Abstract
Brain aging is associated with inflammatory changes. However, data on how the brain arachidonic acid (AA) metabolism is altered as a function of age are limited and discrepant. AA is released from membrane phospholipids by phospholipase A2 (PLA2) and then further metabolized to bioactive prostaglandins and thromboxanes by cyclooxygenases (COX) -1 and -2. We examined the phospholipase A2 (PLA2)/cyclooxygenase (COX)-mediated AA metabolic pathway in the hippocampus and cerebral cortex of 4, 12, 24 and 30 month-old rats. A 2-fold increase in brain thromboxane B2 level in 24 and 30 months was accompanied by increased hippocampal COX-1 mRNA levels at 12, 24, and 30 months. COX-2 mRNA expression was significantly decreased only at 30 months. Hippocampal Ca2+-independent iPLA2 mRNA levels were decreased at 24 and 30 months without any change in Ca2+-dependent PLA2 expression. In the cerebral cortex, mRNA levels of COX and PLA2 were not significantly changed. The specific changes in the AA cascade observed in the hippocampus may alter phospholipids homeostasis and possibly increase the susceptibility of the aging brain to neuroinflammation.
Keywords: aging, cyclooxygenases, calcium-independent phospholipase A2, hippocampus, thromboxane, prostaglandin
1. Introduction
Aging is associated with increased inflammatory responses and vulnerability of neurons to degeneration [17, 18, 22, 39]. Some authors have raised the possibility that inflammation may occur during normal aging and increase the vulnerability to neurodegenerative disorders such as Alzheimer’s disease (AD) [7, 20, 26]. Since the arachidonic acid (AA) cascade plays a key role in neuroinflammation [37], we thought it of interest to identify the changes that occur in this pathway during physiological aging.
AA (20:4n-6) is released from membrane phospholipids by phospholipase A2 (PLA2) enzymes. The PLA2 family can be generally divided into two groups: the Ca2+-independent PLA2 (iPLA2) and the Ca2+-dependent PLA2, which includes secretory (sPLA2) and cytosolic PLA2 (cPLA2) [24]. iPLA2 is thought to mediate homeostatic phospholipid remodeling through fatty acid deacylation/reacylation reactions [49] and may also be involved in cellular signaling [5]. In contrast to cPLA2, which releases predominantly AA, iPLA2 has been suggested to selectively release docosahexaenoic acid (DHA; 22:6n-3), a n-3 polyunsaturated fatty acid highly concentrated in brain membranes [42, 43]. Once released, AA is then converted to bioactive prostaglandins and thromboxanes by cyclooxygenase (COX) enzymes. Two isoforms of COX have been described: COX-1, constitutively expressed in most tissues [30], but also induced by certain inflammatory stimuli in peripheral tissues [46], and COX-2, generally induced by inflammatory stimuli including cytokines, hormones, and mitogens [44], but also constitutively expressed in the central nervous system, especially in pyramidal neurons of hippocampal and cortical circuits [50].
Learning and memory deficits have been well documented in aged F344 rats [14, 21, 41, 45]. Activated microglia and astrocytes [16] and elevated levels of pro-inflammatory cytokines [10, 18, 51] have been described in the rodent brain during normal aging. However, the involvement of COX-mediated AA metabolism in this “pro-inflammatory-status” remains unclear [4, 23, 38]. Aging-related changes in COX-mediated AA metabolism are suggested by age-dependent spatial memory deficits and increased neuronal apoptosis and astrocytic activation in transgenic mice overexpressing COX-2 [2]. We have previously shown in the Rhesus monkey an age-dependant decrease in the levels of cPLA2 protein in the cerebellum and of COX-2 protein in the frontal pole [47]. Taken together, these data suggest that AA metabolism may be disturbed in normal aging. We chose two morphologically and functionally distinct regions, the hippocampus and the cerebral cortex, that have been shown to be affected by the aging process [14, 35]. We assessed age-related changes in the metabolism of AA, with a particular focus on the PLA2/COX pathway across a range of 4 ages (4, 12, 24, and 27–30 months).
2. Materials and methods
2.1 Animals
The study was approved by the National Institutes of Health (NIH) Animal Care and Use Committee in accordance with NIH guidelines on the care and use of laboratory animals. Male Fischer-344 rats, 4, 12, 24 and 27–30 month-old (NIA-sponsored colony at Harlan Sprague-Dawley, Indianapolis, IN) were housed at least one week in our animal facility, maintained at 25°C with a 12 hr light/dark cycle, with free access to food and water. All rats were killed with an overdose of sodium pentobarbital (100 mg/kg, i.p.). Then, rats used to measure prostaglandin levels were subjected to high-energy head-focused microwave irradiation (4.8 kW, 3.4 sec, Cober Electronics, Stanford, CT, USA) to stop metabolism, as reported [8, 9, 33]. Rats used for mRNA analysis were decapitated, hippocampus and cerebral cortex were rapidly dissected out on ice, immediately frozen in −50°C 2-methylbutane, and stored at −80°C until used.
