Abstract
Toll like receptors, the critical receptor family in innate immunity, have been shown to signal via both the ERK 1/2 and the transcription factor NFκB. β-Arrestins 1 and 2 have recently been implicated in modulation of NFκB signaling and ERK 1/2 activation. Using a number of approaches: mouse embryonic fibroblasts (MEF) from wild-type (WT), β-arrestins knockouts (KO), β-arrestins 1 and 2 double KO, and MEFs with reconstituted WT β-arrestins in the double KO cells, RNA interference (siRNA) specific knockdown of β-arrestins, and overexpression of WT β-arrestins, it was demonstrated that β-arrestin 2 positively regulates LPS-induced ERK 1/2 activation and both β-arrestins 1 and 2 negatively regulate LPS-induced NFκB activation. Also β-arrestin 2 positively regulate LPS-induced IL-6 production and both β-arrestin 1 and 2 positively regulate LPS-induced IL-8 production. The specific ERK1/2 inhibitor, PD98059 significantly decreased LPS-induced IL-6 and IL-8 production suggesting that IL-6 and IL-8 production is, in part, mediated by ERK 1/2 activation. Over expression of wild type β-arrestin 1 and 2 had no effect on LPS-induced ERK1/2 activation and LPS-induced IL-8 production suggesting that endogenous β-arrestins 1 and 2 are sufficient to mediate maximum ERK 1/2 activity and IL-8 production. β-arrestins thus not only negatively regulate LPS-induced NFκB activation but also positively regulate ERK 1/2 activation and specific pro-inflammatory gene expression. Understanding the role of β-arrestins in regulation of TLR signaling pathways may provide novel insights into control mechanisms for inflammatory gene expression.
Keywords: β-arrestin, endotoxin shock, MEF, RNAi
1. Introduction
TLRs are an evolutionarily conserved family of pattern recognition receptors that regulate innate immunity and anti-microbial responses (Poltorak et al., 1998; Akira et al., 2006). TLR4 recognizes the Gram-negative bacteria cell wall component LPS by forming a multi-molecular protein complex with CD14 and the secreted protein MD-2 (Akashi et al., 2000). Binding to TLR4 leads to activation of a series of signaling proteins including myeloid differentiation factor 88, TIR domain-containing adaptor protein, interleukin-1 receptor activated kinase, and TNF receptor associated factor 6 (TRAF6). This cascade initiates the NFκB signaling pathway by stimulating IκBα degradation and nuclear translocation of NFκB (Akira and Takeda, 2004). TLRs also activate MAP kinases including ERK1/2, JNK and p38 (O'Neill, 2002). Phosphorylation of ERK1/2, JNK and p38 activate nuclear proteins and transcription factors similar to the IκBα cascade.
Previous studies identified the involvement of post receptor heterotrimeric guanine nucleotide binding regulatory (Gi) proteins in LPS signal transduction (Fan et al., 2004; Fan et al., 2003; Fan et al., 2005; Lentschat et al., 2005; Solomon et al., 1998). In vitro kinase assays performed on human CD14 co-immunoprecipitated proteins demonstrated the presence of Gαi2 and Gαi3 proteins (Solomon et al., 1998). Our studies and others suggest that TLR4 signaling is, in part, Gi protein regulated (Fan et al., 2004; Lentschat et al., 2005). Studies utilizing pertussis toxin, an inhibitor of receptor coupling that catalyzes the ADP-ribosylation of Gi proteins, or mastoparan, a Gi protein antagonist, resulted in inhibition of LPS-induced mediator production in several cell lines and primary macrophages or monocytes (Daniel-Issakani et al., 1989; Ferlito et al., 2002; Ferlito et al., 2001; Wang et al., 1988; Zhang et al., 1993). In addition to pharmacologic inhibition of Gi protein function, we have demonstrated inhibition of TLR4 signaling with mutated constructs of Gαi2 and Gαi3 proteins (Fan et al., 2004), and reduced LPS-stimulated cytokine responses in peritoneal MØ from mice genetically deficient in Gαi2 (Fan et al., 2005). These data suggest that Gi proteins modulate signaling pathways mediating cellular activation in response to LPS.
β-arrestins 1 and 2 are adaptor proteins that regulate Gi protein function by forming complexes with most G protein coupled receptors (GPCRs). This occurs following agonist binding and phosphorylation of receptors by G protein-coupled receptor kinases. They play a central role in the interrelated processes of homologous desensitization and GPCR sequestration that leads to termination of G protein activation by endocytosis in clathrin coated pits (Luttrell and Lefkowitz, 2002; Pitcher et al., 1998; Goodman et al., 1996; Miller et al., 2001). It has also been shown that β-arrestins 1 and 2 function as multifunctional scaffold/adaptor proteins for GPCR activation of MAP kinases including ERK1/2 (Luttrell et al., 2001; DeFea et al., 2000; DeFea et al., 2000), JNK (McDonald et al., 2000), one of the p38 kinases (Sun et al., 2002), and Src family kinases (Luttrell et al., 1999). β-arrestins form complexes with individual members of a particular MAP kinase cassette e.g. ASK for JNK3 and Raf for ERK1/2. In addition to MAP kinase regulation, it has been shown that β-arrestins also modulate NFκB activity (Witherow et al., 2004; Gao et al., 2004). β-arrestins 1 and 2 directly interact with IκBα, preventing its phosphorylation and degradation. Over expression of β-arrestins 1 and 2 attenuated activation of NFκB. More recent studies have implicated β-arrestins in TLR signaling and gene activation. Wang et al. (2006) demonstrated that β-arrestins directly interact with TRAF6 following TLR or IL-1 receptor activation preventing TRAF6 mediated signaling. In β-arrestin 2 KO mice, LPS also stimulated higher expression of pro-inflammatory cytokines in bone marrow derived macrophages and induced higher mortality in galactosamine sensitized β-arrestin 2 KO mice. It was concluded that β-arrestins are essential negative regulators of innate immune activation by TLRs.
Our current studies using β-arrestins knockout and reconstituted MEF cells, siRNA and overexpression of β-arrestins 1 and 2 demonstrate that the role of β-arrestins 1 and 2 in regulation of TLR signaling and pro-inflammatory gene expression is more complex than negative regulation alone. In addition to inhibition of NFκB activation, β-arrestin 2 positively regulates ERK 1/2 activation and IL-6 production while both β-arrestins 1 and 2 positively regulated IL-8 production. Our studies suggest that β-arrestins 1 and 2 differentially regulate TLR4 signaling pathways.
