Abstract
Background
Surgical resection is the only curative treatment for patients with pheochromocytomas, paragangliomas, and other catecholamine-producing tumors. Activation of glycogen synthase kinase 3β (GSK3β) is thought to promote tumor growth and neuroendocrine (NE) peptide secretion in NE tumors. Thus, we hypothesized that inhibition of this signaling pathway with lithium chloride (LiCl), a well-known GSK3β inhibitor, could be a potential therapeutic strategy to control tumor growth and hormone production.
Methods
Pheochromocytoma PC-12 cells were treated with varying concentrations of LiCl [0–30mM]. Levels of active and inactive GSK3β, and NE peptides Chromogranin A (CgA) and mASH1/ASCL1 were determined by Western blot. Cellular growth was measured by MTT cell-proliferation assay
Results
At baseline, PC-12 cells had increased active GSK3β signaling. Treatment of PC-12 cells with increasing dosages of LiCl resulted in dose-dependent inhibition of GSK3β. Importantly, LiCl significantly inhibited pheochromocytoma cellular proliferation. Furthermore, inhibition of GSK3β by LiCl was associated with marked suppression of CgA and mASH1 levels.
Conclusions
These data suggest that GSK3β inhibition may be a novel strategy to treat pheochromocytoma and other catecholamine-producing tumors.
Introduction
Pheochromocytomas, catecholamine producing tumors of the adrenal gland are relatively uncommon. Although these tumors have a reported incidence of only 2 to 8 cases per million annually, the consequence of catecholamine excess can be devastating to patients1. Patients commonly experience hypertension, and it is estimated that pheochromocytomas account for between 0.1% and 1.0% of all hypertensive individuals, representing a potentially curable group2, 3. The clinical manifestations of pheochromocytomas are a result of catecholamine excess and include headaches, diaphoresis, and palpitations in addition to hypertension2. These symptoms can lead to a reduced quality of life for patients suffering from this disease. The only current curative therapy remains surgery, which can usually be done by laparoscopic adrenalectomy; however, some patients present with unresectable disease. Although these patients can often be palliated with alpha and beta-blockade and chemotherapy, there remains significant room for improvement. It is therefore crucial that additional therapies be developed to help those patients with unresectable disease.
The glycogen synthase kinase-3-beta (GSK3β) pathway may be a potential target for therapy in these tumors. Glycogen synthase kinase was first identified as negative regulator of glycogen synthesis. GSK3β is one of the several kinases that phosphorylate glycogen synthase to maintain the enzyme in an inactive state4. GSK3β is a multifunctional serine/threonine protein kinase which regulates numerous cellular processes such as metabolism, cell fate determination, proliferation, and survival5–8. In contrast to other kinases, GSK3β is non-phosphorylated and highly active in unstimulated cells, and becomes inactivated by being phosphorylated in response to signaling cascades9. Active GSK3β is thought to promote tumor growth and vasoactive, neuroendocrine (NE) peptide secretion in pheochromocytomas and other NE tumors.
In this paper we explored the effect of pharmacologically inhibiting the GSK3β pathway in pheochromocytoma cells through the use of Lithium Chloride (LiCl). We show that treatment with LiCl results in progressive phosphorylation of GSK3β, inactivating the protein, and also reduces the amount of chromogranin A (CgA) and mASH1/ASCL1, markers for vasoactive peptides in NE tumors. Furthermore, we report that LiCl successfully inhibits the growth of pheochromocytoma cells.
Materials and Methods
Cell culture
Rat pheochromocytoma cells (PC-12) were obtained from American Type Culture Collection (Manassas, VA) and maintained in Ham’s F12K medium (American Type Culture Collection), supplemented with 15% Horse Serum (Sigma, St. Louis, MO), 2.5% Fetal Bovine Serum (Sigma) and 100 IU/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). The cells were maintained in a humidified atmosphere of 5% CO2 in air at 37°C.
Lithium Chloride treatment
PC-12 cells were plated in 100 mm cell culture dishes and incubated overnight. Cells were treated with LiCl (Sigma) at 0–30mM concentrations. NaCl was used as a control.
