with the fact that BTX binding produces substantial changes in Na+ channel gating function. For example, on binding with BTX the activation process of Na+ channels is shifted to the hyperpolarizing direction by more than 30 mV (for reviews see refs. 4–6). Both fast and slow inactivation of the Na+ channels also are inhibited by BTX. As a result, the Na+ channels open persistently even at the resting potential. Such potent effects suggest that the BTX binding site is coupled to activation as well as inactivation processes and therefore is considered to be very important in Na+ channel gating function.
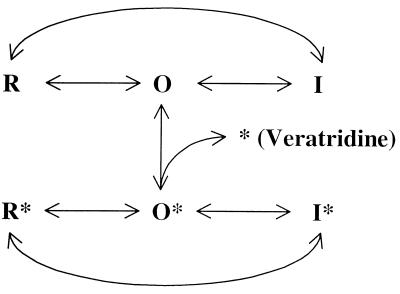
Veratridine (Diagram D1, pKa ≈9.5), which is an alkaloid found in Liliaceae plants, competes with BTX binding in a mutually exclusive manner (4–7). However, the pharmacological effects of veratridine on Na+ channels are quite different from those of BTX (8–10). First, veratridine reduces the single Na+ channel conductance drastically whereas BTX does not. Veratridine therefore is regarded as a partial agonist and BTX as a full agonist of Na+ channels (4). Second, under voltage-clamp conditions BTX binds practically irreversibly to Na+ channels whereas veratridine readily dissociates from its binding site (6, 8). Both of these drugs, however, bind preferentially to the open state of Na+ channels. Remarkably, the BTX-resistant Na+ channels in Phyllobates frogs remain sensitive to veratridine (3), a result that suggests the binding domains for BTX and veratridine do not completely overlap. The precise location of the binding domains for BTX (Kd ≈50 nM) and veratridine (Kd ≈7 μM) (4, 5) within the Na+ channel α-subunit remains unknown.
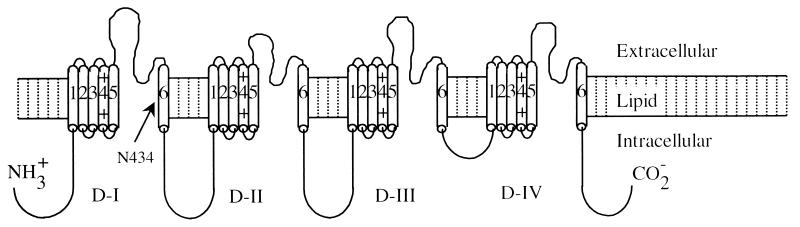
To delineate the BTX binding site, we sought to create Na+ channel mutants that are resistant to BTX. The Na+ channel α-subunit contains four homologous domains (I to IV, Diagram D2) each with six transmembrane segments (S1-S6) (11). Previously, it has been shown that a photoreactive BTX derivative can be crosslinked to segment I-S6 of the Na+ channel α-subunit (12). This finding implies that the BTX binding site is near segment I-S6. In addition, a recent report suggested that the conserved N434 residue of the μ1 channel (also in segment I-S6, see arrow in Diagram D2) is critical for slow inactivation (13). Because BTX modifies slow inactivation of Na+ channels (6, 8, 14), it seems feasible that the N434 residue may be directly involved in binding with BTX and that such binding interactions alter Na+ channel gating function. In this report we focused on the role of the μ1–434 residue in BTX and veratridine binding.
MATERIALS AND METHODS
Site-Directed Mutagenesis.
Mutagenesis of μ1 Na+ channel clone (15) was accomplished on μ1-pcDNA1/Amp by means of the Transformer Site-Directed Mutagenesis Kit (CLONTECH). Two primers (a mutagenesis primer and a restriction primer) were used to generate the desired mutant (13). The mutation was confirmed by DNA sequencing. To avoid unwanted mismatch mutations, we selected two independent clones for μ1-N434K and for μ1-N434A. No differences in phenotype were found in each of the two independent clones. Single clones were generated for all other mutants.