2. Materials and methods
2.1 Reagents, plasmids and cells
Protein free Salmonella minnesota R595 LPS was provided by Dr. Ernst Reitschel, Borstel Germany. LPS (from Salmonella enteritidis) and Poly-L-Lysine solution were purchased form Sigma (St. Louis, MO). Both of these LPS were used in all the experiments and resulted in similar response. PD98059 was obtained from Calbiochem (La Jolla, CA). Flag-β-arrestin 1 and flag-β-arrestin 2 plasmids; A1CT (β-arrestins 1/2) antibody; WT, β-arrestin 2 KO, β-arrestin 1/2 DKO, β-arrestin 1 re-constitution and β-arrestin 2 re-constitution MEF cell lines were kind gifts from Dr. Robert J. Lefkowitz (Duke University Medical Center, Durham, NC) and were described previously (Kohout et al., 2001; Jafri et al., 2006). pcDNA3.1-GFP plasmid and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA). GeneSilencer was purchased from Genlantis (San Diego, CA). The pNFκB-luc plasmid was obtained from Stratagene (La Jolla, CA). The pRL-SV40 plasmid and dual-luciferase reporter assay system were purchased from Promega (Madison WI). Phospho-p44/p42 MAP Kinase antibody was purchased from Cell Signaling (Beverly, MA). Human embryonic kidney 293 cells stably expressing TLR4, CD14 and MD-2 (HEK-TLR4) were purchased from InvivoGen (San Diego, CA).
2.2 Cell culture, transfection and stimulation
MEF cells and HEK-TLR4 cells were grown in Dulbecco's modified Eagle's medium (Gibco Invitrogen Corporation, Carlsbad, CA) supplemented with heat inactivated 10% fetal bovine serum (Cellgro Mediatech Inc., Herndon, VA) in 150 cm2 tissue culture flasks and maintained at 37°C in 5% CO2, 95% air. Antibiotics of 0.05% HygroGold and 0.1% of Blasticidin (InvivoGen, San Diego, CA) were added into the culture medium for HEK-TLR4 cells. The confluent cells were detached by 0.05% trypsin-EDTA (Gibco Invitrogen Corporation, Carlsbad, CA) and passed every 2-3 days. MEF cells and HEK-TLR4 cells within 20 passages were used. Before seeding HEK-TLR4 cells, the 24-well plate was incubated with 100μl of Poly-L-Lysine solution for 10 min to enhance cell adherence.
MEF cells and HEK-TLR4 cells were pretreated with or without PD98059 for 2 hrs followed by stimulation with LPS for 18 hrs. Supernatant IL-6 or IL-8 production was measured by ELISA.
Wild type β-arrestin 1 or β-arrestin 2 plasmids were transfected into HEK-TLR4 cells with Lipofectamine 2000 reagent. Typical transfection reactions consisted of a total of 1 μg plasmid DNA and 0.8 μl of the transfection reagent per well. The negative control wells were transfected with the equivalent amount of vector DNA. pcDNA3.1-GFP plasmid were used to evaluate transfection efficiency that was more than 80%. 24 hrs after plasmid transfection, cells were stimulated with different concentration of LPS for 24 hrs. Luciferase assay or ELISA were performed to examine NFkB activity and IL-8 production, respectively.
2.3 RNA interference
Chemically synthesized, double-stranded siRNAs, with 19-nt duplex RNA and 2-nt 3′ dTdT overhangs, were purchased from Qiagen (Lafayette, CO). The siRNA sequences targeting β-arrestin 1 or β-arrestin 2: 5'-AAAGCCUUCUGCGCGGAGAAU-3' and 5'-AAGGACCGCAAAGUGUUUGUG-3' and a control sequence targeting at 5'-AAUUCUCCGAACGUGUCACGU-3' were designed based upon previous studies (Ahn et al., 2003).
Forty to 50% confluent HEK-TLR4 cells in 6-well plates, split at least 24 h before transfection, were transfected with siRNA using the GeneSilencer transfection reagent (Gene Therapy Systems, San Diego). Briefly, 8.6 μl of the GeneSilencer transfection reagent was added to 50 μl of DMEM, while RNA mixtures containing 12 μl of 20 μM (≈4 μg) RNA, 40 μl of siRNA diluent, and 20 μl of DMEM were prepared. Both solutions were allowed to stand 5-10 min at room temperature and mixed by inversion. After 10-20 min incubation at room temperature, the entire transfection mixture was added to cells. After cells were incubated for 4 h at 37°C, an additional 1 ml of DMEM with 20% FBS and 2% penicillin/streptomycin were added to the plate and incubated for 48 h. After incubation the cells were divided into 12-well plates and incubated for 24 hours and stimulated with different concentrations of LPS for 40 min or 24 hours. The supernatant and protein were collected for analyses. Western blot, luciferase, and ELISA assays were performed on the supernatant or cell lysate to examine ERK1/2 activation, NFκB activation and IL-8 production, respectively.
2.4 Western blot
After stimulation, cells were washed, lysed with ice-cold RIPA lysis buffer (10 mM Tris, pH 7.4, 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM PMSF, 1 μg/ml Aprotinin, 1 μg/ml Leupeptin, and 1 μg/ml Pepstatin A), kept on ice for 30 min, sonicated for 3 s, and centrifuged for 10 min at 4 °C at 10,000 g. An aliquot was taken for protein determination using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). The remaining supernatant was stored at −20 °C until Western blot analysis.
For detection of ERK 1/2 phosphorylation and β-arrestins expression, lysates were added to Laemmli sample buffer and boiled for 4 min. Protein from each sample was subsequently subjected to a 12% SDS-PAGE, and transferred onto a polyvinylidene difluoride membrane. The membranes were washed with TBST (20 mM Tris, 500 mM NaCl, 0.1% Tween 20) and blocked with 5% milk in TBST for 1 h. After washes with TBST, membranes were incubated with primary antibody phospho-p44/p42 MAP Kinase antibody (1:1000 dilution) to detect phosphorylated ERK 1/2. A1CT antibody (1:20,000 dilution) was used to detect β-arrestin 1 and 2 expression, and Anti-ERK 1/2 Ab (1:5000 dilution) that detects total ERK 1/2 protein was used to evaluate equal loading. The blots were washed two times with TBST and incubated for 1 h with horseradish peroxidaseconjugated donkey anti-rabbit-IgG antibody (1:4000 dilution, Amersham Pharmacia Biotech, Inc., Piscataway, NJ) in blocking buffer. Immunoreactive bands were visualized by incubation with ECL Plus detection reagents (Amersham Pharmacia Biotech, Inc., Piscataway, NJ) for 5 min and development of the exposed ECL hyperfilm (Amersham Pharmacia Biotech, Inc., Piscataway, NJ).
