Summary
The addition of different concentrations of sodium bicarbonate had a profound effect on 2,3,4,5-chlorobiphenyl (2,3,4,5-CB) dechlorination in Hudson River sediment cultures. The most extensive dechlorination was observed in cultures to which 100 mg l−1 bicarbonate was added. Cultures amended with 1000 mg l−1 bicarbonate had the least extensive dechlorination, with 2,4-CB and 2,5-CB as predominant end-products. A significant loss of total chlorinated biphenyl mass was observed in cultures to which ≤500 mg l−1 bicarbonate was added, suggesting that degradation beyond chlorinated biphenyls occurred. The dynamics of acetate formation were different among the treatments, with high acetate concentrations detected throughout the 303-day experiment in cultures to which 1000 mg l−1 bicarbonate had been added. Sodium bicarbonate addition also had a significant impact on bacterial community structure as detected by polymerase chain reaction-denaturant gradient gel electrophoresis (PCR-DGGE) of 16S rRNA gene fragments. Three putative polychlorinated biphenyl (PCB) dechlorinators were identified; one Dehalococcoides-like population was detected in all enrichment cultures, whereas two Dehalobacter-like populations were only detected in the enrichment cultures with the most extensive dechlorination. These results suggest that the availability of bicarbonate, and potentially sodium, may affect PCB dechlorination in Hudson River sediment and thus need to be taken into consideration when assessing the fate of PCBs or implementing bioremediation.
Introduction
Polychlorinated biphenyls (PCBs) are ubiquitous contaminants in soils and sediments. For example, from the late 1940s until 1977, an estimated 0.2–1.3 million pounds of PCBs were discharged into the Hudson River (US Environmental Protection Agency, 2000). The stable and hydrophobic nature of PCBs causes them to bioaccumulate and biomagnify through the food chain (Safe et al., 1987). Because of the risk that PCBs pose to human and ecosystem health, remediation technologies have been sought for the clean-up of PCB-contaminated sediments.
Anaerobic PCB dechlorination was first discovered in Hudson River sediment based on altered congener distribution patterns in situ (Brown et al., 1987); PCB dechlorination was subsequently validated in laboratory experiments (Quensen et al., 1988). Although little is known about the physiology of the organisms that anaerobically dechlorinate PCBs, it is believed that PCBs are used as electron acceptors during dehalorespiration (Quensen et al., 1988). Dehalorespiration of PCBs may therefore be similar to that observed with other chlorinated compounds, such as tetrachloroethylene (Maymó-Gatell et al., 1997), 1,1,1-trichloroethane (Sun et al., 2002) and chlorobenzenes (Adrian et al., 2000). Two putative PCB dechlorinators, detected in highly enriched cultures actively dechlorinating PCBs, were phylogenetically related to other dehalorespirers (Cutter et al., 2001; Wu et al., 2002), providing additional support for this hypothesis.
Although the addition of H2 (Sokol et al., 1994) and organic electron donors (Nies and Vogel, 1990; Alder et al., 1993) can stimulate PCB dechlorination, no research has specifically investigated the effect of inorganic carbon on dechlorination. Indeed, bicarbonate has been typically added to the medium at concentrations of 1.2–2.5 g l−1 in studies investigating PCB dechlorination (Shelton and Tiedje, 1984; van Dort et al., 1997; Adrian et al., 1998; Zwiernik et al., 1998; Magar et al., 1999; Zwiernik et al., 1999). Nevertheless, its concentration during incubation has, to our knowledge, not been monitored or replenished. Most hydrogenotrophs utilize aqueous CO2 as their carbon source (Madigan et al., 2003), although Dehalococcoides ethenogenes strain 195 respires tetrachloroethylene while utilizing acetate as a carbon source (Maymó-Gatell et al., 1997). The carbon source for PCB dechlorinators is currently unknown, although H2 appears to be an electron donor for PCB dechlorinators (Sokol et al., 1994; Rysavy et al., 2005), suggesting that aqueous CO2 may be important for the growth of these organisms. Although it is generally assumed that inorganic carbon has little effect on the growth of bacteria, a recent study demonstrated that higher-than-ambient partial pressures of CO2 (5%) improved the growth of Acidobacteria (Stevenson et al., 2004). Whether or not PCB dechlorinators are autotrophs, the presence of elevated concentrations of CO2 may improve the growth and subsequent activity of these organisms because they live in sediments that contain regions of higher-than-ambient levels of CO2. It is therefore important to determine whether CO2 can in fact affect the growth of PCB dechlorinators so that this information can be exploited for either bioremediation or the isolation of these organisms.
In this study, the impact of aqueous CO2 on the reductive dehalogenation of 2,3,4,5-chlorobiphenyl (2,3,4,5-CB) was investigated in Hudson River sediment cultures. Our hypothesis was that the amendment of bicarbonate to the cultures could directly stimulate reductive dechlorination by providing additional inorganic carbon for the growth of PCB dechlorinators. Alternatively, the addition of bicarbonate could stimulate homoacetogenesis, which would generate acetate (another possible carbon source). Because it may be possible to alter the inorganic and organic carbon concentrations in sediment or in an active sediment cap, this research could lead to substantially improved knowledge-based designs for sediment bioremediation. In addition, if the effect of inorganic carbon on PCB dechlorinators is known, then aqueous CO2 concentrations could be optimized to enrich and eventually isolate these important organisms.
Results
2,3,4,5-CB dechlorination
Dechlorination of 2,3,4,5-CB was observed in all live microcosms after 136 days of incubation (Fig. 1); no dechlorination was observed in the sterile controls (data not shown). The use of a single congener (2,3,4,5-CB) enabled us to identify the active dechlorination processes under different conditions. Because of the extremely low solubility of PCBs, evenly distributing them in sediment cultures was a challenge, initially resulting in poor recoveries of 2,3,4,5-CB and high variability in the beginning of the experiment (Fig. 1). This problem, however, was solved by continued incubation and homogenization with time (Fig. 1).
Fig. 1.