Transient Transfection.
The culture of Hek293t cells and their transient transfection were performed as described (16). Cells were grown to 50% confluence in DMEM (GIBCO) containing 10% fetal bovine serum (HyClone), 1% penicillin and streptomycin solution (Sigma), 3 mM taurine, and 25 mM Hepes (GIBCO) and then transfected by a calcium phosphate precipitation method in a Ti25 flask. Transfection of μ1-pcDNA1/Amp (10 μg) and reporter plasmid CD8-pih3m (1 μg) for 15 hr was satisfactory (13). Cells were replated 15 hr after transfection, maintained at 37°C in a 5% CO2 incubator, and used for experiments after 1–4 days in culture. Transfection-positive cells were identified by immunobeads (CD8-Dynabeads, Lake Success, NY).
Whole-Cell Voltage Clamp.
Whole-cell currents were recorded in cells coated with CD-8 beads (16, 17). Patch electrodes contained 100 mM NaF, 30 mM NaCl, 10 mM EGTA, and 10 mM Hepes adjusted to pH 7.2 with CsOH. The advantages of using high internal Na+ ions have been discussed before (18). The electrodes had a tip resistance of 0.5–1.0 MΩ. Experiments were performed at room temperature (22–24°C) under either a reversed Na+ gradient (bath solution: 150 mM choline Cl, 2 mM CaCl2, and 10 mM Hepes adjusted to pH 7.4 with tetramethyl hydroxide) or an equal external/internal Na+ solution (130 mM NaCl, 20 mM choline Cl, 2 mM CaCl2, and 10 mM Hepes adjusted to pH 7.4 with tetramethyl hydroxide). No CdCl2 was included in bath solution. Residual outward currents were evident in some cells at voltages >+30 mV; these currents were present in untransfected cells (13) and were insensitive to tetrodotoxin. Stock solutions of BTX (0.5 mM) and veratridine (20 mM) were dissolved in dimethyl sulfoxide. BTX was a generous gift from John Daly (National Institutes of Health, Bethesda, MD), and veratridine was purchased from Sigma. Whole-cell currents were filtered at 3 kHz and collected by pClamp software (Axon Instruments, Foster City, CA). An unpaired Student’s t test was used to evaluate estimated parameters (means ± SEM or fitted values ± SE of the fit); P values of <0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Mutation μ1-N434K Yields a BTX-Resistant Phenotype with Normal Na+ Current Kinetics.
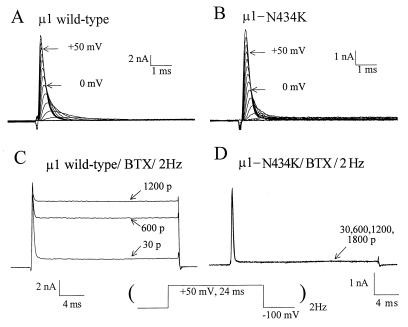
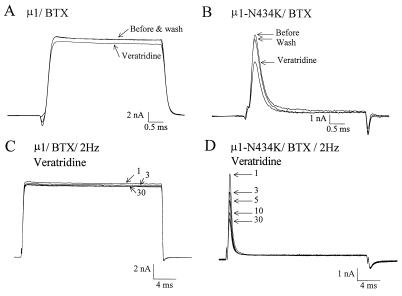
The α-subunit of the rat muscle Na+ channel (μ1; refs. 16 and 19), when transiently expressed in human embryonic kidney cells (Hek293t), displays fast activation and fast inactivation current kinetics under a reversed Na+ gradient (Fig. 1A). Wild-type μ1 Na+ currents first activated around the threshold of −50 to −40 mV. For comparison, Fig. 1B shows the current family recorded from a cell transfected with μ1-N434K mutant Na+ channels. The threshold for activation was also near −50 to −40 mV and the τ value of the fast-decaying phase at +50 mV measured 0.23 ± 0.01 ms (mean ± SEM) for both wild type (n = 8) and N434K mutant (n = 10). With 5 μM BTX in the pipette, this toxin had limited effects on μ1 wild-type channels