Abstract
Pleiotrophin (PTN, Ptn) is an 18 kDa cytokine expressed in human breast cancers. Since inappropriate expression of Ptn stimulates progression of breast cancer in transgenic mice and a dominant negative PTN reverses the transformed phenotype of human breast cancer cells that inappropriately express Ptn, it is suggested that constitutive PTN signaling in breast cancer cells that inappropriately express Ptn activates pathways that promote a more aggressive breast cancer phenotype. Pleiotrophin signals by inactivating its receptor, the Receptor Protein Tyrosine Phosphatase (RPTP)β/ζ, and, recently, PTN was found to activate Anaplastic Lymphoma Kinase (ALK) through the PTN/RPTPβ/ζ signaling pathway in PTN-stimulated cells, not through a direct interaction of PTN with ALK and thus not through the PTN-enforced dimerization of ALK. Since full-length ALK is activated in different malignant cancers and activated ALK is a potent oncogenic protein, we examined human breast cancers to test the possibility that ALK may be expressed in breast cancers and potentially activated through the PTN/RPTPβ/ζ signaling pathway; we now demonstrate that ALK is strongly expressed in different histological subtypes of human breast cancer; furthermore, ALK is expressed in both nuclei and cytoplasm and, in the “dotted” pattern characteristic of ALK fusion proteins in anaplastic large cell lymphoma. This study thus supports the possibility that activated ALK may be important in human breast cancers and potentially activated either through the PTN/RPTPβ/ζ signaling pathway, or, alternatively, as an activated fusion protein to stimulate progression of breast cancer in humans.
Keywords: PTN; RPTPβ/ζ, ; ALK; breast cancer; tumor progression
Introduction
Breast cancers progress through genetic and epigenetic mutations that deregulate oncogenic pathways that initiate a more aggressive breast cancer phenotype [1, 2]. The identification of these mutations is of major importance, since, once identified, it is likely their identification will lead to new targets for therapeutic development and potentially target directed therapy.
Anaplastic Lymphoma Kinase (ALK) is a receptor-type transmembrane tyrosine kinase (RTK) that is a member of the insulin receptor superfamily. ALK is functionally important in embryonic development [3] and in determination of cell survival and cell fate [4]. However, ALK was originally discovered when it was found that the chimeric N-terminal nucleophosmin (NPM) domain/cytoplasmic (catalytic) ALK domain fusion protein (NPM-ALK) is the oncoprotein underlying the pathogenesis of anaplastic large cell lymphomas (ALCL) [5, 6]. NPM-ALK results from the (2;5) (p23;q35) chromosomal translocation, however, other translocations also lead to constitutive activation of ALK and other malignancies and furthermore, wild-type ALK has been postulated to be involved in the pathogenesis of rhabdomyosarcomas [7], neuroblastoma and neuroectodermal tumors [8, 9], glioblastomas [10], and melanomas [11].
ALK is activated through autophosphorylation [12] that has been presumed to result from ligand-enforced dimerization of ALK, a mechanism in common with other receptor tyrosine kinases (RTKs) [13]. Recently, ALK was proposed to be the physiological receptor of the 136 amino acid cytokine pleiotrophin (PTN, Ptn) [14]. However, these data were not reconciled with earlier studies that demonstrated that the Receptor Protein Tyrosine Phosphatase (RPTP)β/ζ is the functional receptor of PTN [15]; in those earlier studies, PTN was shown to inactivate RPTPβ/ζ and to increase tyrosine phosphorylation of the substrates of RPTPβ/ζ, which results from phosphorylation of these substrates by kinases that phosphorylate the same sites dephosphorylated by RPTPβ/ζ when cells are not stimulated by PTN. The levels of tyrosine phosphorylation of β-catenin [15] and of other substrates of RPTPβ/ζ, including β-adducin [16, 17], Fyn [18], GIT1/Cat-1 [19], and P190RhoGAP [20], all are increased in PTN-stimulated cells.