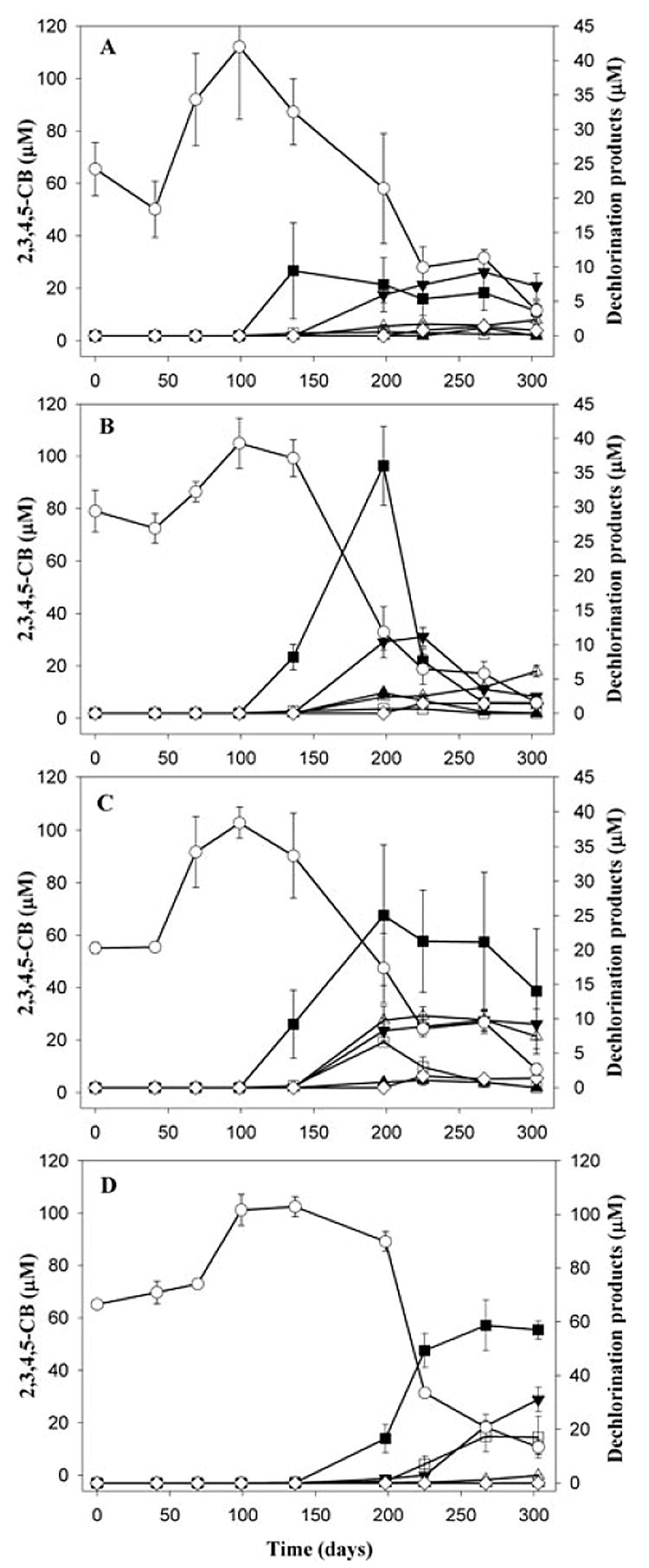
Patterns of 2,3,4,5-CB dechlorination in microcosms amended with 0 mg l−1 (A), 100 mg l−1 (B), 500 mg l−1 (C) and 1000 mg l−1 (D) bicarbonate. Error bars represent the standard deviation of triplicate microcosms. ○, 2,3,4,5-CB; ■, 2,3,5-CB; □, 2,4,5-CB; ▲ = 2,3-CB; △, 2,4-CB; ▼, 2,5-CB; ◇, 2-CB.
Dechlorination began with the removal of doubly flanked (DF) meta and para chlorines (forming 2,3,5-CB and 2,4,5-CB). This was followed by the removal of singly flanked (SF) meta or para chlorines (forming 2,4-CB and 2,5-CB). The concentration dynamics of 2,3,5-CB, 2,5-CB and 2-CB (Fig. 1) suggest that the majority of 2,5-CB was formed from 2,3,5-CB dechlorination, and that the majority of 2-CB was formed from 2,5-CB dechlorination. In treatments to which ≤ 500 mg l−1 bicarbonate was added, the increase of 2,3,5-CB dechlorination products was not proportional to the decrease of 2,3,5-CB. This suggests the concomitant production and degradation of 2,3-CB, 2,5-CB and 2-CB.
There were substantial differences in the dechlorination pattern of 2,3,4,5-CB (Fig. 1) and the resulting product distributions (Fig. 2). In the microcosms to which 500 or 1000 mg l−1 bicarbonate was added, 2,3,5-CB dechlorinated either slowly or not at all (Fig. 1C and D). In contrast, 2,3,5-CB was formed and dechlorinated rapidly in the microcosms fed with 100 mg l−1 bicarbonate (Fig. 1B). The majority of the other observed dechlorination products (2,4,5-CB, 2,5-CB, 2,3-CB) were subsequently dechlorinated, with only 2,4-CB and 2-CB forming and persisting at very low concentrations (approximately 7 and 2 μM respectively) (Fig. 1B). In the microcosms fed with no additional bicarbonate (Fig. 1A), 2,3,4,5-CB appeared to be dechlorinated more slowly, but all of the observed dechlorination products appeared to be formed and dechlorinated simultaneously, as they did not accumulate to a great extent. The dechlorination of 2,3,5-CB, 2,5-CB and 2,4-CB appeared to be slower than in the microcosms amended with 100 mg l−1 bicarbonate (Fig. 1A and B).
Fig. 2.
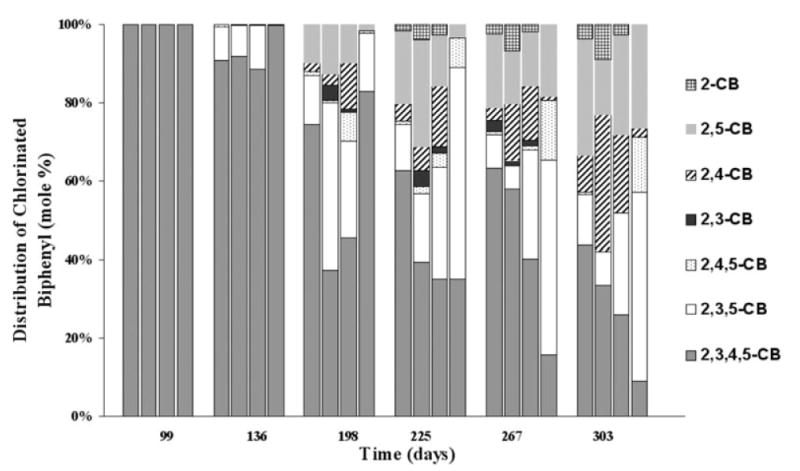
Molar distribution of 2,3,4,5-CB and its dechlorination products. For each four-column group at a certain time point, the first to fourth columns represent microcosms with 0, 100, 500 and 1000 mg l−1 bicarbonate added respectively. The molar percentages presented here are average values of the triplicate microcosms.
The total quantity of chlorinated biphenyls varied substantially over time (averaged over triplicate microcosms) in all of the treatments (Fig. 3). Initially, the mass of total chlorinated biphenyls (present as 100% 2,3,4,5-CB on day 0) measured in all of the microcosms, including the sterile control, was low (6.8 ± 1.4 μmol) compared with the quantity added (12 μmol). This is likely a result of the hydrophobicity of PCBs and the fact that they are very difficult to homogenize upon addition to sediment microcosms. Over time, however, the total mass of chlorinated biphenyls increased as the PCBs homogenized more completely and more representative slurry samples were taken.
Fig. 3.
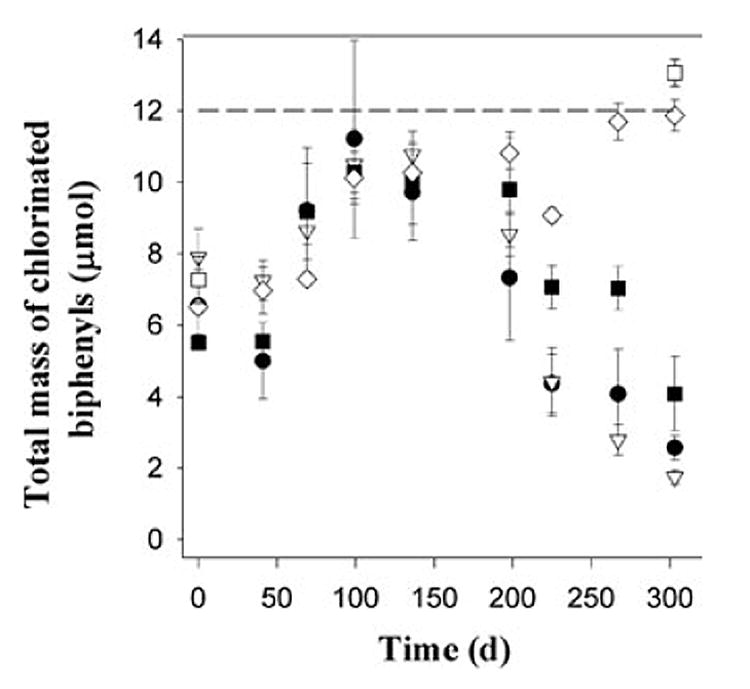
Total measured mass of 2,3,4,5-CB and its identified dechlorination products (2,3,5-CB, 2,4,5-CB, 2,3-CB, 2,4-CB, 2,5-CB and 2-CB). Treatments are: ●, 0 mg l−1 bicarbonate; ▽, 100 mg l−1 bicarbonate; ■, 500 mg l−1 bicarbonate; ◇, 1000 mg l−1 bicarbonate, □, sterile control. The dashed line represents the calculated mass of 2,3,4,5-CB added to the microcosms.