2.2 Determination of brain prostaglandin E2 (PGE2) and thromboxane B2 (TXB2) concentration
After microwave fixation, the amount of the brain extract required to perform analysis did not allow measuring prostaglandin levels in individual brain regions without pooling samples from different animals. Therefore, we measured PGE2 and TXB2 concentration in the whole brain. Microwaved brains were weighed and extracted with 18 volumes of hexane: 2-propanol (3:2, by volume) using a glass Tenbroeck homogenizer. The prostanoids were purified from the lipid extract as described by Radin [34] and the concentrations of PGE2 and TXB2 were determined using specific enzyme-linked immunosorbent assay kits (Oxford Biomedical, Oxford, MI, USA), as previously reported [8]. According to the manufacturer, the sensitivity of the PGE2 and TXB2 assays are 0.2 ng/ml and 0.009 ng/ml at 80% B/Bo, respectively.
2.3 Real-Time Quantitative PCR
Total RNA was extracted from hippocampus or cerebral cortex using RNeasy Lipid Tissue Midi Kit (Qiagen, Valencia, CA, USA) as directed by the manufacturer. Five μg of total RNA was reverse transcribed using a High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA). Five μg of each RNA sample was incubated similarly in the absence of reverse transcriptase to ensure that PCR products resulted from amplification from the specific mRNA rather than from genomic DNA contamination.
The levels of gene expression of COX-1, COX-2, cPLA2, iPLA2, glial fibrillary acidic protein (GFAP) were measured by real-time quantitative RT-PCR, using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Specific primers and probes were purchased from the available Assays-on-Demands (Applied Biosystems). We used glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an endogenous control, as its expression has been shown to remain unchanged during aging [40]. Data were analyzed using sequence detection systems software (Applied Biosystems). Results were expressed as the amount of target gene normalized to the endogenous control (GAPDH) and relative to the 4 month-old rats using the ΔΔCT method [25].