More recently, we demonstrated that ALK is phosphorylated independently of a direct interaction of ALK with PTN (Perez-Pinera et al. Submitted 1); it was demonstrated that ALK phosphorylated in tyrosine in PTN-stimulated cells is a substrate of RPTPβ/ζ and its levels of tyrosine phosphorylation increase in PTN-stimulated cells as also was found with each of the substrates of ALK identified above. It was demonstrated that phosphorylation of ALK in PTN-stimulated cells requires RPTPβ/ζ, and that chemically enforced dimerization of RPTPβ/ζ stimulates phosphorylation of both full-length ALK and ALK that lacks an extracellular domain. Our data furthermore demonstrated RPTPβ/ζ dephosphorylates the same site in ALK that is autophosphorylated in ALK. Thus, the PTN-dependent inactivation of RPTPβ/ζ in PTN-stimulated cells permits unchecked autophosphorylation of ALK and its activation.
Pleiotrophin is expressed in breast cancers and in cell lines derived from human breast cancers [21]; since targeting constitutive PTN signaling by a dominant negative PTN reversed the malignant phenotype of human breast cancer cells in vivo [22], it is suggested that constitutive PTN signaling contributes to the pathogenesis of advanced breast cancer. Recently, different models to determine the role of inappropriate expression of Ptn in breast cancer were tested (Chang et al. Submitted 2); it was found that inappropriate expression of Ptn that was targeted to breast epithelial cells by the mouse mammary tumor virus (MMTV) promoter does not induce breast cancer in a MMTV-Ptn transgenic mice breast cancer model; however, MMTV-driven PTN signaling cooperated with the oncoprotein polyoma middle T-antigen (PyMT) to promote progression of breast cancer in MMTV-PyMT-Ptn bitransgenic mice. It was furthermore found that secretion of PTN alone through activation of stromal cells and induction of marked remodeling of the microenvironment was sufficient to account for significant features of breast cancer progression, thus, the data supports potentially a very important role of PTN signaling in promoting a more aggressive breast cancer phenotype.
Based on our recent findings that inappropriate expression of Ptn in transgenic mice stimulates breast cancer progression (Chang et al. Submitted 2), that PTN signaling may be important in breast cancer [22], and that PTN activates the potent oncoprotein ALK through the PTN/RPTPβ/ζ signaling pathway in PTN-stimulated cells (Perez-Pinera et al. 1), we explored different databases to learn whether ALK is expressed in human breast cancers; only a single manuscript described ALK expression in breast cancer cell lines using RT-PCR [11]. The significance of the transcripts of ALK detected by PCR is unknown and, in the database search, we failed to uncover that ALK is expressed in human breast cancer tissues.
We have now analyzed expression of ALK in tissues derived from human breast cancers using immunohistochemistry. We demonstrate that ALK is highly expressed in each of the different subtypes of human breast cancer studied; we furthermore observed that the cellular location and patterns of ALK expression in the breast cancer cells differs significantly from its pattern of expression in normal breast tissues; this study thus is consistent with the possibility that ALK may be activated through a constitutively activated PTN/RPTPβ/ζ signaling pathway in breast cancers that inappropriately express Ptn; the data also raise the alternative possibility that constitutively activated ALK fusion oncoproteins may contribute to the pathogenesis of breast cancers.
Methods
Breast cancer tissue arrays (Catalog number CC08-01-005) were obtained from Cybrdi (Frederick, Maryland). Tissue slides were deparaffinized (2×10min) in xylene, and hydrated (2×10min) with 100% 95% (2×10min), (1×10min) 90%, (1×10min) 70% ethanol and distilled water (10 min). The slides were then incubated in antigen retrieval solution (Trypsin 0.05%, CaCl2 0.1% pH 7.8) for 20 minutes at 37°C and then for 10 minutes at room temperature in a humidified chamber as previously described [23]. Endogenous peroxidase was quenched by incubating the sections with 3% hydrogen peroxide for 5 minutes and the tissues were permeabilized by incubating the samples in Tris-buffered saline (TBS, 10 mM Tris pH 7.6, 150 mM NaCl) with 1% Triton X-100 for 30 minutes.
Non-specific binding of the antibodies was reduced by incubating the sections for 30 minutes in a blocking solution containing 2% bovine calf serum, 2% goat serum, 1%BSA, 0.1% gelatin, 0.1% Triton X-100, 0.05% Tween 20 in 10 mM PBS, pH 7.2. The sections were incubated overnight with anti-ALK antibodies (Zymed, currently Invitrogen, Carlsbad, CA) diluted 1:100 in PBS pH 7.2, 1% BSA, 0.1% gelatin overnight. The slides were then washed with permeabilization solution (2×10min), incubated with SuperPicTure polymer from Zymed for 30 minutes, washed in PBS (2×3min), and developed with DAB provided with the in the SuperPicTure kit Zymed. The slides were rinsed in distilled water 10 min and dehydrated with 70% (1×10min), 90% (1×10min), 95% (2×10min), 100% ethanol (2×10min), and cleared in xylene (2×10min), mounted, observed with a Nikon TE2000U microscope coupled with a Confocal Cell Imaging CARV system, and photographed.