Western blot analysis
Cellular extracts were prepared and quantified by BCA protein assay kit (Pierce, Rockford, IL) as previously described10, 11. 30μg of denatured proteins from each sample underwent electrophoresis on a 10% Bis-Tris pre-cast polyacrylamide gel (Invitrogen) and transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH). Membranes were then blocked for 1 hour in milk solution (1x Tris Buffered Saline, 5% non-fat dry milk, 0.05% Tween-20) and then incubated at 4°C overnight with primary antibodies. The following primary antibody dilutions were used: phospho-GSK3β (1:1,000); GSK3β (1:1,000), (Cell Signal Technology, Beverly, MA); Chromogranin A (1:1,000), (Zymed Laboratories, San Francisco, CA); MASH-1 (1:1,000), (BD Biosciences Pharmingen, San Jose, CA); and G3PDH (1:10,000), (Trevigen, Gaithersburg, MD). After primary antibody incubation, membranes were washed either 3 × 5 minutes (phospho- GSK3β and GSK3β) or 3 × 10 minutes (CgA, MASH-1 and G3PDH) in 1X TBS-T buffer (1X TBS, 0.05% Tween 20). The membranes were then incubated with 1:2,000 dilution of horse-radish peroxidase conjugated anti-rabbit (phospho- GSK3β, GSK3β, CgA and G3PDH) or anti-mouse (MASH-1) secondary antibody (Cell Signal Technology) at room temperature for 1 hour. The membranes were washed 3 × 5 minutes (phospho-GSK3β and GSK3β) or 3 × 10 minutes (CgA, MASH-1 and G3PDH) in 1XTBS-T buffer and developed by Immunstar™ (phospho-GSK3β, GSK3β, CgA and G3PDH) (Bio-rad Laboratories, Hercules, CA) or Supersignal® West Femto (MASH-1) (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s directions.
Cell Proliferation Assay
Proliferation of PC-12 cells following treatment with LiCl was measured using a 3,4-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) (Sigma) as previously described10, 11. Cells were plated in triplicate into 24 well plates, allowed to adhere overnight and then treated with LiCl [0–30mM] and incubated. The media were changed every 2 days with new treatment. At each time point, cell growth rates were analyzed following addition of MTT reagent to the cultured cells as described11. Absorbance was measured using spectrophotometer at a wavelength of 540 nm.
Results
Lithium Chloride inhibits GSK3β
Western analysis was used to demonstrate that GSK3β phosphorylation and inactivation is achieved by treatment with LiCl in PC-12 cells. At baseline, PC-12 cells have little or no phosphorylated GSK3β, demonstrating that most of the GSK3β is in the active form. After two days treatment with LiCl, inactivation of GSK3β was increased compared to control treatments as demonstrated by protein phosphorylation (figure 1). Phosphorylation was initially demonstrated at concentrations as low as 5mM LiCl, with full inactivation of GSK3β seen by 10mM LiCl (figure 1). Subsequent experiments revealed that LiCl was effective at inactivating GSK3β at concentrations as low as 1mM LiCl (not shown). This data shows that pheochromocytoma cells are particulary sensitive to LiCl treatment.
Figure 1.
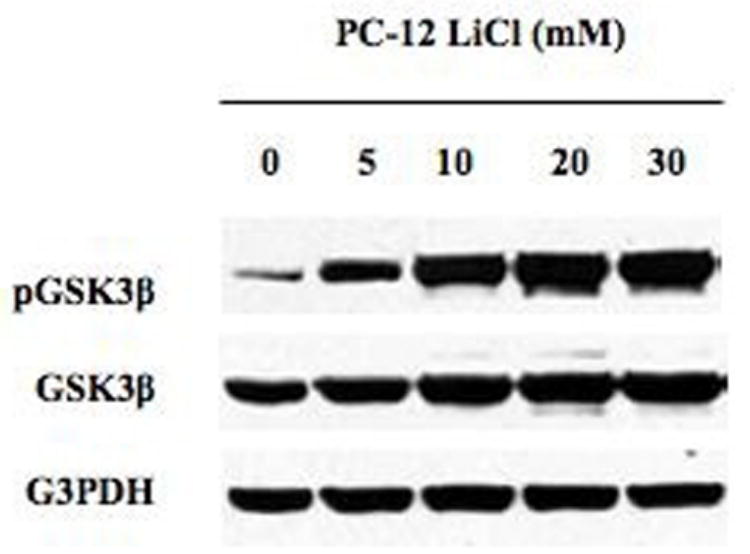
Western analysis for GSK3β inhibition in response to LiCl treatment. Protein extracts from PC-12 cells treated with LiCl [0–30mM] for 2 days. Treatment with LiCl led to inhibition of the GSK3β pathway as shown by increased levels of inactive phosphorylated GSK3β compared to total GSK3β. Equal loading of samples is shown by G3PDH.