2.5 Luciferase assay
pNFκB-luc plasmid (Stratagene, La Jolla, CA) for evaluation of NFκB-dependent transcription and the pRL-SV40 plasmid (Promega, Madison WI) for evaluation of transfection efficiency were co-transfected to HEK-TLR4 cells. Lipofectamine 2000 reagent (Invitrogen Corporation, Carlsbad, CA) was used for transfection according to manufacturer's recommendations. Typical transfection reactions consisted of a total of 1.1 μg plasmid DNA and 0.8 μl of the transfection reagent per well of 24-well plate. The transfection efficiency is more than 80% as evaluated by transfection with pcDNA3.1-GFP plasmid. The ratio of pNFκB-luc plasmid:pRL-SV40 plasmid was 10:1. Reporter luciferase were analyzed on cell lysates using dual luciferase reporter assay kit (Promega, Madison WI). Luminescence was read in SpectraMax M5 plate reader.
2.6 Assay for IL-6 and IL-8 production
IL-6 and IL-8 production were measured using an ELISA with mouse IL-6 ELISA kits (eBioscience, San Diego, CA) and human IL-8 ELISA kits (Diaclone, Stamford, CT).
2.7 Statistical analysis
Data are expressed as the mean ± SEM. Statistical significance was determined by ANOVA followed by a posthoc test with Fisher's probable least-squares difference test using Statview software (SAS Institute Inc., Cary, NC). p< 0.05 was used to reject the null hypothesis.
3. Results
3.1 β-arrestin 2 mediates LPS-induced IL-6 production in MEFs through ERK 1/2 activation
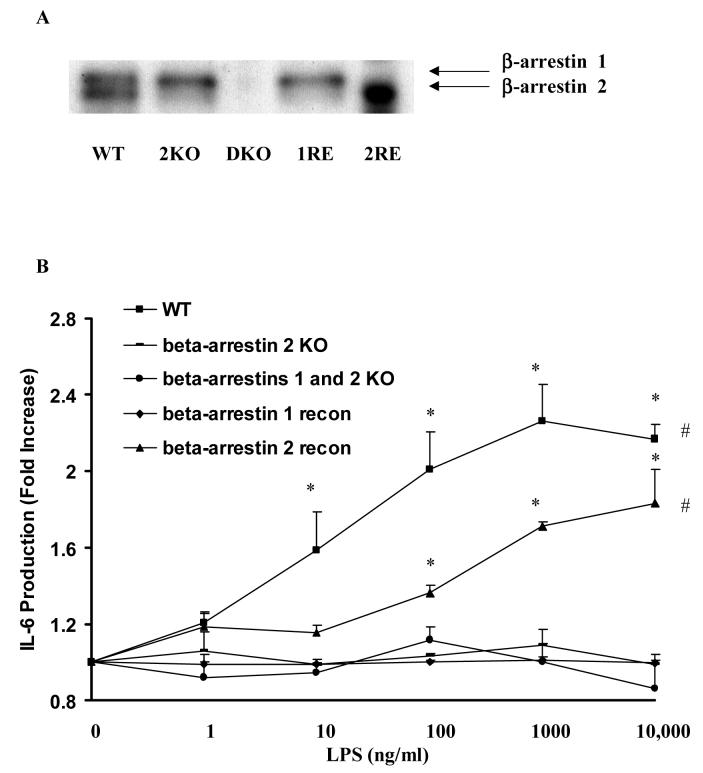
To determine the contribution of β-arrestins to LPS-induced IL-6 production, WT, β-arrestin 2 KO, β-arrestins 1 and 2 double KO, and β-arrestin 1 or 2 re-constitution MEF cell lines were stimulated with LPS. Western blot confirmed β-arrestins expression in MEF cell lines (Fig. 1A). In MEF cells stimulated with LPS for 18 hours, LPS concentration dependently induced IL-6 production (maximum 2.3±0.2 fold p<0.05) in WT MEF cells. β-Arrestin 2 KO, β-arrestins 1 and 2 double KO, and β-arrestin 1 reconstituted MEFs were not responsive to LPS stimulation. In contrast, LPS significantly induced IL-6 production (maximum1.8±0.2 fold, p<0.05) in a concentration dependent manner in β-arrestin 2 re-constituted MEF cells (Fig. 1B).
Figure 1. Effect of altered β-arrestins 1/2 expression on LPS-induced IL-6 production in mouse embryonic fibroblasts.
MEFs from WT mice, β-arrestin 2 knockout mice, β-arrestin 1/2 double knockout mice and re-constitution of wild-type β-arrestin 1 or 2 in the double knockout MEF cells were stimulated with LPS (0-10μg/ml). LPS-induced IL-6 production was studied (A). β-arrestin 1 and 2 expression in these MEF cell lines were examined by Western blot analysis (B). Data represent means ± SE from three independent experiments. *, p<0.05 compared to naïve cells; #, p<0.05 compared to β-arrestin 2 KO, β-arrestin 1/2 DKO, and β-arrestin 1 re-constituted MEF cells.