Slides with breast cancers from mouse mammary tumor virus (MMTV)-Polyoma Middle T antigen (PyMT) mice were used as positive control for ALK expression. Samples from MMTV-PyMT mice in which primary antibodies were omitted were used as negative control.
Results
Expression of ALK protein was tested in 63 samples of human breast cancer from 22 subjects using immunohistochemistry. The histological phenotypes of the breast cancers studied included infiltrating duct carcinomas, infiltrating lobular carcinomas, medullary carcinomas, mucinous adenocarcinomas, intraductal carcinomas, comedocarcinoma, and Paget’s disease. ALK was expressed in all breast cancer sections analyzed (Figure 1). It was expressed in the breast cancer cells themselves and, in all cases analyzed, ALK also was expressed in the tumor associated fibroblasts within the foci of breast cancer and in the arterial smooth muscle cells and nerve fibers. ALK also is expressed in samples of normal tissues (Figure 1H); it is expressed in the normal breast epithelium and in the smooth muscle cells of the blood vessels. The distribution of ALK in breast epithelial cells was localized to the cytoplasm, with higher expression in the apical surface in direct relationship to the ductal lumen.
Figure 1. Expression of ALK in different human breast cancers.
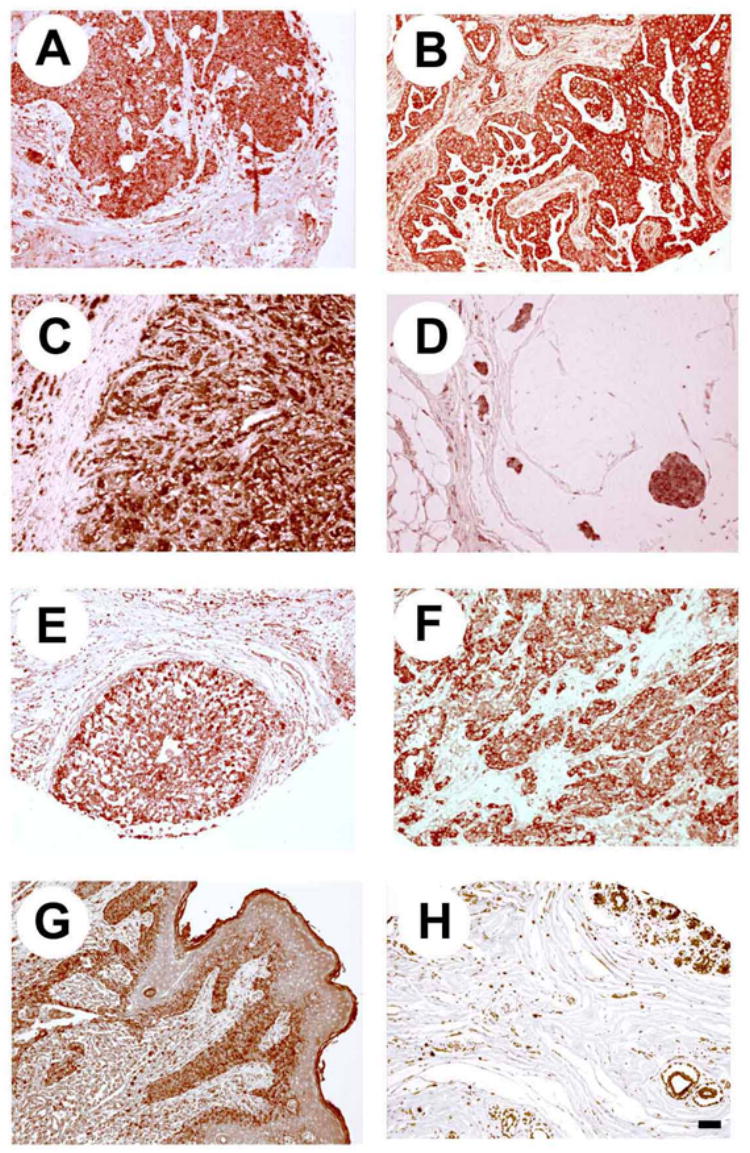
Low level magnification (×100). A. Infiltrating ductal carcinoma B. Infiltrating ductal carcinoma (papillary pattern). C. Infiltrating lobular carcinoma. D. Mucinous adenocarcinoma. E. Intraductal carcinoma. F. Medullary carcinoma. G. Paget’s disease. H. Normal breast tissue. Bar = 250 μm.