By day 99, the total quantity of chlorinated biphenyls was much higher and closer to 12 μmol as expected (10.5 ± 2.7 μmol). From day 0 to day 136, the quantity of total chlorinated biphenyls measured in all of the microcosms at a given time point was relatively consistent, with an average standard deviation over all of the microcosms of 15.6%. Therefore, although the total mass measured was lower than expected until around day 100, all of the microcosms behaved similarly with respect to the total quantity of chlorinated biphenyl present and extractable/ measurable.
On day 225, however, the total measured mass of chlorinated biphenyls began to significantly decrease in the microcosms to which 500 mg l−1, 100 mg l−1 and no bicarbonate were added (Fig. 3) and began to deviate significantly from the calculated total molar mass (12 μmol). The total measured chlorinated biphenyls in the microcosms amended with 1000 mg l−1 bicarbonate [9.1 ± 0.4 μmol (day 225), 11.7 ± 1.0 μmol (day 267) and 11.9 ± 0.9 μmol (day 303)], and in the sterile control on day 303 (13.1 ± 0.7 μmol) remained relatively constant over days 225–303 (11.4 ± 1.7 μmol) and close in value to the calculated total molar mass of chlorinated biphenyl added to the microcosms (12 μmol). The mass of chlorinated biphenyls measured on day 303 in the microcosms receiving ≤ 500 mg l−1 bicarbonate was significantly lower (P < 0.05) than that in the microcosms receiving 1000 mg l−1 bicarbonate. The greatest mass balance deficit was observed in microcosms receiving 100 mg l−1 bicarbonate; the differences in the quantity of total chlorinated biphenyls present on day 303 among all live treatments were statistically significant.
Acetogenesis and methanogenesis
All live microcosms produced similar quantities of methane, whereas no methane was produced by the sterile control (data not shown). The production of acetate, however, varied substantially between the different treatments in the live microcosms (Fig. 4). The initial acetate concentration in all microcosms on day 0 was very low (0.077 ± 0.018 mM). On day 69, the acetate concentration increased to about 2 mM in all microcosms, presumably as a result of the fermentation of sediment organic matter and subsequent acetogenesis. After day 197, the acetate concentrations declined and remained low except in the microcosms receiving 1000 mg l−1 bicarbonate, in which acetate concentrations were significantly higher than those in all of the other microcosms.
Fig. 4.
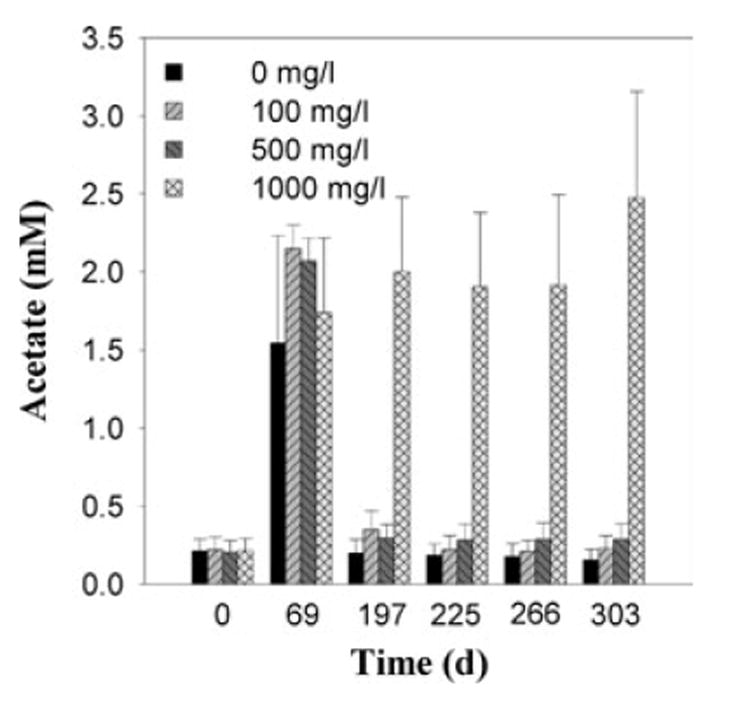
Acetate concentrations in the microcosms amended with 2,3,4,5-CB as a function of time. Results are the mean ± standard deviation of triplicate microcosms.
Community analysis
Polymerase chain reaction-denaturant gradient gel electrophoresis (PCR-DGGE) was used to fingerprint the bacterial community structures of the microcosms receiving different amounts of bicarbonate on day 225, when different dechlorination patterns were first observed (Fig. 5A). No defined bands were detectable in a sample of Hudson River sediment that was used to inoculate the cultures, indicating the presence of numerous bacterial populations of low densities (Nakatsu et al., 2000). Numerous defined bands were discernible, however, in the PCB-amended enrichment cultures. Four bands (Bands A, B, H and J) were common to all of the experimental microcosms, whereas several populations were found in only a few of the enrichments. For example, Band E was only present at a discernible density in microcosms containing bicarbonate ≤ 500 mg l−1. Principal component analysis demonstrated that different concentrations of bicarbonate addition had a significant impact on bacterial community structure (Fig. 5B).
Fig. 5.