unless repetitive pulses were applied (Fig. 1C, 30 vs. 1,200 pulses). This enhancement in BTX binding by repetitive pulses is caused by preferential binding of BTX to the open state of the Na+ channel (14, 20). A significant fraction of the peak μ1 Na+ current was modified by BTX at +50 mV (83.8 ± 4.1%, n = 6) and became noninactivating after 1,200 repetitive pulses (Fig. 1C). Evidently, BTX inhibited strongly the fast inactivation of μ1 Na+ channels. By comparison, even after 1,800 repetitive pulses BTX did not elicit significant changes in the μ1-N434K current in >20 cells tested (Fig. 1D). The N434K mutant channel was thus completely resistant to BTX at 5 μM. The parameters for the activation and the steady-state inactivation (h∞) of N434K mutant were strikingly comparable to those of the wild type (Table 1), except a small +3 mV shift in the h0.5 (P < 0.05). These data, along with fast-rising and fast-decaying current kinetics (Fig. 1), demonstrated that BTX-resistant μ1-N434K channels were functionally normal.
Figure 1.
Na+ current families were recorded in Hek293t cells expressing either μ1-wild-type (A) or μ1-N434K channels (B) under reversed Na+ gradient conditions. Cells were dialyzed by internal solution for 10 min, and current traces were superimposed from −70 to +80 mV at 10-mV step. With 5 μM BTX in the pipette, repetitive pulses (Lower) were applied at 2 Hz and current traces of μ1-wild-type (C) or μ1-N434K channels (D) were superimposed. The pulse numbers are labeled; 30 p represents the 30 pulses applied to monitor Na+ currents during cell dialysis.
Table 1.
Voltage dependence of activation and steady-state inactivation in μ1 wild-type and μ1-N434K channels
| Parameter | μ1 Wild type | μ1-N434K | |
|---|---|---|---|
| Activation | E0.5 | −39.2 ± 2.3 mV (n = 11) | −35.0 ± 2.5 mV (n = 9) |
| ka factor | 7.8 ± 1.0 mV (n = 11) | 8.7 ± 0.8 mV (n = 9) | |
| Steady-state inactivation (h∞) | h0.5 | −76.6 ± 0.9 mV (n = 10) | −73.3 ± 1.1 mV (n = 10)* |
| kh factor | 7.2 ± 0.3 mV (n = 10) | 6.5 ± 0.3 mV (n = 10) |
Cells were dialyzed by internal solution for 30 min, and the activation parameters were determined by I/V curves in equal internal/external Na+ ion concentrations. The peak conductance, g = INa/(E − Erev) where INa is the peak current and Erev is the estimated reversal potential, was normalized, plotted against the corresponding voltage E and least-squares fitted with a Boltzmann equation, g/gmax = 1/{1 + exp(E0.5 − E)/ka} where E0.5 is the voltage at which g/gmax = 0.5, ka is the slope factor and gmax is the maximal conductance (13). The h∞ parameters were determined by a two-pulse protocol. Peak currents were measured, normalized, and plotted against the prepulse potentials. The data were least-squares-fitted with a Boltzmann equation, y = 1/{1 + exp[(Epp − h0.5)/kh]}, where h0.5 is the voltage at which y = 0.5 and kh is the slope factor (13). The fitted values and SEM of the individual fit are presented.
P < 0.05.
The Effects of Veratridine on BTX-Resistant Mutant Channels.