LiCl inhibits cell proliferation in pheochromocytoma tumor cells
When PC-12 cells were treated with LiCl at concentrations above 5mM LiCl, cellular proliferation was suppressed as shown by MTT cell proliferation assay (figure 2). Increased growth suppression appears to vary directly with increased concentration of LiCl treatment. Complete inhibition of growth was observed in cells treated with 30mM concentrations. Beginning at 8 days, significant growth suppression was seen, and this continued out to 10 days. In addition, 10mM concentration treatment proved to greatly reduce the growth of PC-12 cells, observed at both 8 and 10 days. While there was not complete suppression of growth with 5mM concentration, the cells did not grow to the same extent as the control cells. Overall, 10 and 30mM concentrations of LiCl produce significant growth reduction after 8 days.
Figure 2.
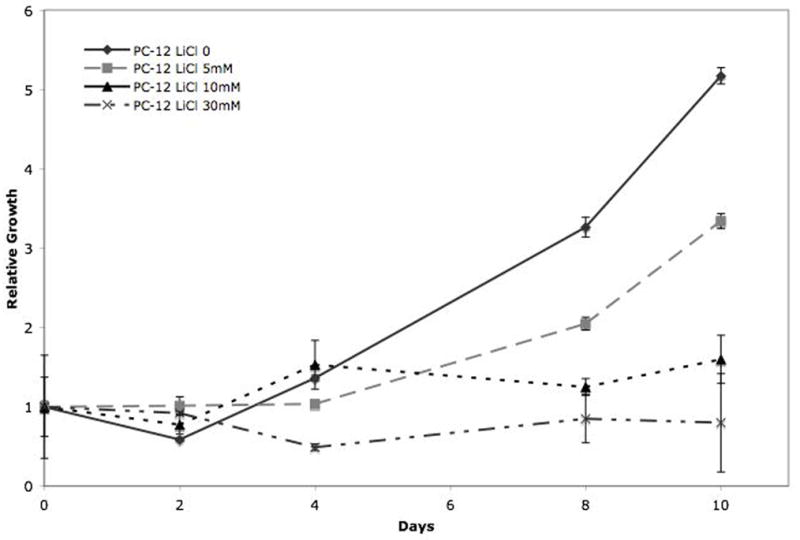
Cell proliferation analysis of PC-12 cells treated with LiCl by MTT assay. PC-12 cells treated with 0, 5 mM, 10 mM, and 30 mM LiCl at 2 day intervals for 10 days. LiCl suppressed cellular proliferation in PC-12 pheochromocytoma cells. Cell growth was significantly inhibited by day 8 in the all LiCl treatments.
LiCl reduces NE vasoactive hormone production in pheochromocytoma tumor cells
It has been well established that changes in CgA and mASH1/ASCL1 levels are representative of changes in other NE hormones such as histamine and serotonin10, 12. Therefore, to determine the effect of GSK3β inhibition with LiCl on vasoactive peptide production in pheochromocytoma cells we performed western blot analysis for CgA and mASH1. Untreated PC-12 cells display high amounts of protein. Subsequent treatment with LiCl results in a complete elimination of the markers as shown by western analysis (figure 3). This finding is again demonstrated with concentrations as low as 1mM LiCl again indicating that pheochromocytoma cells are very sensitive to GSK3β inhibition with LiCl (not shown). However, for the growth inhibition, higher concentrations are required.
Figure 3.
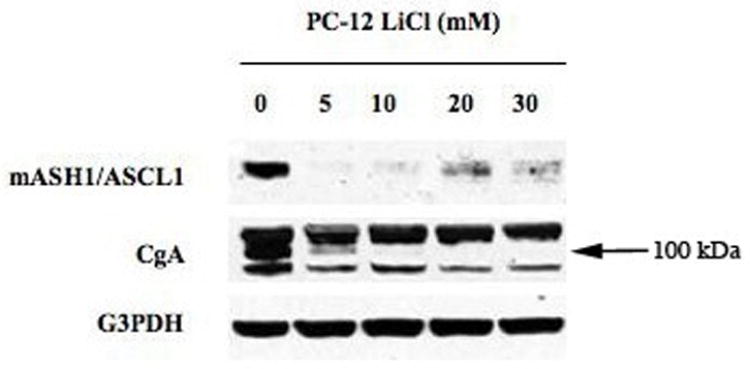
Western analysis of the effect of LiCl treatment on chromogranin A (CgA) and MASH-1. Protein extracts from PC-12 cells treated with LiCl [0–30 mM] for 2 days. Treatment with LiCl led to a reduction in the NE markers CgA and MASH-1. Equal loading of samples is shown by G3PDH. Reduction in CgA, quantified by Quant= 42% at 5mM, 42% at 10 mM, 49% at 20mM, and 50% at 30mM.