The pattern of expression and subcellular localization of ALK was then examined at 600X in the different histological phenotypes of the breast cancer (Figure 2). The pattern of expression of ALK was variable in different cells in invasive ductal carcinomas. ALK was homogeneously distributed in the cytoplasm in the papillary subtype of invasive ductal carcinoma (Figure 2C) whereas the pattern of expression was “dot-like” in other invasive ductal carcinomas (Figure 2A). ALK was localized both in cytosol and nuclei in different invasive lobular carcinomas (Figure 2B), intraductal carcinomas (Figure 2F), and, in some samples of mucinous carcinomas (Figure 2G). In other samples of mucinous carcinoma and in comedocarcinoma (Figure 2E) ALK was distributed in a “dot-like” pattern. ALK is largely expressed in the cytoplasm of the cancer cells in medullary carcinomas, although low to moderate levels of ALK also were found in nuclei (Figure 2D). In samples from patients with Paget’s disease of the breast, ALK is expressed in very high levels in the cytoplasm and in nuclei of stromal fibroblasts and in the inflammatory cells, whereas in the epithelial cells ALK is localized in the cytoplasm (Figure 2H). These extraordinary differences in the patterns of expression of ALK in breast cancer has been previously described in studies of the different ALK fusion oncogenic proteins that characteristically are observed in ALCLs [24] and differ very significantly from normal breast tissues.
Figure 2. Different patterns of ALK expression in human breast cancers.
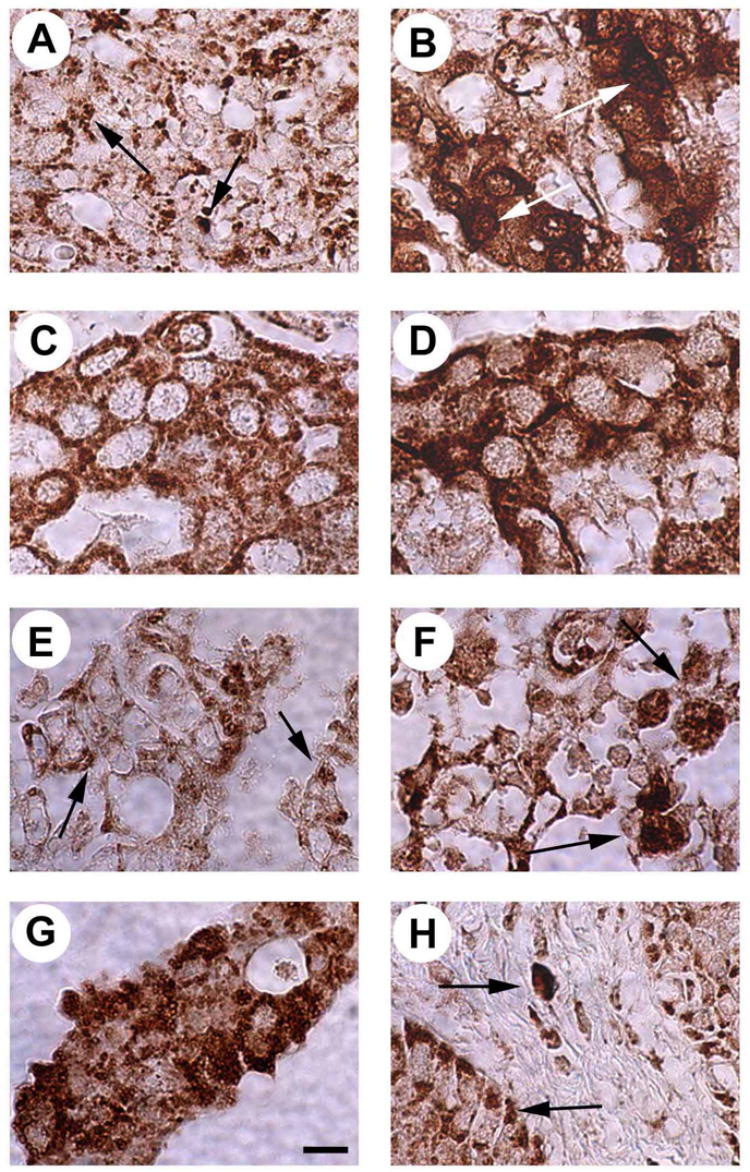