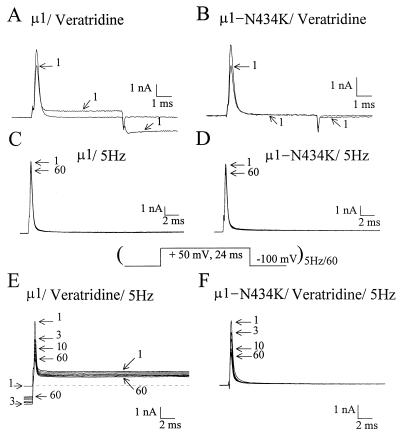
BTX and veratridine are likely to share an overlapping binding site (4, 8) within the Na+ channel α-subunit. To test whether μ1-N434K channels are sensitive to veratridine, we applied 200 μM of this alkaloid from the external side under equal internal/external Na+ ion concentrations. Fig. 2 A and B show that veratridine inhibits the peak μ1 and μ1-N434K currents equally by about 25–35% (28.7 ± 2.0%, n = 8, and 31.7 ± 2.4%, n = 8, respectively, P = 0.32). The effects elicited by veratridine were readily reversible upon superfusion of drug-free external solution. One possible explanation for this reduction of the peak Na+ current amplitude by veratridine is that it is caused by toxin binding during Na+ channel opening. According to Hille (6), veratridine gains access to its receptor only through the open state of Na+ channels (O in Diagram D3). Resting and inactivated states (R and I in Diagram D3) show little binding toward veratridine. The veratridine-modified μ1 Na+ current became noninactivating during the 6-ms test pulse (Fig. 2A, trace 1). This noninactivating current is carried by veratridine-modified Na+ channels that have a reduced single-channel conductance (refs. 9 and 10; O* in Diagram D3). The single-channel conductance previously was estimated 16 pS and 4 pS for unmodified and for veratridine-modified neuronal Na+ channels, respectively (9). In addition, a persistent inward tail current appeared at the holding potential (−100 mV) in the wild type (Fig. 2A, trace 1) caused by the shift in activation of the O* state to the hyperpolarizing direction. In contrast, the N434K mutant lacked the noninactivating current and had little veratridine-induced tail current (Fig. 2B, trace 1). This phenotype suggests that veratridine-modified N434K mutant Na+ channels may have an even smaller conductance (i.e., ≪4 pS) or, alternatively, they may display gating behavior different from their wild-type counterparts (such as infrequent opening or with a rather brief open duration). Even with an increase in the amplifier gain by 20- to 40-fold the records did not show any noninactivating veratridine-induced N434K channel activity during the pulse. Without veratridine, repetitive pulses at 5 Hz elicited little use-dependent inhibition of wild-type and μ1-N434K Na+ currents (Fig. 2 C and D). In the presence of veratridine, repetitive pulses elicited significant use-dependent inhibition of both wild-type and μ1-N434K peak currents (Fig. 2 E and F). This phenomenon is well known for veratridine (8, 9). Repetitive pulses provide frequent opening of the Na+ channel, increase the probability of veratridine binding, and cause the apparent use-dependent inhibition of peak currents (9). The persistent tail current at the holding potential appeared after the first pulse. The inward tail current decayed slowly at −100 mV and remained even before the second pulse (Fig. 2E, before outward current) and its amplitude increased from pulse 2 to 5 and then decreased gradually. The reason for the gradual decrease of veratridine-induced tail current during repetitive pulses (Fig. 2E) is unclear but may be caused by severe loading (21) of Na+ ions within the small Hek-293t cells. Internal loading of Na+ ions would change the reversal potential and the driving force on Na+ ions. Furthermore, slow inactivation of veratridine-bound Na+ channels (I* in Diagram D3) and the continuing dissociation of veratridine from channels (O* →O + * in Diagram D3) also may contribute to this phenomenon (6, 8). In our assay, veratridine therefore is equally effective in inhibiting the peak μ1-N434K and wild-type Na+ currents, despite the fact that veratridine-modified μ1-N434K channels may carry very little current (Fig. 2 A vs. B). This phenotype is comparable with the effects of veratridine on the BTX-resistant skeletal muscle of Phyllobates frogs. The depolarization of the resting membrane potentials in Phyllobates muscle cells by veratridine is reduced significantly (3), as if their veratridine-modified channels also carry a smaller current than normal channels in frogs of Rana pipiens. To explain this phenotype we suggest that the N434K mutation does not abolish veratridine binding but does appear to affect the channel conductance or the gating behavior on veratridine binding. Further detailed studies on this phenotype are needed to substantiate this interpretation.