2.4 Statistical analysis
Results are expressed as mean ± SEM. Statistical analysis was performed using One-Way ANOVA followed by post-hoc Bonferroni test. P < 0.05 was considered statistically significant.
3. Results
3.1 TXB2 levels are specifically increased in the aged brain
Levels of TXB2, a stable product of the very short half-lived TXA2, were increased by 2 fold [F(3,19) = 4.89, p = 0.010] in 24 and 30 month-old group compared to the 4 month-old group (Fig. 1A). PGE2 levels (Fig. 1B) showed a trend towards an increase at 24 months, which did not reach statistical significance among the different groups [F(3,19) = 2.71, p = 0.072].
Fig. 1.
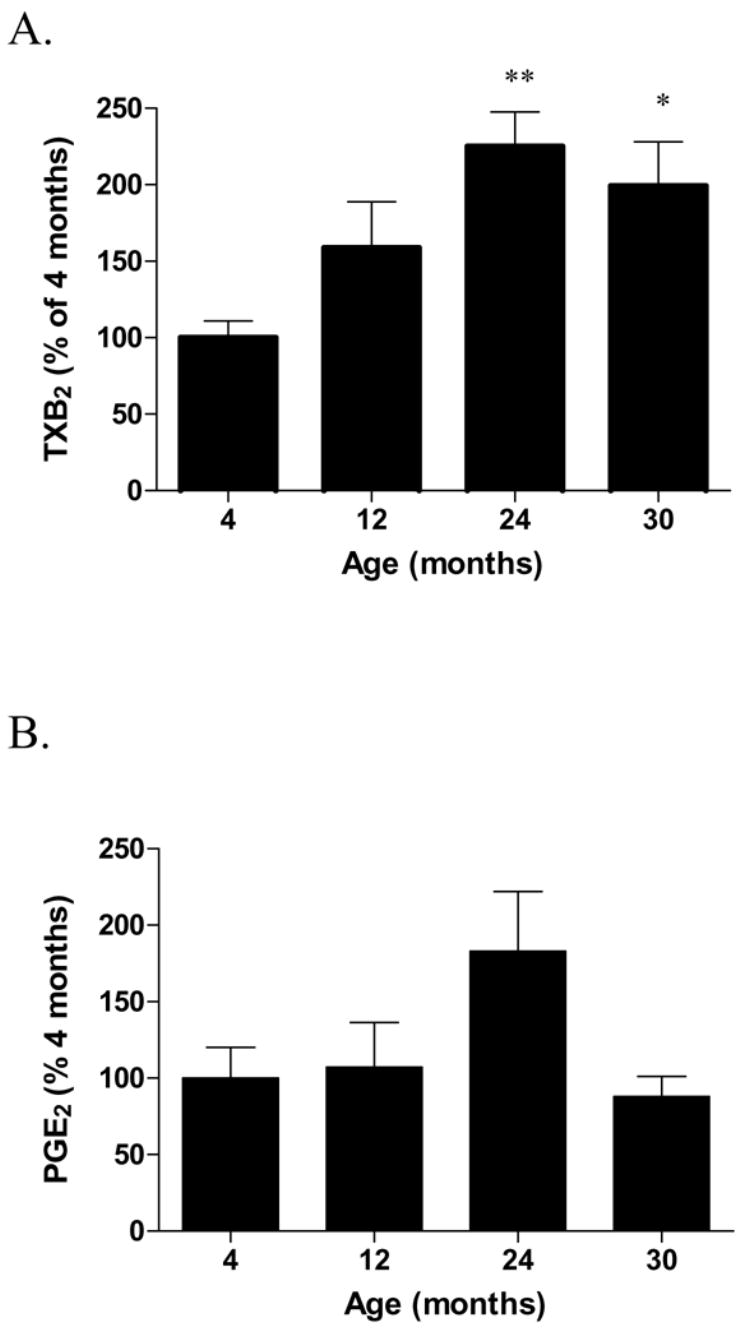
Thromboxane B2 (A) and prostaglandin E2 (B) levels in the microwaved brain of 4, 12, 24 and 30 month-old F344 rats. Data (mean ± SEM, n= 5–6) are expressed as the percentage of the 4 month-old rats. p values are expressed as the comparison of aged rats with 4-month-old rats using One-Way ANOVA (*p<0.05, **p<0.01; post-hoc Bonferroni test).
3.2 COX-1 mRNA expression is upregulated whereas COX-2 mRNA expression is downregulated in the hippocampus during aging
Hippocampal COX-1 mRNA expression (Fig. 2A) was increased by 20% at 12 months and by 27% at 24 and 30 months compared to the 4 months group [F(3,19) = 28.03, p < 0.0001]. In contrast, hippocampal COX-2 mRNA expression (Fig. 2B) was statistically reduced by 15% in the 30 months group compared to the 4 months group [F(3,19) = 4.47, p = 0.015]. COX mRNA expressions (Table 1) were not changed in the cerebral cortex of all age groups examined [COX-1: F(3,19) = 0.37, p = 0.773; COX-2: F(3,19) = 2.97, p = 0.058].
Fig. 2.
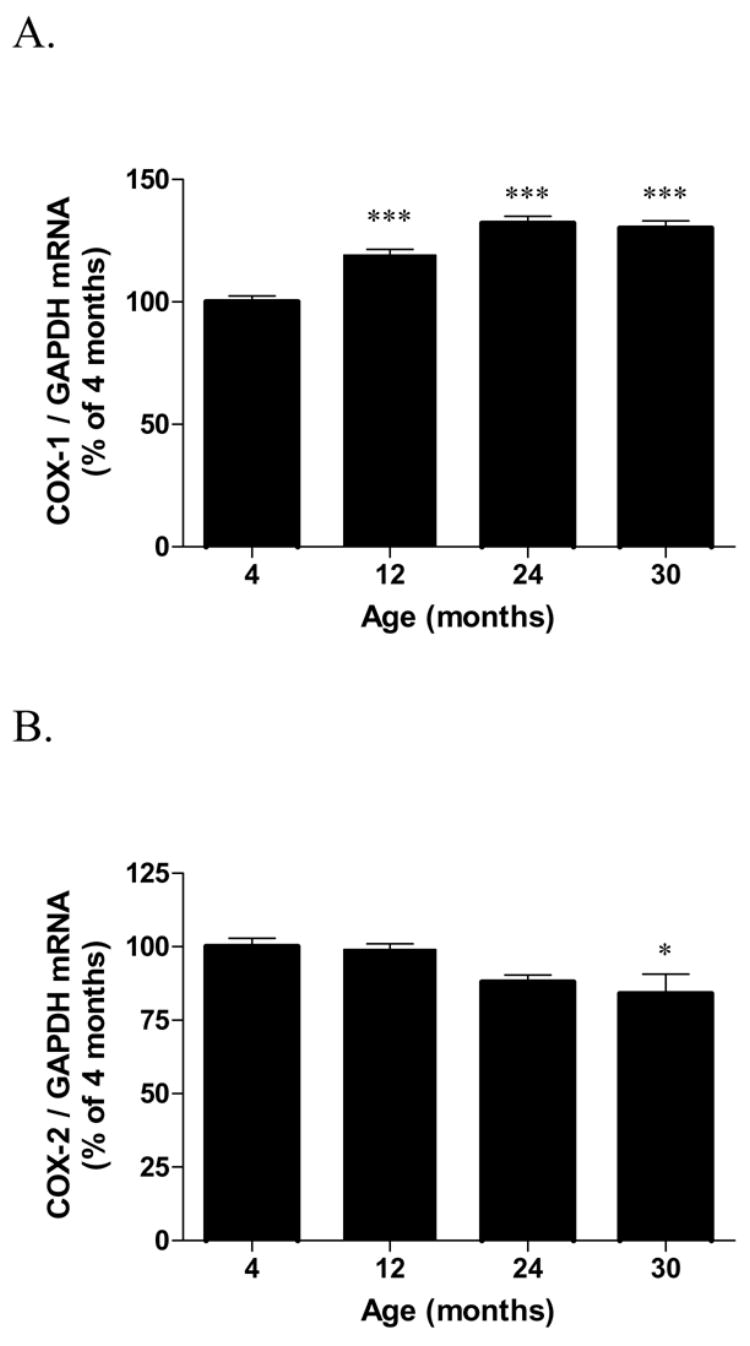
COX-1 and COX-2 mRNA expression in the hippocampi (A-B) of 4, 12, 24 and 30 month-old F344 rats. Data (mean ± SEM, n=5–6) are normalized to the level of the internal control, GAPDH and are expressed as the percentage of the 4 month-old rats. p values are expressed as the comparison of aged rats with 4-month-old rats using One-Way ANOVA (*p<0.05, ***p<0.001; post-hoc Bonferroni test).