A. “Dot-like” expression (arrows) pattern of ALK in an infiltrating ductal carcinoma. B. Mixed nuclear and cytoplasmic expression pattern of ALK in an infiltrating lobular carcinoma (arrows point high level expression of ALK in nuclei). C. Cytoplasmic expression pattern of ALK in an infiltrating ductal carcinoma (papillary subtype). D. Cytoplasmic expression pattern of ALK in a medullary carcinoma with low to moderate levels of expression of ALK in the nuclei. E. “Dot-like” expression (arrows) pattern of ALK in a comedocarcinoma. F. Mixed nuclear and cytoplasmic expression pattern of ALK in an intraductal carcinoma (arrows point high levels of expression of ALK in the nuclei). G. Mixed nuclear and cytoplasmic expression pattern of ALK in a mucinous adenocarcinoma. H. ALK expression in Paget’s disease of the breast localized in the cytoplasm and nuclei of fibroblasts and in the cytoplasm of epithelial cells. Bar = 20 μm.
The levels of expression of ALK were scored according to the intensity of immunoreactivity observed using light microscopy using a scale of 1 (low) to 3 (high). The results demonstrated that 75% of the infiltrating ductal carcinomas, 50% of the infiltrating lobular carcinomas, 50% of the medullary carcinomas, 100% of the mucinous carcinomas, and 50% of the intraductal carcinomas express high levels of ALK immunoreactivity. Interestingly, 100% of the cases of Paget’s disease were found to express high levels of ALK as well (Figure 3). In contrast, the single sample of comedocarcinoma of the breast only weakly expressed ALK.
Figure 3. Levels of expression of ALK in different human breast cancers.
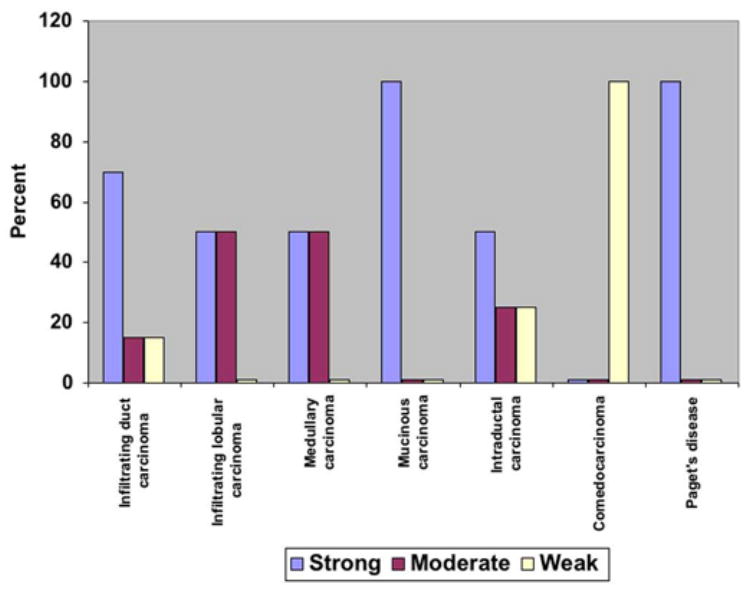
The levels of expression of ALK were quantified using light microscopy and scored in a scale from 1 to 3. The results demonstrated that 75% infiltrating duct carcinomas, 50% infiltrating lobular carcinomas, 50% medullary carcinomas, 100% mucinous carcinomas, 50% intraductal carcinomas, and 100% of the cases of Paget’s disease express high levels of ALK.