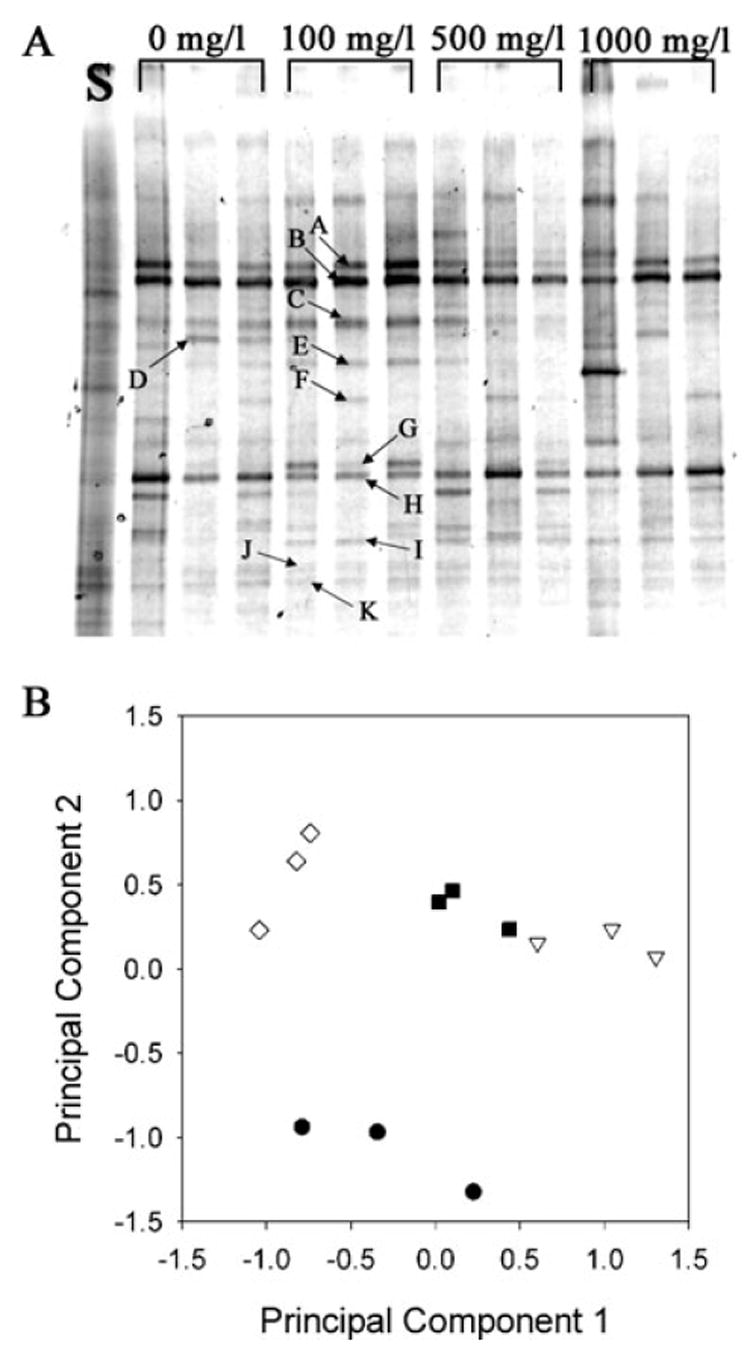
A. Fingerprints of the bacterial community structures of microcosms (day 225) amended with 2,3,4,5-CB and different concentrations of bicarbonate as determined by PCR-DGGE of PCR-amplified 16S rRNA gene fragments. Lane S is the initial community profile for Hudson River sediment before amendment with 2,3,4,5-CB. All other lanes are identified by the concentration of bicarbonate amended to the microcosm. Letters and arrows identify specific bands that were excised from the gel and sequenced. The results of the nucleotide sequence analysis for these PCR-DGGE bands are shown inTable 1.
B. Principal component analysis of PCR-DGGE fingerprints. Principal components 1 and 2 represent 52% of the total variance in the community fingerprints. ●, 0 mg l−1 bicarbonate; ▽, 100 mg l−1 bicarbonate; ■, 500 mg l−1 bicarbonate; ◇, 1000 mg l−1 bicarbonate.
Numerous prominent bands were excised from this gel and their nucleotide sequences were determined (Table 1). Phylogenetic analysis of the nucleotide sequences suggested three putative dechlorinating populations. Band B was phylogenetically related to Dehalococcoides strain CBDB1, which can dehalogenate chlorinated benzenes (Adrian et al., 2000). This band was present in all microcosms. Bands C and E were phylogenetically related to Dehalobacter restrictus, which can. dehalogenate chlorinated aliphatic compounds (Wild et al., 1996; Holliger et al., 1998; Sun et al., 2002). Both of these Dehalobacter-like populations were more prominent in the microcosms amended with 100 mg l−1 bicarbonate compared with the other microcosms (Fig. 5A).
Table 1.
The phylogenetic affiliations of bacterial populations detected by PCR-DGGE (Fig. 5A).
| Band | Sequence length | Band intensity (%) | Phylogenetic affiliation
|
||
|---|---|---|---|---|---|
| Organism (accession No.) | % Identity | Phylum | |||
| A | 154 | 12.3 | Environmental clone WCHB1-29 (AF050544) | 96.1 | Bacteroidetes |
| B | 136 | 32.4 | Dehalococcoides strain CBDB1 (AF230641) | 100 | Chloroflexi |
| C | 161 | 20.3 | Dehalobacter restrictus (Y10164) | 100 | Firmicutes |
| D | 135 | Environmental clone WCHB1-80 (AF050563) | 99.2 | Chloroflexi | |
| E | 161 | 7.8 | Dehalobacter restrictus (Y10164) | 99.3 | Firmicutes |
| F | 138 | 5.3 | Bdellovibrio bacteriovorus (AF148941) | 92.7 | δ-Proteobacteria |
| G | 135 | 1.5 | Clostridium sp. 13A1 (AY554421) | 100 | Firmicutes |
| H | 135 | 9.8 | Sedimentibacter sp. BRS2 (AY221992) | 94.8 | Firmicutes |
| I | 138 | 4.8 | Environmental clone G18 (AF407701) | 100 | Firmicutes |
| J | 135 | 2.6 | Environmental clone LBS18 (AF445645) | 90.3 | Not known |
| K | 136 | 3.3 | Environmental clone LBS18 (AF445645) | 88.9 | Planctomycetales |
Because PCR-DGGE provides only limited phylogenetic information of the detected populations, the bacterial community in one of the microcosms receiving 100 mg l−1 bicarbonate (day 225) was also investigated by PCR cloning of nearly complete 16S rRNA genes. A total of 65 clones were screened by PCR-DGGE (data not shown) to give 19 different 16S rRNA gene sequences (Table 2). The population with the highest frequency of appearance in the clone library (clone Z42) was closely related to Dehalococcoides strain CBDB1, and was identical in sequence to Band B. Two other populations (clones Z29 and Z40) were phylogenetically related to Dehalobacter restrictus and were identical in sequence to Bands C and E respectively.
Table 2.
The phylogenetic affiliations of the bacterial populations detected by PCR cloning from a Hudson River sediment microcosm (day 225) amended with 2,3,4,5-CB and 100 mg l−1 of bicarbonate.