Figure 2.
The inhibition of peak μ1-wild-type (A) and μ1-N434K (B) Na+ currents by external 200 μM veratridine was measured by a test pulse (+50 mV for 6 ms) under equal internal/external Na+ ion concentrations. Trace 1 represents the current recorded 3 min after drug application; control traces before drug are not labeled. Repetitive pulses (shown below) elicited only a small use-dependent inhibition of peak μ1-wild-type (C) and μ1-N434K (D) Na+ currents. With superfusion of 200 μM veratridine for 5 min, repetitive pulses elicited significant use-dependent inhibition of peak μ1-wild-type (E) and μ1-N434K (F) Na+ currents. Current traces corresponding to 1, 3, 5, 10, 20, 30, 40, 50, and 60 pulses are superimposed.
Loss of BTX Binding in BTX-Resistant Channels.
Because BTX and veratridine are competitive and mutually exclusive in binding and because BTX binding with native Na+ channels is practically irreversible under voltage-clamp conditions (6), it is possible to perform protection experiments with these two toxins in wild-type and in μ1-N434K mutant channels. To determine whether BTX binding is truly abolished in the μ1-N434K channel, we included 5 μM BTX in the pipette and applied 1,200 repetitive pulses. Fig. 3 A and B shows that the inhibition of BTX-modified wild-type μ1 channels by veratridine is drastically reduced (6.6 ± 1.5%, n = 6, vs. 28.7 ± 2.0% without BTX; P < 0.05), but the inhibition of BTX-pretreated μ1-N434K current persists (32.5 ± 1.9%, n = 5, vs. 31.7 ± 2.4% without BTX, n = 8) under identical reversed Na+ gradient conditions. Likewise, the use-dependent inhibition of the μ1-N434K peak current (55% inhibition) by veratridine remains with BTX present (Fig. 3D) but is nearly abolished (3% inhibition) in BTX-modified wild-type μ1 channels (Fig. 3C). This result supports the previous conclusion that in Na+ channels BTX and veratridine are mutually exclusive in binding (4) and implies that BTX does not bind to μ1-N434K mutant channels.
Figure 3.
Traces of BTX-pretreated μ1-wild-type (A) and μ1-N434K (B) Na+ currents under a reversed Na+ gradient are superimposed before and after external 200 μM veratridine application. Current traces after washout also are shown. To prevent Na+ ion loading by inward currents we included no Na+ ions in bath solution. BTX at 5 μM was included in the pipette and 1,200 repetitive pulses (+50 mV for 24 ms at 2 Hz) were applied before veratridine application. With superfusion of 200 μM veratridine, repetitive pulses were reapplied to elicit BTX-treated μ1-wild-type (C) and μ1-N434K (D) currents. Pulse numbers corresponding to current traces are labeled.
Other N434 Mutations that Affect BTX Binding.
Under physiological conditions, a lysine residue (K) probably carries a positive charge that may directly interfere with the toxin’s binding through charge-charge or charge-dipole repulsion or through a disruption of hydrophobic interactions between the channel and BTX. Consistent with this direct involvement idea, an identical BTX-resistant, veratridine-sensitive phenotype was found for the μ1-N434R (arginine) mutant (tested in seven cells). Additionally, we observed that an equivalent mutation to μ1-N434K in human heart Na+ channels (22), hH1-N406K, also renders these channels completely resistant to BTX (tested in nine cells), thereby demonstrating that a single N→K mutation in segment I-S6 produces the same BTX-resistant phenotype regardless of Na+ channel isoform. This hH1-N406K channel also remains sensitive to 200 μM veratridine in a manner similar to μ1-N434K channel.