Table 1.
mRNA expression of genes involved in the PLA2/COX pathway in the rat cerebral cortex during aging using quantitative real–time PCR.
| Genes1 | 4 months (n=6) | 12 months (n=6) | 24 months (n=6) | 30 months (n=5) |
|---|---|---|---|---|
| COX-1 | 100 ± 2 | 100 ± 5 | 105 ± 9 | 108 ±7 |
| COX-2 | 100 ± 5 | 126 ±12 | 100 ± 3 | 97 ± 8 |
| cPLA2 | 101 ± 5 | 113 ± 6 | 118 ± 6 | 105 ± 7 |
| iPLA2 | 100 ± 3 | 96 ± 4 | 90 ± 6 | 88 ± 6 |
| GFAP | 101 ± 6 | 122 ± 4 | 206 ± 12 *** | 241 ± 11 *** |
Data (mean ± SEM, n=5–6) are normalized to the level of the internal control, GAPDH, and are expressed as the percentage of values in the 4 month-old rats. p values are expressed as the comparison of aged rats with 4-month-old rats using One-Way ANOVA
p<0.01; post-hoc Bonferroni test.
3.3 Hippocampal iPLA2 (VI) mRNA expression is downregulated in aged rats
Hippocampal iPLA2 mRNA expression (Fig. 3A) was decreased by 15% at 24 and 30 months compared to the 4 months group [F(3,19) = 4.67, p = 0.013]. Hippocampal cPLA2 mRNA expression level (Fig. 3B) was not changed in all age groups examined [F(3,19) = 0.32, p = 0.81], neither did cortical cPLA2 [F(3,19) = 1.89, p = 0.165] and iPLA2 mRNA expression levels [F(3,19) = 1.37, p = 0.283] (Table 1).
Fig. 3.
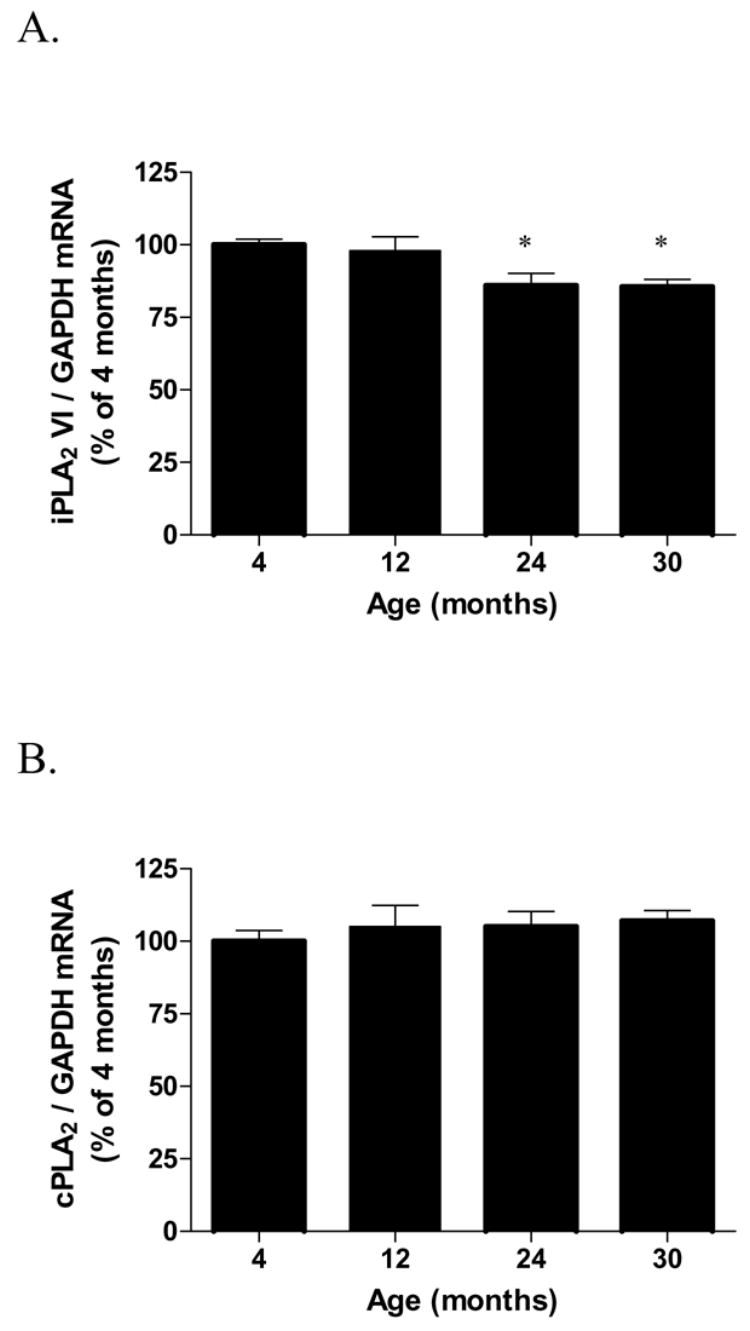
iPLA2 (VI) and cPLA2 mRNA expression in the hippocampi (A-B) of 4, 12, 24 and 30 month-old F344 rats. Data (mean ± SEM, n=5–6) are normalized to the level of the internal control, GAPDH and are expressed as the percentage of the 4 month-old rats. p values are expressed as the comparison of aged rats with 4-month-old rats using One-Way ANOVA ( *p<0.05; post-hoc Bonferroni test).