| Clone | Sequence length | Clone frequency (%) | Phylogenetic relationship
|
||
|---|---|---|---|---|---|
| Species (accession No.) | % Identity | Phylum | |||
| Z9 | 852 | 3.7 | Clostridium cylindrosporum (Y18179) | 91.6 | Firmicutes |
| Z17 | 1476 | 7.4 | Environmental clone KD3-5 (AY188305) | 97.2 | Bacteroidetes |
| Z20 | 1415 | 3.7 | Chimeric | ||
| Z29 | 1447 | 11.1 | Dehalobacter restrictus (Y10164) | 99.5 | Firmicutes |
| Z30 | 815 | 3.7 | Environmental clone GR25 (AY150887) | 97.2 | Ambiguous |
| Z35 | 1456 | 7.4 | Sedimentibacter hydroxybenzoicus (L11305) | 93.7 | Firmicutes |
| Z39 | 873 | 3.7 | Pelospora glutarica (AJ251214) | 93.6 | Firmicutes |
| Z40 | 1446 | 3.7 | Dehalobacter restrictus (Y10164) | 99.0 | Firmicutes |
| Z42 | 1449 | 14.8 | Dehalococcoides strain CBDB1 (AF230641) | 99.8 | Chloroflexi |
| Z43 | 824 | 3.7 | Environmental clone DEL55 (AJ616285) | 97.9 | Planctomycetales |
| Z44 | 838 | 3.7 | Environmental clone NMW3.210WL (AY043958) | 87.5 | Ambiguous |
| Z45 | 853 | 3.7 | Environmental clone BIOEST-22 (AJ548911) | 98.3 | Firmicutes |
| Z52 | 849 | 3.7 | Sedimentibacter hydroxybenzoicus (L11305) | 92.1 | Firmicutes |
| Z54 | 862 | 11.1 | Clostridium sp. 9B4 (AY554416) | 96.8 | Firmicutes |
| Z57 | 820 | 3.7 | Environmental clone WCHB1-40 (AF050549) | 96.2 | Spirochaetes |
| Z60 | 853 | 3.7 | Arthrobacter globiformis (AB098573) | 99.5 | Actinobacteria |
| Z62 | 1470 | 3.7 | Environmental clone BSA1B-04 (AB175358) | 98.5 | Firmicutes |
| Z63 | 818 | 3.7 | Environmental clone BIOEST-22 (AJ548911) | 97.0 | Firmicutes |
Discussion
This study demonstrates that the addition of exogenous sodium bicarbonate to Hudson River sediment cultures had a profound effect on PCB dechlorination. Microcosms amended with an intermediate amount of sodium bicarbonate (100 mg l−1 as HCO3−) appeared to exhibit more extensive dechlorination of 2,3,4,5-CB (Figs 2 and 3) and more rapid dechlorination of its daughter products (Fig. 1) than either the control microcosms to which no additional sodium bicarbonate was added or the microcosms to which higher amounts of sodium bicarbonate (500 or 1000 mg l−1 as HCO3−) were added. The microcosms to which ≤ 500 mg l−1 bicarbonate was added also contained three putative dechlorinating populations. The two Dehalobacter-like populations were more prominent in the microcosms to which 100 mg l−1 bicarbonate was added (Fig. 5A) and in which more rapid and extensive dechlorination occurred. These results suggest that the addition of a small quantity of sodium bicarbonate can stimulate reductive dehalogenation of PCBs and help select for a more diverse dehalogenating bacterial community, whereas the addition of excessive sodium bicarbonate can adversely affect dechlorination processes.
Although both the sodium and bicarbonate concentrations changed in the various treatments (from approximately 2.5–18.9 mM sodium and from approximately 0–16.4 mM bicarbonate), we believe that it was the changing bicarbonate, rather than sodium concentrations, that altered the observed dechlorination. Sodium is known to be important for acetogenesis (Heise et al., 1989) and methanogenesis (Blaut et al., 1985), and if limiting in these experiments, varying sodium concentrations would have been expected to affect the rates of methanogenesis and acetogenesis in the microcosms. This was not observed, suggesting that bicarbonate was indeed the critical variable.
The results presented herein also suggest that a substantial fraction (> 50%) of the originally added 2,3,4,5-CB was degraded beyond chlorinated biphenyls (Fig. 3). This interpretation is supported by a mass balance deficit that occurred only in microcosms where 2-CB was produced and in which substantial sequential dechlorination past the trichlorobiphenyls was observed. In previous studies of PCB dechlorination, either similar mass balance deficits or anaerobic dechlorination of PCB congeners to biphenyl were also observed (Rhee et al., 1993a,b; Williams, 1994; Natarajan et al., 1996). Our results suggest that the addition of a small quantity of sodium bicarbonate enhanced the extent of anaerobic 2,3,4,5-CB degradation beyond chlorinated biphenyls.
The bacterial community structures of the sediment cultures were also substantially affected by sodium bicarbonate concentration. Of particular importance is the selection of several putative dechlorinating populations as a result of the changes in sodium bicarbonate concentration. Although populations with subtly different or even identical 16S rRNA gene sequences can have markedly different physiologies (Jaspers and Overmann, 2004), numerous researchers have isolated Dehalococcoides-like populations capable of the dehalorespiration of both aliphatic (Maymó-Gatell et al., 1997; He et al., 2003) and aromatic compounds (Adrian et al., 2000; Fennell et al., 2004), or enriched for Dehalococcoides-like populations that appeared to be associated with PCB dechlorination (Cutter et al., 2001; Wu et al., 2002). In addition, work in our laboratory has shown that in paired microcosm experiments in which one set of microcosms received PCBs and another did not, Dehalococcoides-like populations developed in microcosms to which PCBs were added, but were absent in microcosms to which no PCBs were added (Yan et al., 2006), suggesting that these organisms were associated with PCB dechlorination. Previous researchers have also characterized Dehalobacter spp. as obligate hydrogen-oxidizing, dehalorespiring organisms (Wild et al., 1996; Holliger et al., 1998; Sun et al., 2002); Dehalobacter-like populations have also been detected in a 1,2-dichloropropane-dechlorinating bioreactor (Schlotelburg et al., 2002). The Dehalococcoides-like populations in these microcosms did not appear to be affected by the changing sodium bicarbonate concentrations. This is consistent with current evidence that suggests that these organisms use acetate as a carbon source, rather than fixing inorganic carbon (Maymó-Gatell et al., 1995; Holliger et al., 1998). The presence of Dehalobacter-like populations, however, was affected by the different sodium bicarbonate concentrations. Our PCB-dechlorinating sediment cultures contained as many as two Dehalobacter-like populations, which were only present when 2-CB was formed and the bicarbonate concentration was ≤ 500 mg l−1. In addition, these populations appeared to be more prominent in the microcosms to which 100 mg l−1 sodium bicarbonate (as HCO3−) was added and in which the most rapid and extensive dechlorination occurred. To the knowledge of the authors, the present study is the first to detect Dehalobacter-like populations in association with PCB dechlorination and is also the first to simultaneously detect both Dehaloccoides-like and Dehalobacter-like populations in PCB-dechlorinating enrichment cultures.
The addition of very high concentrations of sodium bicarbonate resulted in increased acetate concentrations and a reduced rate and extent of dechlorination. We speculate that the high sodium bicarbonate concentrations either decreased the consumption of acetate, which had some negative effect on the development of a niche for PCB dechlorinators, or stimulated homoacetogenesis, which altered the flow of nutrients and electron donor away from dehalorespiration and specifically created an environment unfavourable to the enrichment of Dehalobacter-like populations.
In conclusion, considerable attention has focused on the use of appropriate electron donors to stimulate the dechlorination of environmental contaminants (e.g. Fennell et al., 1997; Yang and McCarty, 1998). Little work, however, has been conducted on the effect of carbon dioxide or bicarbonate concentrations on such processes, which may provide information that can be exploited for the bioremediation of contaminated sediments and is important for understanding the physiology of PCB dechlorinators and for use in their isolation (Stevenson et al., 2004). In this research we observed that 2,3,4,5-CB dechlorination in Hudson River sediment could be stimulated by the amendment of low levels of sodium bicarbonate. Because the amendment of higher quantities of sodium bicarbonate adversely affected dechlorination, however, it is critical to continue to probe the mechanisms behind this phenomenon so that appropriate enrichment techniques or in situ biostimulation techniques can be developed. This research has practical implications in that it may be possible to alter the inorganic and organic carbon concentrations in contaminated sediments or active caps to stimulate PCB bioremediation. The control of inorganic carbon concentrations could also allow one to better enrich and potentially isolate PCB dechlorinators.
Experimental procedures
Experimental set-up
Sediment samples collected from the Hudson River near Moreau, New York [North American Datum of 1983 (NAD83) Northing Coordinate (ft) 1609914.52 and NAD83 Easting Coordinate (ft) 733570.10] were transported under anaerobic conditions and stored in a glovebag (COY labs, MI) upon arrival. Reduced anaerobic mineral medium was prepared as described by Shelton and Tiedje (1984), except that 2 mM l-cysteine was used as a reducing agent instead of Na2S and no bicarbonate was added to the medium. The pH of the medium was adjusted to 7.0 with approximately 2.5 mM NaOH. The medium was sterilized by autoclaving and stored in the glovebag before use.
