Abstract
Variable use of pain scale anchors may influence recalled pain ratings, rating consistency, and agreement between actual rating change and ratings of pain relief. This investigation examined change in events that represent maximal pain scale anchors. Participants (N = 68, 50% women) provided events for maximal anchors of 0-100 pain scales and cold pressor pain was rated using self-selected event/s and an investigator-provided event. Then, participants were allowed to change their self-selected event/s. Then the revised event/s or original events were used to rate a second cold pressor trial. Forty-one percent of participants changed event/s and the new event/s was more likely to involve cold or heat, but the painfulness of events and the pain ratings of the second trial did not change. The cold pressor pain ratings were higher when rated based on self-selected event/s than the investigator- provided event for intensity (M = 80.13, SD = 19.30 & M = 60.81, SD = 27.45) and unpleasantness (M = 80.84, SD = 19.07 & M = 59.07, SD = 27.53), which could be due to the submaximal painfulness of the investigator-provided event. Therefore, the width of numerical scales is stable with maximal events regardless of the actual events.
Perspective:
This report identifies change in physical events that are used by participants to represent maximal pain scale anchors and suggests that the maximal nature of the events' painfulness is more important than variability in the actual events. We conclude that the numerical pain scales we used are understandable and stable, but we suggest that instructional sets for pain measurement may be improved by evaluation of the painfulness of events that respondents use to conceptualize maximal pain scale anchors.
Keywords: measurement, reliability, ratings, numerical scales, endpoint
Introduction
Maximal pain scales anchors need to be extreme to avoid ceiling effects without being so extreme that respondents cannot conceptualize or use them,18 but few investigations have actually compared different maximal pain scale anchors. An exception is a study that evaluated the clinical pain ratings of dental patients using visual analog scales with different maximal anchors.37 As the maximal anchor became more extreme, the pain ratings decreased and the anchor of “worst pain imaginable” produced a distribution of scores that most closely resembled normal. Thus, as expected, the maximal pain scale anchor affected pain ratings.
More research has been conducted on the meaning of maximal pain scale anchors. A few qualitative studies have reported that specific painful events are used to represent maximal pain anchors,25,45 but they type of painful events was found to vary between participants.25 For example, our group detected that men and women differed in the categories of events selected to represent the most intensely painful event imaginable for one's self with childbirth being selected most often by women and injuries being selected most often by men.35 Also some respondents may imagine an event that they think would be painful while other respondents may recall a painful event that they have personally experienced.45 Systematic differences between intact groups in the use of events to represent maximal pain scale anchors could influence pain ratings.
Variability within subjects in the events used to represent maximal pain scale anchors across time could also influence pain ratings. Discordance between actual change in pain ratings throughout treatment and retrospective ratings of pain relief could be due to such variability.5,8,16,17,26,30 In addition, inaccurate memories of chronic pain15 and fluctuating clinical pain ratings could be influenced by inconsistent use of events across time.24 One potential explanation for these findings is that change occurs in the way pain scales are used across time. However we did not locate any investigations of change in use of maximal pain scale anchors across time.
Certainly fluctuations occur in factors that may affect the interpretation of maximal pain scale anchors.3,45,25 For example, current activity interference3,45 and current pain levels4,14,16,19,20,38,41 have affected recalled pain ratings. In addition, previous pain responses have been associated with subsequent pain responses.9,10,27,31,34 However, postoperative pain patients' visual analog scale ratings of “mild,” “moderate,” and “severe” pain did not differ from the ratings of healthy controls39 and the pain responses of women have not been found to change before and after childbirth.13,44 Thus, additional research is needed on the effects of pain experiences on interpretation of maximal pain scale anchors.
The present investigation examined the occurrence of change in events used to represent maximal pain scale anchors and the influence of these events on ratings of pain from a controlled stimulus – cold pressor. We hypothesized that participants could conceptualize specific maximal pain scale anchors by generating events that are maximally rated (i.e., 100 on a 0-100 numerical scale). We also expected that providing participants with a specific event would subsequently alter pain ratings. Finally, we anticipated that the type of events used to represent the maximal pain scale anchors would be influenced by the type of pain experienced during the study. In summary, we believed the results of this study would establish that participants could conceptualize specific maximal pain scale anchors and they would do so in a variable manner that would subsequently affect pain ratings.