3.4 GFAP mRNA expression is increased in hippocampus and cerebral cortex during aging
To confirm aging-related astrocytic activation, we measured the expression of GFAP, a specific marker of astrocytes. Hippocampal GFAP mRNA expression (Fig. 4) was increased by 46% at 24 months and by 72 % at 30 months compared to the 4 months group [F(3,19) = 31.21, p < 0.0001]. Cortical GFAP mRNA expression (Table 1) was increased by 2 fold at 24 months and by 2.4 fold at 30 months compared to the 4 months group [F(3,19) = 60.74, p < 0.0001].
Fig. 4.
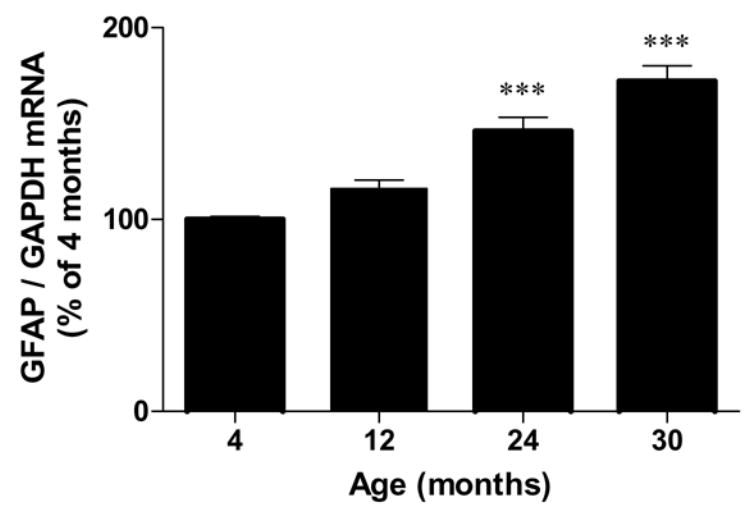
GFAP mRNA expression (B) in the hippocampi of 4, 12, 24 and 30 month-old F344 rats. Data (mean ± SEM, n=5–6) are normalized to the level of the internal control, GAPDH, and are expressed as the percentage of the 4 month-old rats. p values are
4. Discussion
In this study, we found that brain TXB2 level was increased by 2 fold in 24 and 30 month-old rats compared to 4 month-old rats and we also showed that parts of the AA cascade are specifically altered in the hippocampus during normal aging: namely 1) COX-1 expression is increased, 2) COX-2 expression is decreased, and 3) iPLA2, but not cPLA2, expression is decreased. In contrast, we did not find any significant change in the expression of COX or iPLA2 in the cerebral cortex from aged rats.