Microcosms were set up in 160 ml serum bottles containing 100 ml medium. Because sediment has been shown to be important for the development of diverse PCB-dechlorinating activities (e.g. Morris et al., 1990; Wu and Wiegel, 1997), Hudson River sediment (5 g dry weight equivalent) was used as the inoculum. Sediment that had been stored under anaerobic conditions was weighed and transferred into the bottles under strict anaerobic conditions. The sediment inorganic carbon was below the detection limit (0.15% by mass) and the organic carbon was 2.12% (by mass). The congener 2,3,4,5-CB (12.5 μmoles in 0.4 ml of hexane) was added to each bottle. The use of 2,3,4,5-CB as the model congener prevented interference from any naturally present PCBs, as 2,3,4,5-CB and its daughter products are unlikely to be present as a result of industrial contamination. Hexane was allowed to evaporate by keeping the bottles uncapped in the glovebag (gas composition: 4% H2 and 96% N2) for about 10 min until the added hexane was no longer visible as a separate layer. Bottles were then capped with Teflon-lined rubber stoppers, sealed with aluminum crimps and flushed with N2 to remove H2 from the headspace. Bottles were shaken on a wrist-action shaker for 2 h for PCB distribution, after which they were incubated on a rotating shaker (120 r.p.m.). Initial samples were collected in the glovebag for PCB analysis 24 h after 2,3,4,5-CB addition.
After initial samples were taken, bottles were resealed and again flushed with N2. At this time, bottles were amended with a pre-reduced (with 20 mM l-cysteine) bicarbonate stock solution containing 50 g l−1 NaHCO3 and 20 mM phosphate at a pH of 7.2 to generate the following treatments: 0, 100, 500 and 1000 mg l−1 bicarbonate. This added a small additional quantity of cysteine (0–0.4 mM) to the microcosms, but this was not thought to be significant. An additional treatment containing 1000 mg l−1 bicarbonate was autoclaved for 50 min on two consecutive days to serve as a sterile control. Each treatment consisted of triplicate microcosms. H2 (0.06 ml) was injected into the headspace (60 ml) of each bottle to reach a headspace H2 partial pressure of 0.001 atm (0.1% H2). Additional H2 (0.06 ml) was injected into all of the microcosms weekly. The concentration of H2 was not monitored between weekly additions; nevertheless, no accumulation of H2 was observed in the live microcosms when the headspace gas was analysed monthly.
Microcosms were incubated at 25°C in the dark on a rotating shaker (120 r.p.m.). The pH of the culture was measured and adjusted to 7.0 when bottles were opened for sediment sampling. The pH values in all microcosms before adjustment ranged from 6.76 to 7.53 and no distinct trends were observed among the different treatments (data not shown). The concentration of CO2 in the headspace was measured; the bicarbonate concentration in the medium was calculated assuming equilibrium; and if needed, additional bicarbonate was added to maintain the desired bicarbonate concentration after resealing the microcosms. All gases used in these experiments (N2, H2 and He) were ultra-high purity grade.
Sample collection
Microcosms were completely mixed for 2 min before the bottles were opened in the glovebag. Approximately 1.5 ml of the sediment slurry was withdrawn from the bottles using a glass pipette. The bottles were opened and resealed quickly to limit gas exchange. Samples were placed in 20 ml serum vials and the exact weight of the samples was determined. For bacterial community analysis, sediment slurry samples were collected in sterile 1.6 ml microcentrifuge tubes, centrifuged at 19 000 g for 5 min, and the pellet was stored at −70°C until DNA extraction. Supernatant samples (2 ml) were also withdrawn from the microcosms and filtered (0.45 μm) for immediate acetate analysis.
Chemical analysis
Headspace concentrations of CH4 and CO2 were measured before the microcosms were opened for withdrawal of the sediment slurry. Headspace gas samples (200 μl) were analysed using a gas chromatograph (GC) [Hewlett Packard (HP) 6890 Series] equipped with a thermal conductivity detector. Separation of the components in the gas sample was accomplished on a packed column (8 ft Hayesep Q, 8 ft × 0.125 in) with He as the carrier gas (flow rate: 20 ml min−1). Acetate was analysed on an ion chromatograph (761 compact IC, Metrohm) with an anion separation column (Metrosep A supp5, Metrohm).
The sample extraction method for PCB analysis was based on the method of Quensen and colleagues (1988) and is described elsewhere (Rysavy et al., 2005). Briefly, sediment slurry samples were weighed and then extracted, first with acetone (10 ml) for 2 min, followed by two hexane-acetone (1:1) (10 ml) extractions, each for 2 min. The pooled extracts (30 ml) were mixed with 10 ml of a 2% (v/v) NaCl solution for 2 min and the hexane layer was removed. The hexane layer was then mixed with 4 ml of a 30% (v/v) sulfuric acid solution for 2 min, after which the hexane layer was removed. This layer was again mixed with 10 ml of a 2% (v/v) NaCl solution, after which the hexane layer was removed, dried with Na2SO4 and filtered through a florisil-copper (25% copper w/w) column. The hexane extract was adjusted to a volume of 25 ml. The extracted PCBs were analysed on a GC equipped with an electron capture detector (ECD). An HP-1 capillary column (25 m × 0.200 mm × 0.11 μm film thickness) was used for congener separation. The identification of 2,3,4,5-CB and its dechlorination products was primarily accomplished by comparing retention times with those of authentic standards (AccuStandard) on GC-ECD. Congeners 2,4-CB and 2,5-CB have very close retention times; therefore, identification was accomplished by analysing replicate GC samples and standards. Products were verified using a GC (HP 5890 Series II) equipped with an HP 5972 mass selective detector.
Characterization of sediment carbon
Sediment characterization was performed by the Research Analytical Laboratory in the Department of Soil, Water and Climate at the University of Minnesota. The total organic carbon was determined by dry combustion and subsequent measurement of CO2 by infrared (IR) spectrum absorption using a Skalar Primacs carbon furnace. The inorganic carbon was converted to CO2 with phosphoric acid and then measured by IR spectrum absorption. The detection limits were 0.10% and 0.15% for total organic carbon and inorganic carbon respectively.
DNA extraction
Cell lysis was accomplished by a combination of chemical and physical approaches. Briefly, 450 μl of phosphate buffer (100 mM, pH = 8) and 450 μl of lysis buffer [100 mM NaCl, 500 mM Tris (pH = 8), 10% (w/v) SDS] were added to the sediment pellets. Samples were incubated at 70°C for 90 min and then subjected to bead-beating to help ensure complete cell lysis. Genomic DNA was purified using a FastDNA kit for soil (Q-BIOgene; Vista, CA).
Polymerase chain reaction-denaturant gradient gel electrophoresis (PCR-DGGE)
Polymerase chain reaction was performed using a PTC 100 thermal cycler (MJ Research; Watertown, MA). Partial 16S rRNA genes were amplified from the extracted genomic DNA using primers 338F (5′-ACT CCT ACG GGA GGC AGC AG-3′) (Lane, 1991) and 518R (5′-ATT ACC GCG GCT GCT GCT GG-3′) (Muyzer et al., 1993) with a GC clamp attached to the forward primer (Muyzer et al., 1993). The final 50 μl of reaction mixture contained: 1× PCR buffer (Promega; Madison, WI), 175 μmol MgCl2, 4 nmol deoxynucleoside triphosphates, 2% bovine serum albumin, 25 pmol forward and reverse primers, 1.25 units of Taq polymerase (Promega) and ∼1 ng of template DNA. The PCR protocol included a 5 min initial denaturation at 94°C, 30 cycles of 92°C for 30 s, 55°C for 30 s and 72°C for 30 s, and a final extension for 10 min at 72°C.