Discussion
We recently demonstrated that ALK is a substrate of RPTPβ/ζ and that the PTN-dependent inactivation of RPTPβ/ζ is a mechanism through which ALK is activated (Perez-Pinera et al. Submitted 1). This mechanism of activation of ALK is unique; it is independent of the classically described direct interaction of a growth factor with its cognate receptor tyrosine kinase (RTK) and has been termed “alternative mechanism of RTK activation”. Since PTN is expressed in both human [21] and mouse (Chang et al. Submitted 2) breast cancers, it is possible ALK is activated through the PTN/RPTPβ/ζ signaling pathways in these breast cancer cells. Importantly, targeting PTN by a dominant negative PTN reverses the malignant phenotype of human breast cancer cells in vivo [22], thus the PTN signaling pathway is essential for the aggressive phenotype in these breast cancer cells. The demonstration that ALK is also expressed in breast cancers is consistent with the hypothesis that ALK also maybe important in human breast cancers and potentially may be activated through the PTN/RPTPβ/ζ signaling pathway.
The present studies establish that ALK is expressed in high levels in many histological types of breast cancers. Different patterns of expression and subcellular localization of ALK in different tumors were found in contrast to the sites of expression of ALK in normal ductal breast epithelial cells. Surprisingly, in some cases noted above, ALK was identified in the nucleus, and, in other cases, its expression was “dot-like”, patterns of ALK expression characteristically seen in ALCLs in which fusion proteins of ALK establish constitutive activation of ALK kinase and its downstream activated oncogenic signaling pathways. The data thus also suggest the possibility that ALK fusion proteins may alternatively be responsible for the abnormal distribution of ALK within the breast cancer cells, and furthermore, ALK fusion proteins also have important roles in the pathogenesis of breast cancer.
Acknowledgments
This is manuscript number 18839 from the Scripps Research Institute. This work was supported by grant CA88440 from The National Institutes of Health. The MEM core laboratory is supported by Sam and Rose Stein Endowment Fund. Pablo Perez-Pinera was supported by grant 2 T32 DK007022-26 from the National Institute of Health. Yunchao Chang was supported by Skaggs training grant.
Footnotes
P. Perez-Pinera, W. Zhang, Y. Chang, J.A. Vega and T.F. Deuel. Anaplastic Lymphoma Kinase (ALK) is Activated Through the Pleiotrophin (PTN)/Receptor Protein Tyrosine Phosphatase (RPTP)β/ζ Signaling Pathway: An “Alternative Mechanism of Receptor Tyrosine Kinase (RTK) Activation”. Submitted.
Y. Chang, M. Zuka, P. Perez-Pinera, A. Astudillo, J. Mortimer, J.R. Berenson, Z. Wang, and T.F. Deuel. Inappropriate Expression of Pleiotrophin (PTN) Stimulates Breast Cancer Progression Through Secretion of PTN and PTN-dependent Remodeling of the Tumor Microenvironment. In preparation.
This is manuscript number 18839 from the Scripps Research Institute. This work was supported by grant CA88440 from The National Institutes of Health. The MEM core laboratory is supported by Sam and Rose Stein Endowment Fund. Pablo Perez-Pinera was supported by grant 2 T32 DK007022-26 from the National Institute of Health. Yunchao Chang was supported by Skaggs training grant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 2.Lakhani SR. Molecular genetics of solid tumours: translating research into clinical practice. What we could do now: breast cancer. Mol Pathol. 2001;54:281–284. doi: 10.1136/mp.54.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwahara T, Fujimoto J, Wen D, Cupples R, Bucay N, Arakawa T, Mori S, Ratzkin B, Yamamoto T. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 4.Mourali J, Benard A, Lourenco FC, Monnet C, Greenland C, Moog-Lutz C, Racaud-Sultan C, Gonzalez-Dunia D, Vigny M, Mehlen P, Delsol G, Allouche M. Anaplastic lymphoma kinase is a dependence receptor whose proapoptotic functions are activated by caspase cleavage. Mol Cell Biol. 2006;26:6209–6222. doi: 10.1128/MCB.01515-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 6.Le Beau MM, Bitter MA, Larson RA, Doane LA, Ellis ED, Franklin WA, Rubin CM, Kadin ME, Vardiman JW. The t(2;5)(p23;q35): a recurring chromosomal abnormality in Ki-1-positive anaplastic large cell lymphoma. Leukemia. 1989;3:866–870. [PubMed] [Google Scholar]