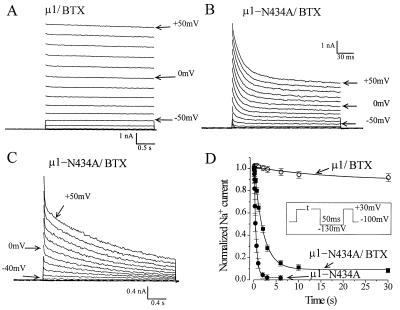
Previously, we reported that a mutation in μ1-N434A enhances slow inactivation and accelerates fast inactivation (13). Because alanine at N434A is a nonpolar residue, it may not abolish BTX binding as charged lysine at N434K does. Fig. 4 shows that BTX still binds with μ1-N434A channels but its effects differ considerably from those in wild-type channels. Unlike typical BTX-modified μ1 Na+ currents (Fig. 4A), BTX-modified N434A currents continued to decline with a multiphasic kinetic (Fig. 4 B and C). It is possible that this decaying phase of BTX-modified μ1-N434A currents is caused by the fast and slow inactivation of BTX-modified μ1-N434A channels. To test this possibility we investigated the slow inactivation of BTX-modified μ1-N434A channels during a prolonged depolarization by a two-pulse protocol as shown in Fig. 4D (Inset). A 50-ms gap of −130 mV was inserted to allow the recovery of BTX-modified μ1-N434A channels from the observed fast inactivation shown in Fig. 4B. The result shows that slow inactivation in wild type is nearly abolished by BTX (Fig. 4D, ○, n = 9) even with a 30-s conditioning pulse at +30 mV. In contrast, more than 90% of BTX-modified N434A mutant currents are inactivated under the same pulse protocol. The development of this slow inactivation follows a single exponential decay with a time constant of 2.06 ± 0.07 s at +30 mV (fitted value and SE of the fit, n = 6, Fig. 4D, ▪), a value slower than that of mutant channels without BTX (0.46 ± 0.01, n = 9, Fig. 4D, •). Accordingly, the remnant of this slow inactivation process is likely to be the cause of the slow decaying phase of the BTX-modified μ1-N434A currents shown in Fig. 4C (with a time constant of 1.56 s at +30 mV). For wild type, the time constant measured 2.11 ± 0.10 s without BTX (n = 9). Thus, the alanine (A) substitution at the μ1–434 position is critical not only in accelerating both fast and slow inactivation (13) but is also important in coupling between BTX binding and its subsequent changes in the channel gating behavior.
Figure 4.
Superimposed traces of μ1-wild-type (A) and μ1-N434A (B and C) Na+ current families at various voltages were recorded under reversed Na+ gradient conditions. Repetitive pulses (+50 mV for 24 ms at 2 Hz) were applied for a total of 1,200 pulses before recording. Frequent rests during repetitive pulses were allowed in μ1-N434A for recovery from slow inactivation (13). The pipette solution contained 5 μM BTX. The BTX-modified μ1-N434A currents with multiple decaying phases shown with two different time scales are evident (B and C; τf1 = 12.8 ms, τf2 = 86.4 ms, and τs = 1.56 s at +30 mV). Note that BTX-modified wild-type and μ1-N434A Na+ channels were activated at potentials 30–40 mV more negative than the unmodified wild-type channels. (D) The time courses of slow inactivation in BTX-modified μ1 and μ1-N434A channels were measured by a conventional two-pulse protocol (Inset). Note that >90% of BTX-modified N434A currents were inactivated whereas little inactivation occurred in BTX-modified μ1 wild-type currents. For comparison, the time course of slow inactivation of μ1-N434A channels without BTX present also is shown.
The Role of N434 in Na+ Channel Gating.