The upregulation of COX-1 mRNA expression appeared quite early in adulthood (12 months) and therefore can be viewed as part of the normal aging process. In contrast, COX-2 mRNA level was downregulated only at 30 months. Previous studies have examined hippocampal and cortical COX-2 mRNA expression in different strains of rats aged from 3 to 27 months and reported no change [4, 23]. Therefore, COX-2 gene expression appears to become affected only in the advanced phase of the aging process. COX-1 upregulation as a function of age may have different implications. First, it may be responsible for the increased brain levels of TXB2, an AA-derived prostaglandin, via the subsequent metabolism of COX and thromboxane synthase. Indeed, evidence indicates that thromboxane synthase preferentially couples with COX-1 for TXB2 production [11]. Although brain microvessels also contribute to COX-1 derived TXB2 synthesis, since microvessels represent only 0.1 % of the whole brain [48] the increase in TXB2 that we observed in this study is unlikely significantly contributed by the cerebral circulation. However, since in this study TXB2 and PGE2 levels were determined in the whole brain, it would be interesting to further examine the regional distribution of the changes in prostaglandin during normal aging. An increase in COX activity has been reported in the cerebrum of F344 rats at 24 months versus 6 month-old [4], corresponding approximately to the age at which we found increased levels of TXB2 in this study. Since COX-1 is mainly localized in glia, as opposed to COX-2 which is mainly in neurons [13, 50, 52], the upregulation of COX-1 could be associated with the aging-related glial activation. In particular, the age-related increase of GFAP mRNA that we show has been well documented in human and rodent brain [19, 29, 32]. Glial activation may contribute to neuronal dysfunction as both microglia and astrocytes become activated early during physiological aging [12, 16]. It remains unclear why only the hippocampus is responsive to the age-related astrocytic activation which occurred in several brain regions. It is unlikely that COX-1 upregulation in the hippocampus alone could account for the increase of TXB2 level in the whole brain. It is possible that other cerebral regions that we did not examine may have an upregulated COX-1 expression. Another possibility is that increased hippocampal COX-1 mRNA expression is accompanied by an upregulation in thromboxane synthase activity in the hippocampus and/or in other regions. Even though age-related changes occur in the whole brain, the aging process can exhibit regional specificity, the hippocampus being especially sensitive [6, 14].
Only few reports have addressed changes in brain prostanoid production with aging. Using brain microwaving to stop lipid metabolism in vivo [3, 9], we demonstrated an increased level of TXB2 in the brains of 24 and 30 month-old animals compared to 4 month-old rats. The same tendency was observed for PGE2 levels, although it did not reach statistical significance. Prostaglandins have been shown to have numerous effect on astrocytes in vitro including upregulating GFAP expression [31]. Thus, it should be further clarified if the astrocytic activation observed during aging is either the consequence or the cause of altered AA metabolism. COX-1 upregulation with aging might play a role in altering the neuroinflammatory response and increasing vulnerability to neurodegenerative diseases with a marked inflammatory component [28].
We also found a decrease in the Ca2+-independent iPLA2 mRNA levels in the hippocampus of 24 and 30 month-old rat. In contrast to cPLA2, which preferentially cleaves AA from membrane phospholipids, iPLA2 is thought to show selectivity towards docosahexaenoic acid (DHA) [42]. DHA, the major n-3 polyunsaturated fatty acid found in cerebral membranes, plays a crucial role in physiological functions such as neurotransmission, membrane fluidity, ion channel and regulation of enzyme activity and gene expression [1], and it may protect against cognitive decline [27]. The decrease in iPLA2 expression observed in this study is consistent with evidence that the DHA content in cerebral phospholipids is decreased during normal aging [15] and that cortical iPLA2 is downregulated in DHA-deprived rats [36]. Considering the role of iPLA2 in regulating homeostatic phospholipid levels [49], the downregulation of iPLA2 expression during aging might alter phospholipid homeostasis by decreasing the release of DHA in the hippocampus. Therefore, the age-related decrease of AA and DHA in membrane phospholipids [15], coupled with the downregulation of iPLA2, could contribute to increase the ratio of free AA/DHA and consequently alter the brain prostanoid profile. These changes may exert profound effects in the hippocampus, a region highly vulnerable to the aging process [6, 14].