- 7.Pillay K, Govender D, Chetty R. ALK protein expression in rhabdomyosarcomas. Histopathology. 2002;41:461–467. doi: 10.1046/j.1365-2559.2002.01534.x. [DOI] [PubMed] [Google Scholar]
- 8.Lamant L, Pulford K, Bischof D, Morris SW, Mason DY, Delsol G, Mariame B. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am J Pathol. 2000;156:1711–1721. doi: 10.1016/S0002-9440(10)65042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li XQ, Hisaoka M, Shi DR, Zhu XZ, Hashimoto H. Expression of anaplastic lymphoma kinase in soft tissue tumors: an immunohistochemical and molecular study of 249 cases. Hum Pathol. 2004;35:711–721. doi: 10.1016/j.humpath.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Powers C, Aigner A, Stoica GE, McDonnell K, Wellstein A. Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J Biol Chem. 2002;277:14153–14158. doi: 10.1074/jbc.M112354200. [DOI] [PubMed] [Google Scholar]
- 11.Dirks WG, Fahnrich S, Lis Y, Becker E, MacLeod RA, Drexler HG. Expression and functional analysis of the anaplastic lymphoma kinase (ALK) gene in tumor cell lines. Int J Cancer. 2002;100:49–56. doi: 10.1002/ijc.10435. [DOI] [PubMed] [Google Scholar]
- 12.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 13.Schlessinger J. Cellular signaling by receptor tyrosine kinases. Harvey Lect. 1993;89:105–123. [PubMed] [Google Scholar]
- 14.Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, Caughey DJ, Wen D, Karavanov A, Riegel AT, Wellstein A. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 15.Meng K, Rodriguez-Pena A, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci U S A. 2000;97:2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pariser H, Herradon G, Ezquerra L, Perez-Pinera P, Deuel TF. Pleiotrophin regulates serine phosphorylation and the cellular distribution of beta-adducin through activation of protein kinase C. Proc Natl Acad Sci U S A. 2005;102:12407–12412. doi: 10.1073/pnas.0505901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pariser H, Perez-Pinera P, Ezquerra L, Herradon G, Deuel TF. Pleiotrophin stimulates tyrosine phosphorylation of beta-adducin through inactivation of the transmembrane receptor protein tyrosine phosphatase beta/zeta. Biochem Biophys Res Commun. 2005;335:232–239. doi: 10.1016/j.bbrc.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 18.Pariser H, Ezquerra L, Herradon G, Perez-Pinera P, Deuel TF. Fyn is a downstream target of the pleiotrophin/receptor protein tyrosine phosphatase beta/zeta-signaling pathway: regulation of tyrosine phosphorylation of Fyn by pleiotrophin. Biochem Biophys Res Commun. 2005;332:664–669. doi: 10.1016/j.bbrc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Kawachi H, Fujikawa A, Maeda N, Noda M. Identification of GIT1/Cat-1 as a substrate molecule of protein tyrosine phosphatase zeta/beta by the yeast substrate-trapping system. Proc Natl Acad Sci U S A. 2001;98:6593–6598. doi: 10.1073/pnas.041608698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura H, Fukada M, Fujikawa A, Noda M. Protein tyrosine phosphatase receptor type Z is involved in hippocampus-dependent memory formation through dephosphorylation at Y1105 on p190 RhoGAP. Neurosci Lett. 2006;399:33–38. doi: 10.1016/j.neulet.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 21.Fang W, Hartmann N, Chow DT, Riegel AT, Wellstein A. Pleiotrophin stimulates fibroblasts and endothelial and epithelial cells and is expressed in human cancer. J Biol Chem. 1992;267:25889–25897. [PubMed] [Google Scholar]
- 22.Zhang N, Zhong R, Wang ZY, Deuel TF. Human breast cancer growth inhibited in vivo by a dominant negative pleiotrophin mutant. J Biol Chem. 1997;272:16733–16736. doi: 10.1074/jbc.272.27.16733. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Pinera P, Garcia-Suarez O, Prieto JG, Germana A, Ciriaco E, del Valle ME, Vega JA. Thymocyte depletion affects neurotrophin receptor expression in thymic stromal cells. J Anat. 2006;208:231–238. doi: 10.1111/j.1469-7580.2006.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulford K, Morris SW, Turturro F. Anaplastic lymphoma kinase proteins in growth control and cancer. J Cell Physiol. 2004;199:330–358. doi: 10.1002/jcp.10472. [DOI] [PubMed] [Google Scholar]