The N residue at segment I-S6 is conserved in Na+ channel α-subunit isoforms responsible for generating action potentials in excitable membranes. Assuming that position μ1-N434 is a part of the BTX binding site and that it is critical for the BTX-induced phenotype in the wild type, one expects that the N434 mutation will alter Na+ channel gating drastically. Why then does the μ1-N434K-mutation produce little changes in Na+ channel gating (Fig. 1B and Table 1)? The N → K substitution at 434 position apparently retains its proper function with respect to the movement of the four individual S4 segments (activation gating; ref. 23) and the closing of the inactivation particle located at the III-IV linker (fast inactivation gating; ref. 24). This paradox resembles the puzzling fact that BTX-resistant veratridine-sensitive Phyllobates frogs survive in the wild (3). Together we conclude that BTX-resistant Na+ channels can be created with minimal gating changes. Despite this finding, we are unable to define the role of conserved N434 residue in Na+ channel gating by way of allosteric action. Further studies are needed to address such a complicated question.
Other Lysine Mutants Within Segment I-S6 that Affect BTX Binding.
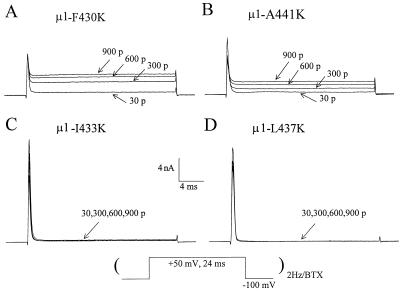
Dependent on the secondary structure of segment I-S6 (e.g., ref. 25), residues adjacent to N434 position also may play a significant role in BTX binding and in coupling with distinct gating processes. Residues within segment I-S6 from position μ1-F430 to position μ1-A441 were individually replaced with the K residue. Three of these 12 mutants did not express sufficient currents (L432K, A438K, and V440K residues). Preliminary results show that seven of the nine remaining mutants expressed functional channels that were sensitive to 5 μM BTX (e.g., Fig. 5 A and B). Inhibition of peak Na+ currents during repetitive pulses was observed in some of these mutants even without BTX presence. Such inhibition during repetitive pulses may slow the binding rate of BTX toward the open channel as shown in Fig. 5B. Surprisingly, we found that only two mutants, μ1-I433K and μ1-L437K, expressed Na+ channels that were completely resistant to 5 μM BTX (Fig. 5 C and D). These results suggest that μ1-I433, N434, and L437 may orient to the same face of an α-helix structure (25) and form a part of the BTX binding site. Although μ1-F430K and μ1-A441K residues are located at the same face, they apparently do not exhibit the BTX-resistant phenotype (Fig. 5 A and B) and are probably outside of the BTX binding domain.
Figure 5.
Na+ current traces from mutants of μ1-F430K (A), μ1-A441K (B), μ1-I433K (C), and μ1-L437K (D) were recorded during repetitive pulses at 2 Hz (protocol shown below). The pipette solution contained 5 μM BTX. The pulse numbers are labeled; 30 p represents the 30 pulses applied to monitor Na+ currents during cell dialysis. Significant use-dependent inhibition of peak currents from μ1-A441K and μ1-I433K mutants occurred during repetitive pulses. This use-dependent inhibition was not caused by BTX because the same phenomenon was found in the absence of BTX (also see ref. 13).
In summary, our results demonstrate that the μ1-N434 residue in segment I-S6 is critical for BTX binding and that an introduction of a positively charged amino acid (K or R) at this position disrupts BTX binding completely. A direct involvement of the μ1-N434 residue in BTX binding is likely because a photoreactive BTX derivative can be covalently linked to amino acids near segment I-S6 (12). Within 12 adjacent mutants tested only two other lysine mutants (I433K and L437K) exhibit BTX-resistant phenotype, suggesting that these three residues are within the BTX binding domain. It will be worthwhile to determine the naturally occurring Na+ channel mutation(s) in Phyllobates frogs (3) by using reverse transcription–PCR. Such Phyllobates mutants along with mutants in segment I-S6 and mutants within other adjacent transmembrane segments eventually may help to elucidate how BTX alters Na+ channel gating at the molecular level.