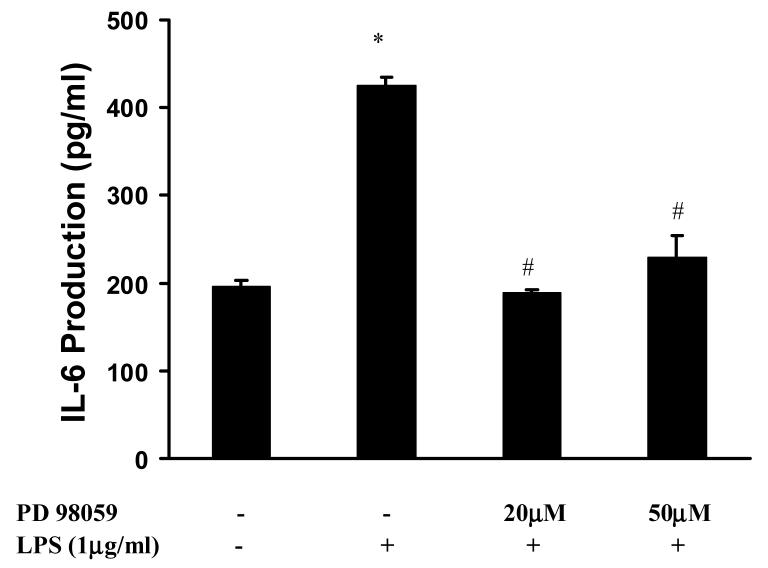
To further examine the role of ERK 1/2 in LPS-induced IL-6 production, a specific MEK inhibitor PD98059 was employed. LPS significantly induced IL-6 production (2.2±0.1 fold over basal p<0.05) in WT MEF cells. Pretreatment of WT MEF cells with PD98059 significantly decreased LPS-induced IL-6 (56±1% and 46±6% inhibition, p<0.05) (Fig. 2).
Figure 2. Effect of PD98059 on LPS-induced IL-6 production in MEFs.
MEFs from WT mice were pretreated with PD98059 (20μM-50μM) a specific ERK1/2 inhibitor followed by stimulation with LPS (1μg/ml) for 18 hours. Data represent means ± SE from three independent experiments. *, p<0.05 compared to basal cells; #, p<0.05 compared to LPS stimulated groups.
3.2 β-arrestin 2 mediates LPS-induced ERK 1/2 activation
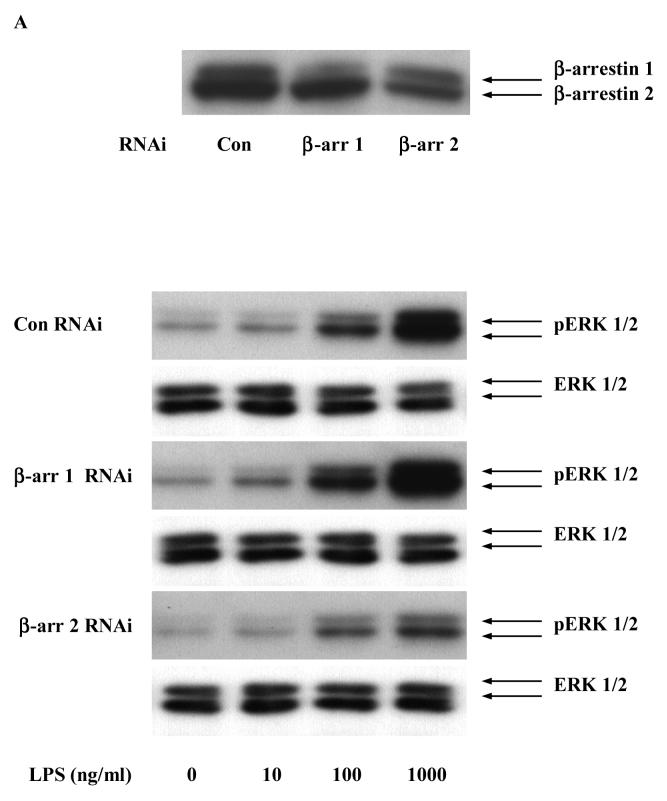
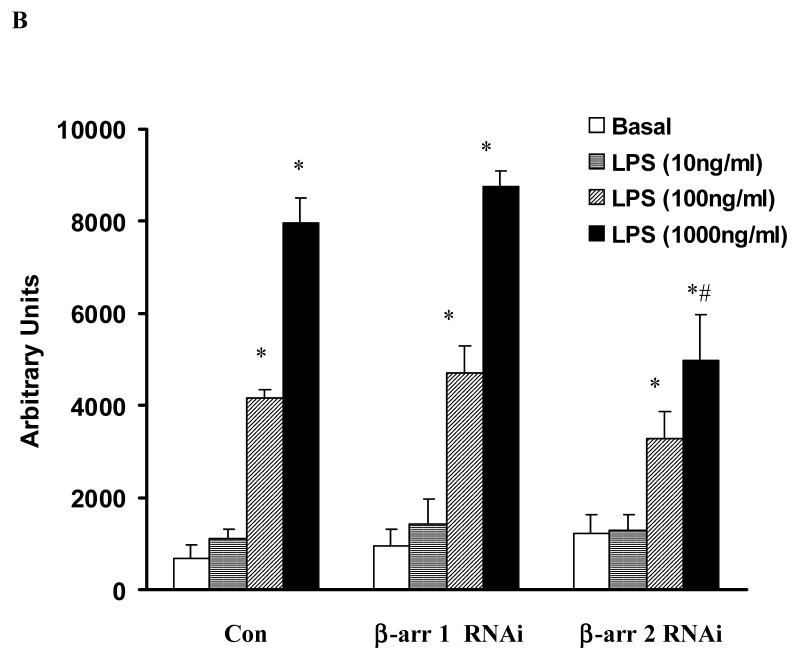
To further investigate the role of β-arrestins in LPS-induced ERK 1/2 activation, HEKTLR4 cells were transfected with RNA interference specifically targeted to β-arrestin 1 or 2 (both β-arrestin 1 and 2 were depleted by more than 50%, Fig. 3A). β-arrestin 2 siRNA transfection resulted in a 37±12% decreases in LPS-induced ERK 1/2 activation (p<0.05). β-arrestin 1 siRNA transfection had no effect on LPS-induced ERK 1/2 activation (Fig. 3A, 3B). Overexpression of wild type β-arrestin 1 and 2 had no effect on LPS-induced ERK 1/2 activation (data not shown).
Figure 3. Effect of β-arrestins 1/2 RNAi on LPS-induced ERK 1/2 activation in HEK-TLR4 cells.
HEK 293 cells stably expressing TLR4, CD14 and MD-2 were transfected with RNAi for control, β-arrestin 1 and β-arrestin 2 followed by stimulation with LPS (0-1μg/ml). LPS-induced ERK1/2 activation was studied (A). Statistical analysis was performed on the densitometry results (B). Data represent means ± SE from three independent experiments. *, p<0.05 compared to basal cells; #, p<0.05 compared to LPS stimulated groups.