In summary, our results indicate that the mRNA expression of three important enzymes in the AA metabolic pathway, COX-1, COX-2, and iPLA2, is altered in the hippocampus during normal aging. These changes may alter brain phospholipids homeostasis and possibly increase the susceptibility of the aging brain to neuroinflammation. Future studies should address the functional consequences of the changes in gene expression described here by examining protein expression and activity of the AA cascade enzymes, as well as regional distribution of COX-1 within all brain regions during aging.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Langelier B, Aid S, Poumes-Ballihaut C, Champeil-Potokar G, Lavialle M. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reprod Nutr Dev. 2004;44:509–38. doi: 10.1051/rnd:2004063. [DOI] [PubMed] [Google Scholar]
- 2.Andreasson KI, Savonenko A, Vidensky S, Goellner JJ, Zhang Y, Shaffer A, Kaufmann WE, Worley PF, Isakson P, Markowska AL. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J Neurosci. 2001;21:8198–209. doi: 10.1523/JNEUROSCI.21-20-08198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton RF, Wallis C, Randall CL. In vivo regional levels of PGE and thromboxane in mouse brain: effect of decapitation, focused microwave fixation, and indomethacin. Prostaglandins. 1983;26:421–9. doi: 10.1016/0090-6980(83)90177-6. [DOI] [PubMed] [Google Scholar]
- 4.Baek BS, Kim JW, Lee JH, Kwon HJ, Kim ND, Kang HS, Yoo MA, Yu BP, Chung HY. Age-related increase of brain cyclooxygenase activity and dietary modulation of oxidative status. J Gerontol A Biol Sci Med Sci. 2001;56:B426–31. doi: 10.1093/gerona/56.10.b426. [DOI] [PubMed] [Google Scholar]
- 5.Balsinde J, Balboa MA. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–62. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–8. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 7.Blasko I, Grubeck-Loebenstein B. Role of the immune system in the pathogenesis, prevention and treatment of Alzheimer’s disease. Drugs Aging. 2003;20:101–13. doi: 10.2165/00002512-200320020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bosetti F, Weerasinghe GR, Rosenberger TA, Rapoport SI. Valproic acid down-regulates the conversion of arachidonic acid to eicosanoids via cyclooxygenase-1 and -2 in rat brain. J Neurochem. 2003;85:690–6. doi: 10.1046/j.1471-4159.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- 9.Bosisio E, Galli C, Galli G, Nicosia S, Spagnuolo C, Tosi L. Correlation between release of free arachidonic acid and prostaglandin formation in brain cortex and cerebellum. Prostaglandins. 1976;11:773–81. doi: 10.1016/0090-6980(76)90186-6. [DOI] [PubMed] [Google Scholar]
- 10.Casolini P, Catalani A, Zuena AR, Angelucci L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J Neurosci Res. 2002;68:337–43. doi: 10.1002/jnr.10192. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Langenbach R, Bosetti F. Cyclooxygenase-1 and -2 enzymes differentially regulate the brain upstream NF-kappa B pathway and downstream enzymes involved in prostaglandin biosynthesis. J Neurochem. 2006;98:801–11. doi: 10.1111/j.1471-4159.2006.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- 13.Deininger MH, Schluesener HJ. Cyclooxygenases-1 and -2 are differentially localized to microglia and endothelium in rat EAE and glioma. J Neuroimmunol. 1999;95:202–8. doi: 10.1016/s0165-5728(98)00257-4. [DOI] [PubMed] [Google Scholar]
- 14.Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: A multi-level analysis in the rat. Neuroscience. 2006;139:1173–85. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Favrelere S, Stadelmann-Ingrand S, Huguet F, De Javel D, Piriou A, Tallineau C, Durand G. Age-related changes in ethanolamine glycerophospholipid fatty acid levels in rat frontal cortex and hippocampus. Neurobiol Aging. 2000;21:653–60. doi: 10.1016/s0197-4580(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 16.Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging. 2003;24(Suppl 1):S123–7. doi: 10.1016/s0197-4580(03)00051-4. discussion S131. [DOI] [PubMed] [Google Scholar]
- 17.Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat Med. 1998;4:827–31. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- 18.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 19.Goss JR, Finch CE, Morgan DG. Age-related changes in glial fibrillary acidic protein mRNA in the mouse brain. Neurobiol Aging. 1991;12:165–70. doi: 10.1016/0197-4580(91)90056-p. [DOI] [PubMed] [Google Scholar]
- 20.Griffin WS. Inflammation and neurodegenerative diseases. Am J Clin Nutr. 2006;83:470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- 21.Ingram DK, Spangler EL, Iijima S, Ikari H, Kuo H, Greig NH, London ED. Rodent models of memory dysfunction in Alzheimer’s disease and normal aging: moving beyond the cholinergic hypothesis. Life Sci. 1994;55:2037–49. doi: 10.1016/0024-3205(94)00384-x. [DOI] [PubMed] [Google Scholar]
- 22.Kalehua AN, Taub DD, Baskar PV, Hengemihle J, Munoz J, Trambadia M, Speer DL, De Simoni MG, Ingram DK. Aged mice exhibit greater mortality concomitant to increased brain and plasma TNF-alpha levels following intracerebroventricular injection of lipopolysaccharide. Gerontology. 2000;46:115–28. doi: 10.1159/000022146. [DOI] [PubMed] [Google Scholar]