Polymerase chain reaction products were loaded onto 8% (w/v) polyacrylamide gels with the denaturing gradient ranging from 30% to 55% [100% denaturant contains 7 M urea and 40% formamide in 0.5× Tris-acetate-EDTA (TAE) buffer]. Electrophoresis was performed on a D-Code apparatus (Bio-Rad; Hercules, CA) in 0.5× TAE buffer at 60°C, initially at 20 V for 20 min followed by 200 V for 270 min. Gels were stained with SYBR Green I (Molecular Probes; diluted 1:5000 in 0.5× TAE), visualized on a UV transilluminator and photographed with a digital CCD camera (BioChemi System; UVP; Upland, CA).
Prominent bands were excised and further purified by repeated PCR-DGGE until only a single band was detectable. A final PCR was performed using primers 338F (without the GC clamp) and 518R. These PCR products were purified using a Geneclean Kit (Q-Biogene) before nucleotide sequence determination. In several cases, vertically co-migrating bands were analysed from different gel lanes for quality assurance.
Polymerase chain reaction cloning
Nearly complete 16S rRNA genes were amplified by PCR using primers 27F (5′-AGA GTT TGA TCC TGG CTC AG-3′) (Edwards et al., 1989) and 1522R (5′-AAG GAG GTG ATC CAN CCR CA-3′) (Johnson, 1994). The composition of the PCR mixture was the same as that described above. The PCR protocol included a 5 min initial denaturation at 94°C, 35 cycles of 94°C for 1 min, 55°C for 1 min and 72°C for 2 min, and a final extension for 10 min at 72°C. Polymerase chain reaction amplicons were purified, ligated into the pGEM-T Easy cloning vector (Promega), and transformed into competent Escherichia coli DH5α cells. Plasmids were extracted by the alkaline lysis method (Sambrook et al., 1989). Plasmids were then analysed from unique inserts by PCR-DGGE as described previously (LaPara et al., 2000).
Nucleotide sequence analysis
Nucleotide sequences were determined at the Advanced Genetic Analysis Center (University of Minnesota) using an ABI 3100 Genetic Analyser (Applied Biosystems; Foster City, CA). Polymerase chain reaction-DGGE bands were sequenced using primers 338F and 518R. Plasmids were sequenced using primers 27F, 907R, 907F (Muyzer et al., 1995) and 1522R. Reported nucleotide sequences are the consensus of bi-directional sequence information and do not include the original PCR primer sequences.
Data analysis
A two-sided Student's t-test was performed at the 95% confidence interval to determine whether observed differences between experimental treatments were statistically significant. Principal component analysis was performed using StatistiXL ver. 1.4 (Kalamunda, Australia).
Sequences were compared with sequences in the Gen-Bank database (Benson et al., 2000) using the blastn program (Altschul et al., 1997) to search for their closest phylogenetic relatives. DNA sequences were screened for potential chimera using Chimera-Check from the Ribosomal Database Project II (Maidak et al., 2001). Putative chimeric sequences were manually split into three different components and re-submitted to GenBank to determine whether these segments were from the same phylogenetic group.
Nucleotide sequence accession number
Sequences were deposited in the GenBank database under Accession No. AY754828 to AY754856.
Acknowledgments
Funding for this work was provided by the Office of Naval Research (Grant N00014-99-1-0923), the National Institute of Environmental Health Sciences (Grant ES12810-01) and the Hudson River Foundation (Graduate Fellowship). T.Y. was financially supported by a Sommerfeld fellowship from the University of Minnesota and by a Hudson River Foundation Graduate Fellowship (Grant GF/03/02). The authors thank D. Dzombak for providing the Hudson River sediment and M. Simcik for technical assistance with GC-MS.
References
- Adrian L, Manz W, Szewzyk U, Görisch H. Physiological characterization of a bacterial consortium reductively dechlorinating 1,2,3- and 1,2,4-trichlorobenzene. Appl Environ Microbiol. 1998;64:496–503. doi: 10.1128/aem.64.2.496-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian L, Szewzyk U, Wecke J, Görlsch H. Bacterial dehalorespiration with chlorinated benzenes. Nature. 2000;408:580–583. doi: 10.1038/35046063. [DOI] [PubMed] [Google Scholar]
- Alder AC, Häggblom MM, Oppenheimer SR, Young LY. Reductive dechlorination of polychlorinated biphenyls in anaerobic sediments. Environ Sci Technol. 1993;27:530–538. [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped blast and psi-blast: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL. Genbank. Nucleic Acids Res. 2000;28:15–18. doi: 10.1093/nar/28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaut M, Müller V, Fiebig K, Gottschalk G. Sodium ions and an energized membrane required by Methanosarcina barkeri for the oxidation of methanol to the level of formaldehyde. J Bacteriol. 1985;164:95–101. doi: 10.1128/jb.164.1.95-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JF, Jr, Bedard DL, Brennan MJ, Carnahan JC, Feng H, Wagner RE. Polychlorinated biphenyl dechlorination in aquatic sediments. Science. 1987;236:709–712. doi: 10.1126/science.236.4802.709. [DOI] [PubMed] [Google Scholar]
- Cutter LA, Watts JEM, Sowers KR, May HD. Identification of a microorganism that links its growth to the reductive dechlorination of 2,3,5,6-chlorobiphenyl. Environ Microbiol. 2001;3:699–709. doi: 10.1046/j.1462-2920.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- van Dort HM, Smullen LA, May RJ, Bedard DL. Priming microbial meta-dechlorination of polychlorinated biphenyls that have persisted in Housatonic river sediments for decades. Environ Sci Technol. 1997;31:3300–3307. [Google Scholar]
- Edwards U, Rogall T, Blocker H, Emde M, Bottger EC. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16s ribosomal RNA. Nucleic Acids Res. 1989;17:7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell DE, Gossett JM, Zinder SH. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ Sci Technol. 1997;31:918–926. [Google Scholar]
- Fennell DE, Nijenhuis I, Wilson SF, Zinder SH, Häggblom MM. Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ Sci Technol. 2004;38:2075–2081. doi: 10.1021/es034989b. [DOI] [PubMed] [Google Scholar]
- He J, Ritalahti Kirsti M, Yang KL, Koenigsberg Stephen S, Löffler Frank E. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature. 2003;424:62–65. doi: 10.1038/nature01717. [DOI] [PubMed] [Google Scholar]
- Heise R, Müller V, Gottschalk G. Sodium dependence of acetate formation by the acetogenic bacterium Acetobacterium woodii. J Bacteriol. 1989;171:5473–5478. doi: 10.1128/jb.171.10.5473-5478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger C, Hahn D, Harmsen H, Ludwig W, Schumacher W, Tindall B, et al. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra- and trichloroethene in an anaerobic respiration. Arch Microbiol. 1998;169:313–321. doi: 10.1007/s002030050577. [DOI] [PubMed] [Google Scholar]