Acknowledgments
We thank J. Daly, J. Trimmer, R. Kallen, and S. Cannon for BTX toxin, μ1–2, hH1, and CD-8 clones, respectively. We are grateful to G. R. Strichartz and S. N. Wright for helpful discussion and to C. Quan for technical assistance. This work was supported by the National Institutes of Health (GM-35401 and GM-48090).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviation: BTX, batrachotoxin.
References
- 1.Myers C W, Daly J W, Malkin B. Bull Am Mus Nat Hist. 1978;161:307–360. [Google Scholar]
- 2.Warnick J E, Albuquerque E X, Onur R, Janssen S-E, Daly J, Tokuyama T, Witkop B. J Pharmacol Exp Ther. 1975;193:232–245. [PubMed] [Google Scholar]
- 3.Daly J W, Myers C W, Warnick J E, Albuquerque E X. Science. 1980;208:1383–1385. doi: 10.1126/science.6246586. [DOI] [PubMed] [Google Scholar]
- 4.Catterall W A. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- 5.Strichartz G R, Rando T A, Wang G K. Annu Rev Neurosci. 1987;10:237–267. doi: 10.1146/annurev.ne.10.030187.001321. [DOI] [PubMed] [Google Scholar]
- 6.Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 7.McKinney L C, Chakraverty S, De Weer P. Anal Biochem. 1986;153:33–38. doi: 10.1016/0003-2697(86)90056-4. [DOI] [PubMed] [Google Scholar]
- 8.Ulbricht W. In: Ion Channels. Narahashi T, editor. New York: Plenum; 1990. pp. 123–168. [DOI] [PubMed] [Google Scholar]
- 9.Barnes S, Hille B. J Gen Physiol. 1988;91:421–443. doi: 10.1085/jgp.91.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigel E. Pflügers Arch. 1987;410:112–120. doi: 10.1007/BF00581903. [DOI] [PubMed] [Google Scholar]
- 11.Catterall W A. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 12.Trainer V L, Brown G B, Catterall W A. J Biol Chem. 1996;271:11261–11267. doi: 10.1074/jbc.271.19.11261. [DOI] [PubMed] [Google Scholar]
- 13.Wang S Y, Wang G K. Biophys J. 1997;72:1633–1640. doi: 10.1016/S0006-3495(97)78809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khodorov B, Revenko S V. Neuroscience. 1979;4:1315–1330. doi: 10.1016/0306-4522(79)90159-3. [DOI] [PubMed] [Google Scholar]
- 15.Trimmer J S, Cooperman S S, Tomiko S A, Zhou J, Crean S M, et al. Neuron. 1989;3:33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- 16.Cannon S C, Strittmatter S M. Neuron. 1993;10:317–326. doi: 10.1016/0896-6273(93)90321-h. [DOI] [PubMed] [Google Scholar]
- 17.Hamill O P, Marty E, Neher M E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 18.Cota G, Armstrong C M. J Gen Physiol. 1989;94:213–232. doi: 10.1085/jgp.94.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ukomadu C, Zhou J, Sigworth F J, Agnew W S. Neuron. 1992;8:663–676. doi: 10.1016/0896-6273(92)90088-u. [DOI] [PubMed] [Google Scholar]
- 20.Tanguy J, Yeh J Z. J Gen Physiol. 1991;97:499–519. doi: 10.1085/jgp.97.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubois J M, Coulombe A. J Gen Physiol. 1984;84:25–48. doi: 10.1085/jgp.84.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gellens M E, George A L, Chen L, Chahine M, Horn R, Barchi R L, Kallen R G. Proc Natl Acad Sci USA. 1992;89:554–558. doi: 10.1073/pnas.89.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang N, George A L, Horn R. Neuron. 1996;16:113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- 24.West J W, Patton D E, Scheuer T, Wang Y, Goldin A L, Catterall W A. Proc Natl Acad Sci USA. 1992;89:10905–10909. doi: 10.1073/pnas.89.22.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guy H R, Conti F. Trends Neurosci. 1990;13:201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]