Discussion
Patients with untreatable pheochromocytomas, paragangliomas and other catecholamine-producing tumors often have a poor quality of life. These tumors produce a detrimentally excessive amount of hormones leading to the devastating symptoms characteristic of the disease. Thus patients with pheochromocytomas experience such debilitating symptoms as hypertension, headaches, diaphoresis, and palpitations. Although surgery can be a curative therapy for patients with this disease, unfortunately not all patients are candidates for surgical removal. The incidence of metastatic disease is low in patients with pheochromocytomas; however, for this 10% of patients surgery is not always an option2. These patients can often be palliated with alpha and beta-blockade and chemotherapy, but there is significant room for improvement and therefore alternative methods of therapy would be more advantageous.
We believe that GSK3β pathway inhibition is a potential form of alternative therapy for pheochromocytomas. In this paper we have shown that LiCl can effectively be used to inhibit growth and reduce vasoactive hormone production in pheochromocytoma cells. GSK3β pathway inhibition was achieved through the use of LiCl treatment in PC-12 cells. Interestingly, these cells seem to be very sensitive to LiCl treatment. In PC-12 cells phosphorylation GSK3β was evident at concentrations as low as 1mM LiCl. While we have not yet conducted any animal studies with pheochromocytoma cells and LiCl treatment, we are confident the low concentrations of LiCl needed to produce a growth effect in vitro will be easily achievable in animal models without toxicity.
LiCl treatment to PC-12 cells was effective in inhibiting growth of the cells after 8 days. Observation of psychiatric patients undergoing lithium therapy has previously proven that these patients had a lower incidence of cancers of non-epithelial origin. In addition, there is a significant inverse relationship between the dose of lithium and the incidence of cancers of non-epithelial origin13. This data is supportive of the results we obtained, showing that LiCl inhibits the growth of pheochromocytoma cells. While inhibition of the GSK3β pathway is seen at concentrations as low as 1mM LiCl, substantial growth inhibition of tumor cells does not appear until treatment with 10mM LiCl (figures 1 and 2). It may be that growth inhibition requires longer treatment with LiCl, while inhibition of the GSK3β pathway occurs quite rapidly with initiation of treatment. Furthermore, LiCl is not generally toxic to all cells. In fact, lithium has been shown to promote cell survival in several cell types including neuronal, breast cancer, and renal cells14–17. These doses of lithium also do not inhibit growth of NIH 3T3 cells in our hands.
Finally since the symptoms associated with pheochromocytomas are a result of catecholamine excess, a reduction in hormone production could potentially palliate the symptoms experienced by patients. Pheochromocytoma cells have reduced production of CgA and mASH1/ASCL1, biomarkers for vasoactive hormones, after inhibition of GSK3β with LiCl. PC-12 cells display high levels of mASH1 and CgA at baseline. PC-12 cells display high levels of mASH1 and CgA at baseline. mASH1 is virtually eliminated at 5mM LiCl and although CgA levels are not reduced completely they are greatly reduced by 5mM LiCl treatment. CgA and mASH1 are established to be representative of NE hormones including histamine and serotonin, and these biomarkers are decreased with LiCl treatment. These data suggest that LiCl will effectively and significantly reduce hormone production by pheochromocytoma cells, thus alleviating the symptoms of catecholamine excess.
In summary, GSK3β pathway inhibition by LiCl may be a novel strategy in the treatment of pheochromocytomas and other catecholamine-producing tumors. LiCl treatment could improve the quality of life for patients with this debilitating disease.