- 23.Katafuchi T, Takaki A, Take S, Kondo T, Yoshimura M. Endotoxin inhibitor blocks heat exposure-induced expression of brain cytokine mRNA in aged rats. Brain Res Mol Brain Res. 2003;118:24–32. doi: 10.1016/s0169-328x(03)00331-0. [DOI] [PubMed] [Google Scholar]
- 24.Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCann JC, Ames BN. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr. 2005;82:281–95. doi: 10.1093/ajcn.82.2.281. [DOI] [PubMed] [Google Scholar]
- 28.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 1999;89:687–99. doi: 10.1016/s0306-4522(98)00334-0. [DOI] [PubMed] [Google Scholar]
- 30.Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68–69:165–75. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 31.Morrison RS, De Vellis J, Lee YL, Bradshaw RA, Eng LF. Hormones and growth factors induce the synthesis of glial fibrillary acidic protein in rat brain astrocytes. J Neurosci Res. 1985;14:167–76. doi: 10.1002/jnr.490140202. [DOI] [PubMed] [Google Scholar]
- 32.Nichols NR, Day JR, Laping NJ, Johnson SA, Finch CE. GFAP mRNA increases with age in rat and human brain. Neurobiol Aging. 1993;14:421–9. doi: 10.1016/0197-4580(93)90100-p. [DOI] [PubMed] [Google Scholar]
- 33.Poddubiuk ZM, Blumberg JB, Kopin IJ. Brain prostaglandin content in rats sacrificed by decapitation vs focused microwave irradiation. Experientia. 1982;38:987–8. doi: 10.1007/BF01953694. [DOI] [PubMed] [Google Scholar]
- 34.Radin NS. Extraction of tissue lipids with a solvent of low toxicity. Methods Enzymol. 1981;72:5–7. doi: 10.1016/s0076-6879(81)72003-2. [DOI] [PubMed] [Google Scholar]
- 35.Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–83. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 36.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–7. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem. 2004;88:1168–78. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- 38.Sanguino E, Roglans N, Alegret M, Sanchez RM, Vazquez-Carrera M, Laguna JC. Prevention of age-related changes in rat cortex transcription factor activator protein-1 by hypolipidemic drugs. Biochem Pharmacol. 2004;68:1411–21. doi: 10.1016/j.bcp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Shetty AK, Turner DA. Vulnerability of the dentate gyrus to aging and intracerebroventricular administration of kainic acid. Exp Neurol. 1999;158:491–503. doi: 10.1006/exnr.1999.7107. [DOI] [PubMed] [Google Scholar]
- 40.Slagboom PE, de Leeuw WJ, Vijg J. Messenger RNA levels and methylation patterns of GAPDH and beta-actin genes in rat liver, spleen and brain in relation to aging. Mech Ageing Dev. 1990;53:243–57. doi: 10.1016/0047-6374(90)90042-e. [DOI] [PubMed] [Google Scholar]
- 41.Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15:319–28. doi: 10.1016/0197-4580(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 42.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–22. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strokin M, Chechneva O, Reymann KG, Reiser G. Neuroprotection of rat hippocampal slices exposed to oxygen-glucose deprivation by enrichment with docosahexaenoic acid and by inhibition of hydrolysis of docosahexaenoic acid-containing phospholipids by calcium independent phospholipase A2. Neuroscience. 2006;140:547–53. doi: 10.1016/j.neuroscience.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68–69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 45.van der Staay FJ, Blokland A. Behavioral differences between outbred Wistar, inbred Fischer 344, brown Norway, and hybrid Fischer 344 × brown Norway rats. Physiol Behav. 1996;60:97–109. doi: 10.1016/0031-9384(95)02274-0. [DOI] [PubMed] [Google Scholar]
- 46.Wallace JL, Bak A, McKnight W, Asfaha S, Sharkey KA, MacNaughton WK. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998;115:101–9. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- 47.Weerasinghe GR, Coon SL, Bhattacharjee AK, Harry GJ, Bosetti F. Regional protein levels of cytosolic phospholipase A2 and cyclooxygenase-2 in Rhesus monkey brain as a function of age. Brain Res Bull. 2006;69:614–21. doi: 10.1016/j.brainresbull.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams WM, Chang MC, Hayakawa T, Grange E, Rapoport SI. In vivo incorporation from plasma of radiolabeled palmitate and arachidonate into rat brain microvessels. Microvasc Res. 1997;53:163–6. doi: 10.1006/mvre.1996.1984. [DOI] [PubMed] [Google Scholar]
- 49.Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A(2): structure and function. Biochim Biophys Acta. 2000;1488:28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 50.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–86. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 51.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 52.Yermakova AV, Rollins J, Callahan LM, Rogers J, O’Banion MK. Cyclooxygenase-1 in human Alzheimer and control brain: quantitative analysis of expression by microglia and CA3 hippocampal neurons. J Neuropathol Exp Neurol. 1999;58:1135–46. doi: 10.1097/00005072-199911000-00003. [DOI] [PubMed] [Google Scholar]