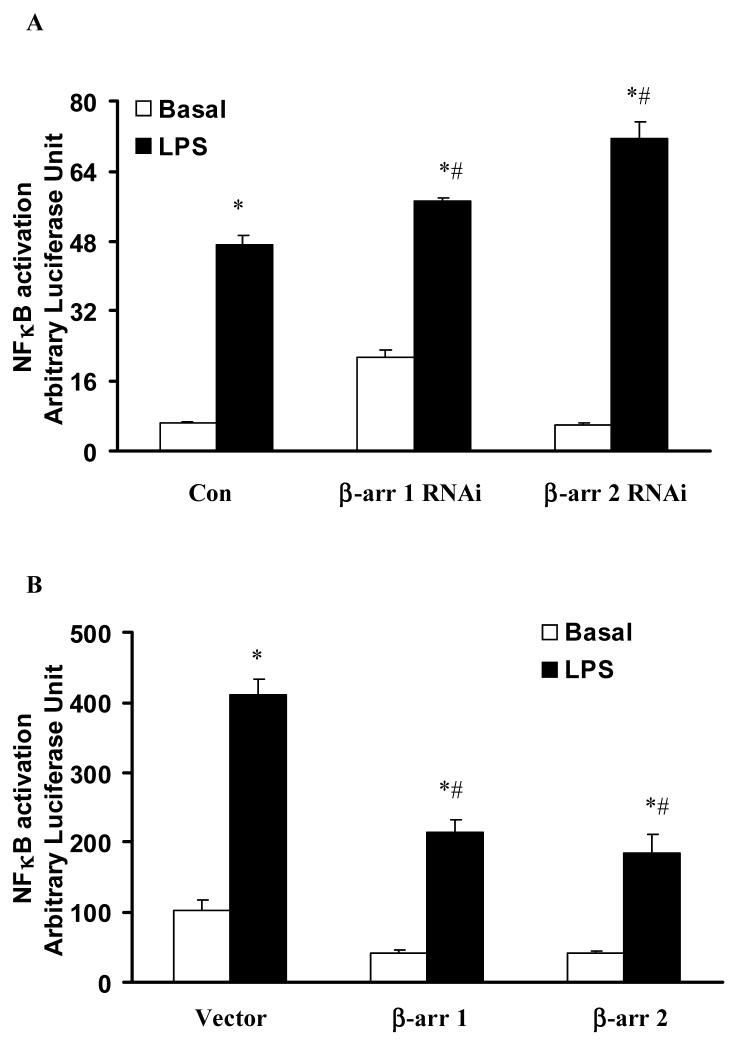
3.3 Both β-arrestin 1 and 2 negatively regulate LPS-induced NFκB activation
To investigate the role of β-arrestins in LPS-induced NFκB activation, a protocol similar to ERK 1/2 activation was used. β-arrestin 1 and 2 siRNA transfection significantly increased LPS-induced NFkB activation 1.2±0.01 fold, and 1.5±0.08 fold respectively (p<0.05) (Fig. 4A). Overexpression of wild type β-arrestin 1 and 2 significantly decreased LPS-induced NFκB activation (52±4%, and 45±7% respectively, p<0.05) (Fig. 4B).
Figure 4. Effect of altered β-arrestins expression on LPS-induced NFκB activation in HEK-TLR4 cells.
HEK 293 cells stably expressing TLR4, CD14 and MD-2 were transfected with RNAi for control, β-arrestin 1 or β-arrestin 2 (A) or empty vector or wild-type β-arrestin 1 or β-arrestin 2 (B) followed by stimulation with LPS (1μg/ml). LPS-induced NFκB activation was studied. Data represent means ± SE from three independent experiments. *, p<0.05 compared to basal cells; #, p<0.05 compared to LPS stimulated groups.
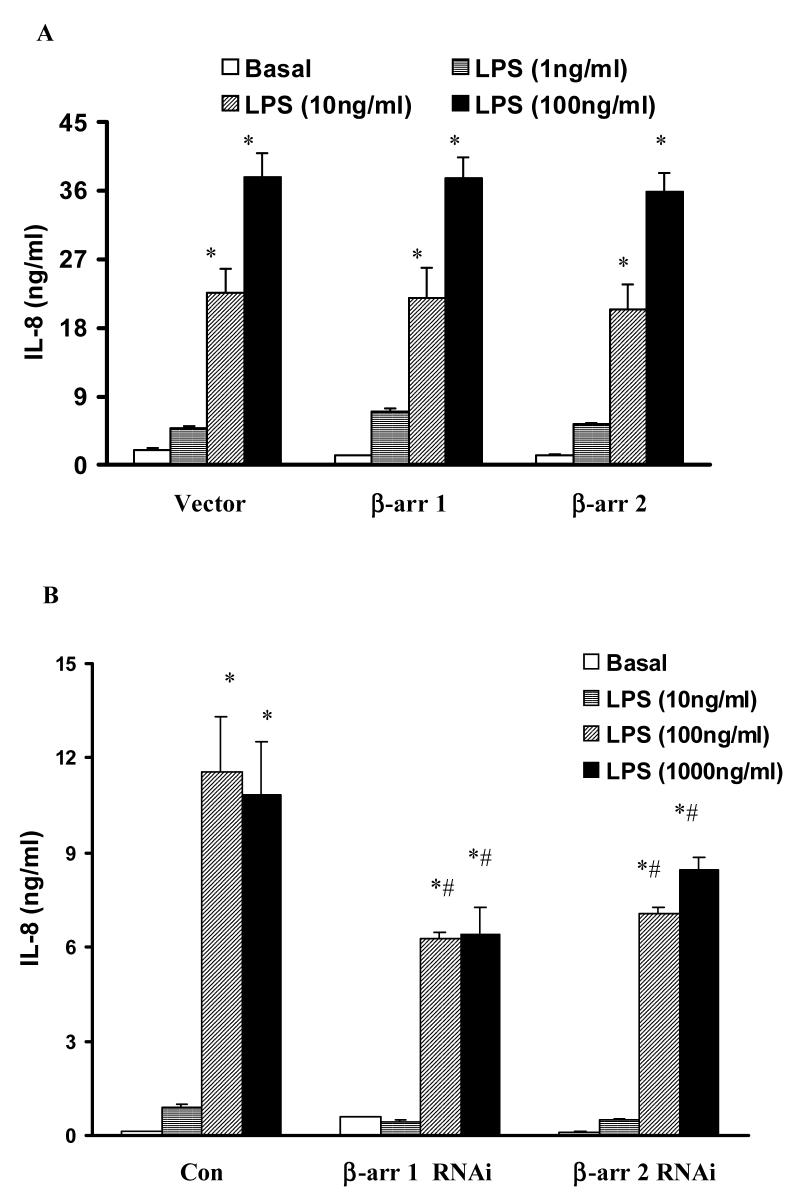
3.4 Both β-arrestin 1 and 2 positively regulate LPS-induced IL-8 production through ERK 1/2 activation
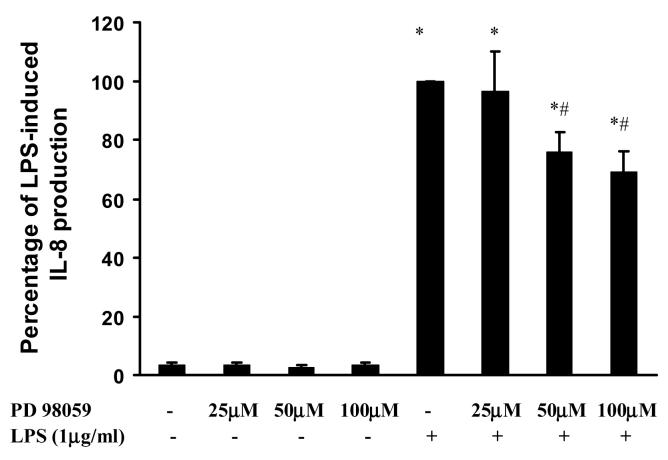
Since overexpression of β-arrestin 1 or 2 decreased NFκB activation, while inhibition of β-arrestin 2 decreased ERK 1/2 activation, we wanted to assess the role of β-arrestin 1 and 2 in LPS-induced IL-8 production. LPS (100 and 1000ng/ml) significantly induced IL-8 production (79±12 fold and 74±12 fold, respectively). Overexpression of wild type β-arrestin 1 and 2 had no effect on LPS-induced IL-8 production (Fig. 5A). However, β-arrestin 1 and 2 depletion by siRNA transfection significantly decreased LPS-induced IL-8 (46±2%, and 39±2%, respectively at 100ng/ml LPS stimulation, p<0.05) (Fig. 5B). As with the MEF cells, pretreatment with PD98059 (50 or 100μM) significantly decreased LPS induced IL-8 production in HEK 293 cells (24±7% and 31±7% inhibition, p<0.05) (Fig. 6)
Figure 5. Effect of altered β-arrestins expression on LPS-induced IL-8 production in HEK-TLR4 cells.
HEK 293 cells stably expressing TLR4, CD14 and MD-2 were transfected with empty vector or wild-type β-arrestin 1 or β-arrestin 2 (A) or RNAi for control, β-arrestin 1 or β-arrestin 2 (B) followed by stimulation with LPS (0-1μg/ml). LPS-induced IL-8 production was measured. Data represent means ± SE from three independent experiments. *, p<0.05 compared to basal cells; #, p<0.05 compared to LPS stimulated groups.