Acknowledgments
This study was supported in part by a Research Scholars Grant from the American Cancer Society (HC), National Institutes of Health grants DK064735 and DK066169 (HC), George H.A. Clowes, Jr., Memorial Research Career Development Award of the American College of Surgeons (HC), a grant from the Shapiro Foundation (AK), and the University of Wisconsin Comprehensive Cancer Center (AK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheps SG, Jiang NS, Klee GG. Diagnostic evaluation of pheochromocytoma. Endocrinol Metab Clin North Am. 1988;17(2):397–414. [Review] [43 refs] [PubMed] [Google Scholar]
- 2.Grant CS. Pheochromocytoma. In: Clark OH, Duh Q, Kebebew E, editors. Textbook of Endocrine Surgery. Philadelphia, PA: Elsevier Saunders Co; 2005. pp. 621–33. [Google Scholar]
- 3.Chen H, Doppman JL, Chrousos GP, Norton JA, Nieman LK, Udelsman R. Adrenocorticotropic hormone-secreting pheochromocytomas: the exception to the rule. Surgery. 1995;118(6):988–94. doi: 10.1016/s0039-6060(05)80104-7. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P, nimmo HG, Proud CG. Biochem Soc Symp. 1978;43:69–95. [PubMed] [Google Scholar]
- 5.Hardt SE, Sadoshima J. Glycogen synthase kinase-3 beta - A novel regulator of cardia hypertrophy and development. Circulation Research. 2002;90:1055–63. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- 6.Krylova O, Messenger MJ, Salinas PC. Dishevelled-1 regulates microtuble stability: A new function medicated by glycogen synthase kinase-3 beta. Journal of Cell Biology. 2000;151:83–93. doi: 10.1083/jcb.151.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harwood AJ, Plyte SE, Woodgett J, Strutt H, Kay RR. Glycogen-Synthase Kinase-3 Regulates Cell Fate in Dictyostelium. Cell. 1995;80:139–48. doi: 10.1016/0092-8674(95)90458-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang QD, Wand XF, Hernandez A, Hellmich MR, Gatalica Z, Evers BM. Regulation of TRAIL expression by the phosphatidylinositol 3-kinase/Akt/GSK-3 pathway in human colon cancer cells. J Biol Chem. 2002;16:3797–804. doi: 10.1074/jbc.M206306200. [DOI] [PubMed] [Google Scholar]
- 9.Cohen P, Frame S. The renaissance of GSK3. Nature Reviews Molecular Cell Biology. 2001;2:769–76. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 10.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G245–54. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 11.Van Gompel JJ, Kunnimalaiyaan M, Holen K, Chen H. ZM336372, a Raf-1 activator, suppresses growth and neuroendocrine hormone levels in carcinoid tumor cells. Mol Cancer Ther. 2005;4(6):910–7. doi: 10.1158/1535-7163.MCT-04-0334. [DOI] [PubMed] [Google Scholar]
- 12.Lal A, Chen H. Treatment of Advanced Carcinoid Tumors. Current Opinion in Oncology. 2006;18:9–15. doi: 10.1097/01.cco.0000198018.53606.62. [DOI] [PubMed] [Google Scholar]
- 13.Cohen Y, Chetrit A, Cohen Y, Sirota P, Modan B. Cancer morbidity in psychiatric patients: influence of lithium carbonate treatment. Medical Oncology. 1998;15(1):32–6. doi: 10.1007/BF02787342. [DOI] [PubMed] [Google Scholar]
- 14.Welshons WV, Engler KS, Taylor JA, Grady LH, Curran EM. Lithium-Stimulated Proliferation and Alteration of Phosphoinositide Metabolites in Mcf-7 Human Breast-Cancer Cells. J Cell Physiol. 1995;165:134–144. doi: 10.1002/jcp.1041650116. [DOI] [PubMed] [Google Scholar]
- 15.Cross DAE, Culbert AA, Chalmers KA, Facci L, Skaper SD, Reith AD. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- 16.Nonaka S, Katsube N, Chuang DM. Lithium protects rat cerebellar granule cells against apoptosis induced by anticonvulsants, phenytoin and carbamazepine. J Pharmacol Exp Ther. 1998;286:539–547. [PubMed] [Google Scholar]
- 17.Sinha D, Wang ZY, Ruchalski KL, Levine JS, Krishnan S, Lieberthal W, Schwartz JH, Borkan SC. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. American Journal of Physiology-Renal Physiology. 2005;288:F703–F713. doi: 10.1152/ajprenal.00189.2004. [DOI] [PubMed] [Google Scholar]