- Jaspers E, Overmann J. Ecological significance of microdiversity: identical 16s rRNA gene sequences can be found in bacteria with highly divergent genomes and ecophysiologies. Appl Environ Microbiol. 2004;70:4831–4839. doi: 10.1128/AEM.70.8.4831-4839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL. Similarity analysis of rRNAs. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR, editors. Methods for General and Molecular Bacteriology. Washington, DC, USA: American Society for Microbiology Press; 1994. pp. 683–700. [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing. In: Goodfellow M, editor. Nucleic Acid Techniques in Bacterial Systematics. New York, USA: John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- LaPara TM, Nakatsu CH, Pantea L, Alleman JE. Phylogenetic analysis of bacterial communities in mesophilic and thermophilic bioreactors treating pharmaceutical wastewater. Appl Environ Microbiol. 2000;66:3951–3959. doi: 10.1128/aem.66.9.3951-3959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan MT, Martinko JM, Parker J. Brock Biology of Microorganisms. Upper Saddle River, NJ, USA: Prentice-Hall; 2003. [Google Scholar]
- Magar VS, Stensel HD, Puhakka JA, Ferguson JF. Sequential anaerobic dechlorination of pentachlorophenol: competitive inhibition effects and a kinetic model. Environ Sci Technol. 1999;33:1604–1611. [Google Scholar]
- Maidak BL, Cole JR, Lilburn TG, Parker CT, Jr, Saxman PR, Farris RJ, et al. The RDP-II (ribosomal database project) Nucleic Acids Res. 2001;29:173–174. doi: 10.1093/nar/29.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maymó-Gatell X, Tandoi V, Gossett JM, Zinder SH. Characterization of an H2-utilizing enrichment culture that reductively dechlorinates tetrachloroethene to vinyl chloride and ethene in the absence of methanogenesis and acetogenesis. Appl Environ Microbiol. 1995;61:3928–3933. doi: 10.1128/aem.61.11.3928-3933.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maymó-Gatell X, Chien YT, Gossett JM, Zinder SH. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science. 1997;276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- Morris PJ, Mohn WW, Quensen JF, III, Boyd SA, Tiedje JM. General Electric corporate research and development program for the destruction of PCBs, ninth progress report. General Electric Corporate Research and Development; 1990. The Establishment and Characterization of an Anaerobic, Aroclor 1242-Dechlorinating Culture. [Google Scholar]
- Muyzer G, De Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G, Teske A, Wirsen CO, Jannasch HW. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16s rDNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- Nakatsu CH, Torsvik V, Øvreas L. Soil community analysis using DGGE of 16s rDNA polymerase chain reaction products. Soil Sci Soc Am J. 2000;64:1382–1388. [Google Scholar]
- Natarajan MR, Wu WM, Nye J, Wang H, Bhatnagar L, Jain MK. Dechlorination of polychlorinated biphenyl congeners by an anaerobic microbial consortium. Appl Microbiol Biotechnol. 1996;46:673–677. [Google Scholar]
- Nies L, Vogel TM. Effects of organic substrates on dechlorination of Aroclor 1242 in anaerobic sediments. Appl Environ Microbiol. 1990;56:2612–2617. doi: 10.1128/aem.56.9.2612-2617.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quensen JF, III, Tiedje JM, Boyd SA. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science. 1988;242:752–754. doi: 10.1126/science.242.4879.752. [DOI] [PubMed] [Google Scholar]
- Rhee GY, Sokol RC, Bethoney CM, Bush B. A long-term study of anaerobic dechlorination of PCB congeners by sediment microorganisms: pathways and mass balance. Environ Toxicol Chem. 1993a;12:1829–1834. [Google Scholar]
- Rhee GY, Sokol RC, Bethoney CM, Bush B. Dechlorination of polychlorinated biphenyls by Hudson River sediment organisms: specificity to the chlorination pattern of congeners. Environ Sci Technol. 1993b;27:1190–1192. [Google Scholar]
- Rysavy JP, Yan T, Novak PJ. Enrichment of anaerobic polychlorinated biphenyl dechlorinators from sediment with iron as a hydrogen source. Water Res. 2005;39:569–578. doi: 10.1016/j.watres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Safe S, Safe L, Mullin M. Polychlorinated biphenyls: environmental occurrence and analysis. Environ Toxin Series. 1987;1:1–13. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schlotelburg C, von Wintzingerode C, Hauck R, von Wintzingerode F, Hegemann W, Gobel UB. Microbial structure of an anaerobic bioreactor population that continuously dechlorinates 1,2-dichloropropane. FEMS Microbiol Ecol. 2002;39:229–237. doi: 10.1111/j.1574-6941.2002.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Shelton DR, Tiedje JM. General method for determining anaerobic biodegradation potential. Appl Environ Microbiol. 1984;47:850–857. doi: 10.1128/aem.47.4.850-857.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RC, Bethoney CM, Rhee GY. Effect of hydrogen on the pathway and products of PCB dechlorination. Chemosphere. 1994;29:1735–1742. doi: 10.1016/0045-6535(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Stevenson BS, Eichorst SA, Wertz JT, Schmidt TM, Breznak JA. New strategies for cultivation and detection of previously uncultured microbes. Appl Environ Microbiol. 2004;70:4748–4755. doi: 10.1128/AEM.70.8.4748-4755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Griffin BM, Ayala-del-Rio HL, Hashsham SA, Tiedje JM. Microbial dehalorespiration with 1,1,1-trichloroethane. Science. 2002;298:1023–1025. doi: 10.1126/science.1074675. [DOI] [PubMed] [Google Scholar]
- US Environmental Protection Agency. Hudson River PCBs Superfund Site: Proposed Plan. New York: United States Environmental Protection Agency; 2000. [Google Scholar]
- Wild A, Hermann R, Leisinger T. Isolation of an anaerobic bacterium which reductively dechlorinates tetrachloroethene and trichloroethene. Biodegradation. 1996;7:507–511. doi: 10.1007/BF00115297. [DOI] [PubMed] [Google Scholar]
- Williams WA. Microbial reductive dechlorination of trichlorobiphenyls in anaerobic sediment slurries. Environ Sci Technol. 1994;28:630–635. doi: 10.1021/es00053a015. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wiegel J. Two anaerobic polychlorinated biphenyl-dehalogenating enrichments that exhibit different para-dechlorination specificities. Appl Environ Microbiol. 1997;63:4826–4832. doi: 10.1128/aem.63.12.4826-4832.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Watts JEM, Sowers KR, May HD. Identification of a bacterium that specifically catalyzes the reductive dechlorination of polychlorinated biphenyls with doubly flanked chlorines. Appl Environ Microbiol. 2002;68:807–812. doi: 10.1128/AEM.68.2.807-812.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, LaPara TM, Novak PJ. The reductive dechlorination of 2,3,4,5-chlorobiphenyl in three different sediment cultures. Evidence for the involvement of phylogenetically similar Dehalococcoides-like bacterial populations. FEMS Microbiol Ecol. 2006;55:248–261. doi: 10.1111/j.1574-6941.2005.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, McCarty PL. Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ Sci Technol. 1998;32:3591–3597. [Google Scholar]
- Zwiernik MJ, Quensen JF, III, Boyd SA. FeSO4 amendments stimulate extensive anaerobic PCB dechlorination. Environ Sci Technol. 1998;32:3360–3365. [Google Scholar]
- Zwiernik MJ, Quensen JF, III, Boyd SA. Residual petroleum in sediments reduces the bioavailability and rate of reductive dechlorination of Aroclor 1242. Environ Sci Technol. 1999;33:3574–3578. [Google Scholar]