Figure 6. Effect of PD98059 on LPS-induced IL-8 production in HEK-TLR4 cells.
HEK-TLR4 cells were pretreated with PD98059 (25μM-100μM) a specific ERK1/2 inhibitor followed by stimulation with LPS (1μg/ml) for 18 hours. Data represent means ± SE from three independent experiments. *, p<0.05 compared to basal cells; #, p<0.05 compared to LPS stimulated groups.
4. Discussion
Our studies demonstrate that β-arrestins differentially regulate LPS-induced ERK 1/2 and NFκB signaling and positively mediate IL-6 and IL-8 production. LPS significantly induces IL-6 production in WT MEF cells, but not in β-arrestin 2 KO, β-arrestin 1 and 2 double KO and β-arrestin 1 reconstituted MEF cells. Reconstituted expression of β-arrestin 2 in double KO MEF cells restores LPS-induced IL-6 production. These data provide the first evidence that β-arrestin 2 positively regulates LPS-induced pro-inflammatory gene expression. A specific ERK 1/2 inhibitor PD98059 attenuates LPS-induced IL-6 production in WT MEF cells demonstrating that LPS-induced IL-6 production is mediated, in part, by the ERK 1/2 signaling pathway. In LPS responsive HEK 293 cells, siRNA depletion of β-arrestin 2 significantly decreases LPS-induced ERK 1/2 activation, while depletion of β-arrestin 1 had no effect. Over expression of β-arrestins 1 and 2 had no effect on LPS-induced ERK 1/2 activation, suggesting endogenous β-arrestins 1 and 2 are sufficient to mediate maximum ERK 1/2 activity.
Our finding that β-arrestin 2 mediates LPS-induced ERK 1/2 activation is consistent with previous studies that β-arrestin 2 is a scaffold, recruiting Raf-1, MEK, and ERK 1/2 to activated receptors and leading to increased ERK 1/2 activity (Luttrell et al., 2001; DeFea et al., 2000; DeFea et al., 2000). β-arrestin 2 binding results in retention of activated ERK 1/2 in the cytoplasm, possibly leading to phosphorylation of cytoplasmic substrates (Luttrell et al., 2001; DeFea et al., 2000; DeFea et al., 2000; McDonald et al., 2000). Our data suggest that β-arrestin 2 but not β-arrestin 1 mediates LPS-induced ERK 1/2 activation. Studies by Ahn et al. (2004) demonstrated that siRNA depletion of β-arrestin 2 inhibits cytoplasmic ERK 1/2 activation while siRNA depletion of β-arrestin 1 augments cytoplasm ERK 1/2 activation upon GPCR activation with angiotensin II. Our findings are the first report that β-arrestin 2 mediates ERK 1/2 activation in TLR signaling.
Our previous studies and others suggest a role of Gi proteins in TLR signaling (Fan et al., 2004; Fan et al., 2005; Lentschat et al., 2005). Gαi proteins regulate LPS-induced ERK1/2 activation and downstream pro-inflammatory mediator production (Fan et al., 2004; Lentschat et al., 2005). β-Arrestins may regulate Gi protein function that further mediates ERK 1/2 activation. It is possible that β-arrestins regulate GPCRs indirectly activated by TLR4. Triantafilou et al. (2001) have proposed that LPS interacts with a cluster of receptors in lipid rafts including Gi protein coupled receptors eg. CXCR4 (Ling et al., 1999), growth factor receptors (Luttrell et al., 1999), and β2 integrins (Saito et al., 2002). It is interesting in this context that β-arrestin 2 regulates CXCR4 agonist-induced MAP kinase activation and down stream signaling events (Sun et al., 2002). Thus both Gi proteins and β-arrestin may be important in LPS-induced signaling. However, other studies suggest that β-arrestins also mediate Gi protein independent signaling pathways upon activation of GPCRs (Shenoy et al., 2006; Gesty-Palmer et al., 2006).
In contrast to ERK 1/2 activation, we found that both β-arrestins 1 and 2 negatively regulate LPS- induced NFκB activation. The siRNA depletion of β-arrestins 1 and 2 increases LPS-induced NFκB activation while over expression decreased it. These findings agree with previous studies that β-arrestin 2 directly interacts with IκBα, thus preventing the phosphorylation and degradation of IκBα (Witherow et al., 2004; Gao et al., 2004). More recent studies have demonstrated that β-arrestins 1 and 2 directly interact with TRAF6 following TLR or IL-1R activation (Wang et al., 2006). The complexes of β-arrestins and TRAF6 prevented its autoubiquitination and activation of NFκB and AP-1 (Wang et al., 2006). These studies describe an inhibitory role for β-arrestins in the regulation of LPS signaling. Our studies are in agreement, demonstrating that overexpression of β-arrestins 1 and 2 decreases LPS-induced NFκB activation and that siRNA depletion of β-arrestins increases LPS-induced NFκB activation.
Since β-arrestins play reciprocal roles in the regulation of LPS-induced ERK 1/2 and NFκB activation, the impact on pro-inflammatory gene expression was examined. We found both β-arrestins 1 and 2 mediate LPS-induced IL-8 production in HEK 293 cells and β-arrestin 2 mediated LPS-induces IL-6 production in MEFs. Previous studies have shown that the chemokine IL-8 gene expression is mediated by both MAP kinase and NFκB signaling pathways (Hoffmann et al., 2002). The NFκB mediated signaling pathway is essential for certain agonist-induced IL-8 gene expression (Hoffmann et al., 2002). However, LPS-induced IL-8 production appears to be primarily mediated by the ERK 1/2 signaling pathway. PD98059 or U0126, specific MEK inhibitors, significantly inhibited LPS-induced IL-8 gene expression in monocytes or epithelial cells (Hoffmann et al., 2002; Warny et al., 2000; Scherle et al., 1998; Reddi et al., 2003). Our studies also showed that PD98059 significantly inhibit LPS-induced IL-6 production in MEF cells suggesting that LPS-induced IL-6 production in MEF cells was also mediated by ERK 1/2 signaling pathways. These findings are consistent with our data that β-arrestin 2 depletion reduces LPS-induced ERK 1/2 activation and that PD98059 attenuated LPS-induced IL-8 production in HEK 293 cells. However, our results demonstrate that β-arrestin 1 regulation of IL-8 is independent of ERK 1/2 activation. Although β-arrestin 1 reconstitution in MEF double KO cells did not affect IL-6 production, depletion of either β-arrestin 1 or β-arrestin 2 by siRNA in HEK 293 cells decreased LPS-induced IL-8 production. This suggests positive regulation of IL-8 production by both β-arrestins. It is possible that β-arrestin 1 may modulate other LPS-induced signaling pathways. Since β-arrestin 1 but not β-arrestin 2 translocates into the nucleus, it is possible that β-arrestin 1 may positively regulate IL-8 production at the transcription level (Kang et al., 2005).
NFκB is undeniably a major transcription factor for most cytokines and chemokines. Since HEK 293 cells produce only IL-8, this precluded examination of other cytokines and/or chemokines that are NFκB regulated. Indeed Wang et al. (2006) demonstrated that bone marrow derived cells following LPS stimulation produce more TNFα, IL-6, and IL-12p40 than WT cells, which is consistent with the notion that β-arrestin is a negative regulator of signaling. Nevertheless we have demonstrated that spleen cells from β-arrestin 2 (−/−) mice produce less TNFα and IL-6 upon LPS stimulation than WT (unpublished data). Thus, there appears to be cellular phenotype differences in the role of β-arrestin 2 in regulation in TLR signaling and its function as a positive or negative regulator of signaling. Additionally, Wang et al. (2006) demonstrated that LPS-induced mortality in galactosamine sensitized mice was enhanced in β-arrestin 2 (−/−) mice. However, it has been argued that galactosamine induced LPS mortality is not identical to LPS shock since the former induces severe hepatic inflammation and are extremely sensitive to stimulation with TNFα (Mignon et al., 1999). In response to higher doses of LPS in the absence of TNFα, we have demonstrated in β-arrestin 2 (−/−) mice reduced hepatic leukosequestration as measured by myeloperoxidase activity relative to WT (unpublished data). Although β-arrestins play a role in desensitization of specific G protein signaling pathway, they have been shown to function as scaffolding proteins to activate ERK signaling in G protein independent pathways. Indeed ligand activators of receptors has been shown to activate distinct signaling pathways dependent upon whether the receptor is in the Gi or Gq configurations as opposed to the G protein independent configuration (Gesty-Palmer et al., 2006). Regardless of the positive and/or negative impact of β-arrestins on cell signaling and cellular phenotype difference, it is clear that β-arrestins modulate TLR4 signaling.
Collectively, these studies provide evidence that β-arrestins play a role in TLR4 signaling and inflammatory gene expression. β-arrestins must be considered in a growing list of signaling proteins that modulate TLR4 activation and require further investigation.
Acknowledgments
This work was supported in part by NIH GM27673 (JAC) and DK55524 (LML).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Pro.c Natl. Acad. Sci. U S A. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J. Biol. Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J. Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Daniel-Issakani S, Spiegel AM, Strulovici B. Lipopolysaccharide response is linked to the GTP binding protein, Gi2, in the promonocytic cell line U937. J. Biol. Chem. 1989;264:20240–20247. [PubMed] [Google Scholar]
- DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc. Natl. Acad. Sci. U S A. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Peck OM, Tempel GE, Halushka PV, Cook JA. Toll-like receptor 4 coupled Gi protein signaling pathways regulate extracellular signal-regulated kinase phosphorylation and AP-1 activation independent of NFkappaB activation. Shock. 2004;22:57–62. doi: 10.1097/01.shk.0000129759.58490.d6. [DOI] [PubMed] [Google Scholar]
- Fan H, Teti G, Ashton S, Guyton K, Tempel GE, Halushka PV, Cook JA. Involvement of G(i) proteins and Src tyrosine kinase in TNFalpha production induced by lipopolysaccharide, group B Streptococci and Staphylococcus aureus. Cytokine. 2003;22:126–133. doi: 10.1016/s1043-4666(03)00122-4. [DOI] [PubMed] [Google Scholar]
- Fan H, Zingarelli B, Peck OM, Teti G, Tempel GE, Halushka PV, Cook JA. Lipopolysaccharide- and gram-positive bacteria-induced cellular inflammatory responses: role of heterotrimeric Galpha(i) proteins. Am. J. Physiol. Cell Physiol. 2005;289:C293–301. doi: 10.1152/ajpcell.00394.2004. [DOI] [PubMed] [Google Scholar]
- Ferlito M, Romanenko OG, Guyton K, Ashton S, Squadrito F, Halushka PV, Cook JA. Implication of Galpha i proteins and Src tyrosine kinases in endotoxin-induced signal transduction events and mediator production. J. Endotoxin Res. 2002;8:427–435. [PubMed] [Google Scholar]
- Ferlito M, Squadrito F, Halushka PV, Cook JA. Signal transduction events in Chinese hamster ovary cells expressing human CD14; effect of endotoxin desensitization. Shock. 2001;15:291–296. doi: 10.1097/00024382-200115040-00007. [DOI] [PubMed] [Google Scholar]
- Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol. Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J. Biol. Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Jafri F, El-Shewy HM, Lee MH, Kelly M, Luttrell DK, Luttrell LM. Constitutive ERK1/2 activation by a chimeric neurokinin 1 receptor-beta-arrestin1 fusion protein. Probing the composition and function of the G protein-coupled receptor “signalsome”. J. Biol. Chem. 2006;281:19346–19357. doi: 10.1074/jbc.M512643200. [DOI] [PubMed] [Google Scholar]
- Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc. Natl. Acad. Sci. U S A. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentschat A, Karahashi H, Michelsen KS, Thomas LS, Zhang W, Vogel SN, Arditi M. Mastoparan, a G protein agonist peptide, differentially modulates TLR4- and TLR2-mediated signaling in human endothelial cells and murine macrophages. J. Immunol. 2005;174:4252–4261. doi: 10.4049/jimmunol.174.7.4252. [DOI] [PubMed] [Google Scholar]
- Ling K, Wang P, Zhao J, Wu YL, Cheng ZJ, Wu GX, Hu W, Ma L, Pei G. Five-transmembrane domains appear sufficient for a G protein-coupled receptor: functional five-transmembrane domain chemokine receptors. Proc. Natl. Acad. Sci. U S A. 1999;96:7922–7927. doi: 10.1073/pnas.96.14.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr. Opin. Cell. Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc. Natl. Acad. Sci. U S A. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignon A, Rouquet N, Fabre M, Martin S, Pages JC, Dhainaut JF, Kahn A, Briand P, Joulin V. LPS challenge in D-galactosamine-sensitized mice accounts for caspase-dependent fulminant hepatitis, not for septic shock. Am. J. Respir. Crit. Care Med. 1999;159:1308–1315. doi: 10.1164/ajrccm.159.4.9712012. [DOI] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- Miller WE, Lefkowitz RJ. Expanding roles for beta-arrestins as scaffolds and adapters in GPCR signaling and trafficking. Curr. Opin. Cell Biol. 2001;13:139–145. doi: 10.1016/s0955-0674(00)00190-3. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr. Top. Microbiol. Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Reddi K, Phagoo SB, Anderson KD, Warburton D. Burkholderia cepaciainduced IL-8 gene expression in an alveolar epithelial cell line: signaling through CD14 and mitogen-activated protein kinase. Pediatr. Res. 2003;54:297–305. doi: 10.1203/01.PDR.0000076661.85928.1D. [DOI] [PubMed] [Google Scholar]
- Saito N, Yamada Y, Sannohe S, Honda K, Adachi T, Kayaba H, Chihara J. Possible involvement of C-C chemokines in functional augmentation of adhesion molecules in asthmatic patients. Lung. 2002;180:251–263. doi: 10.1007/s004080000099. [DOI] [PubMed] [Google Scholar]
- Scherle PA, Jones EA, Favata MF, Daulerio AJ, Covington MB, Nurnberg SA, Magolda RL, Trzaskos JM. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J. Immunol. 1998;161:5681–5686. [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J. Biol. Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- Solomon KR, Kurt-Jones EA, Saladino RA, Stack AM, Dunn IF, Ferretti M, Golenbock D, Fleisher GR, Finberg RW. Heterotrimeric G proteins physically associated with the lipopolysaccharide receptor CD14 modulate both in vivo and in vitro responses to lipopolysaccharide. J. Clin. Invest. 1998;102:2019–2027. doi: 10.1172/JCI4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J. Biol. Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nat. Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- Wang J, Kester M, Dunn MJ. Involvement of a pertussis toxin-sensitive G-protein-coupled phospholipase A2 in lipopolysaccharide-stimulated prostaglandin E2 synthesis in cultured rat mesangial cells. Biochim. Biophys. Acta. 1988;963:429–435. doi: 10.1016/0005-2760(88)90311-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- Warny M, Keates AC, Keates S, Castagliuolo I, Zacks JK, Aboudola S, Qamar A, Pothoulakis C, LaMont JT, Kelly CP. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Invest. 2000;105:1147–1156. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc. Natl. Acad. Sci. U S A. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Morrison DC. Lipopolysaccharide-induced selective priming effects on tumor necrosis factor alpha and nitric oxide production in mouse peritoneal macrophages. J. Exp. Med. 1993;177:511–516. doi: 10.1084/jem.177.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]