Materials and Methods
Participants
Participants were 68 university students (50% women) with an average age of 22 years (SD = 3.36). Seventy-four percent (73.5%) of participants were Caucasian, 5.9% were African American, and 8.8% were Hispanic. Participants were excluded if they had any chronic pain disorders or contraindications for (e.g., Raynauds disease) or previous experience with the cold pressor task. In addition, participants were restricted from having smoked a cigarette in the previous 3 hours, consumed caffeine in the previous 3 hours, or consumed a pain reliever in the previous 12 hours.
Procedures
After providing written informed consent to the protocol, which was approved by the University of Florida's Institutional Review Board, participants provided demographic and health history information. Then the investigator read the following instructional set for the dimensions of pain:
There are two aspects of pain that we are interested in measuring: the intensity, which is how strong the pain feels, and the unpleasantness, which is how disturbing the pain is for you. The distinction between these two aspects of pain might be made clearer if you think of listening to a sound, such as a radio. As the volume of the sound increases, I can ask you how loud it sounds, or how unpleasant it is to you. The intensity of the pain is like loudness; the unpleasantness of the pain depends not only on intensity, but also on other factors that may affect you. Although some pain sensations may be equally intense and unpleasant, we would like you to judge these two aspects of your pain independently. We will be using a numerical rating system - for intensity, 0 = no pain and 100 = most intense pain sensation imaginable and - for pain unpleasantness, 0 = not at all unpleasant and 100 = most unpleasant imaginable.
Next participants described and rated (a) medical conditions or injuries that they believed represented the maximal anchors for the numerical pain scales and (b) the “most painful physical event, medical condition, or injury” that they had ever experienced. (The painfulness of the events were rated using the previously described 0-100 pain scales.22,23) Then participants completed the Situational Pain Questionnaire (SPQ).6 The SPQ assesses the amount of expected pain in response to a number of different events, of which 15 events are considered painful (signals) and 15 events are considered non-painful (blanks). Examples of painful events included “you get a tooth drilled without a pain killer”, “you fall from a 6-foot ladder and hit the cement floor”, and “you spill some boiling water on your hand.” Examples of non-painful events included “you get a mosquito bite”, “you get out of breath trying to catch a bus”, and “your teeth are examined by a dentist in a check-up.” Items were rated using a scale of 1 “not noticeable” to 10 “worst possible pain.” The SPQ was administered to determine the participants' ratings of specific events that would be subsequently substituted by investigators for the participants' own previously selected events.
After these tasks, the participants completed the first of two cold pressor tasks. During both trials, the participant immersed his/her hand up to the wrist into a tank (Thermo Electron Corporation; Waltham, MA) of circulating cold water at 2.0° C (± 0.2° C). Participants were instructed not to move the immersed hand during the task and to stop the task when they could no longer endure it. Three minutes was used as the maximum allowable duration of immersion.
At termination of the first cold pressor trial, participants rated their pain intensity and pain unpleasantness using 0-100 numerical pain scales with their self-selected events to represent the maximal anchors. Then the participants were alternatively asked to rate the pain intensity and pain unpleasantness at tolerance using alternative numerical scales with an investigator-provided alternative event from the SPQ – “falling off a 6-foot ladder and hitting the cement floor” – written in as the maximal anchors.
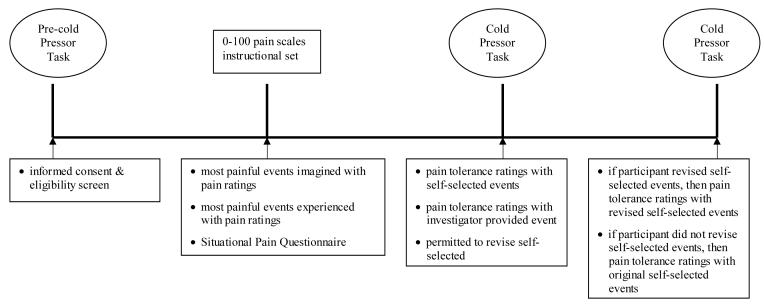
After at least 20 minutes for return to baseline cardiovascular status and hand warming, the participants were asked in an open-ended manner if they wanted to change their previously self-selected events used to represent the maximal anchors. Then they were informed that a second trial of the cold pressor task would be completed. If participants selected new events after the first cold pressor trial, they were instructed to use those new events for rating the second cold pressor trial. If participants did not change their previous self-selected events after the first cold pressor trial, then they were instructed to use their original self-selected events for rating the second cold pressor trial. See Figure 1 for a diagram of the design.
Figure 1.
Diagram of the research design.
Data Analyses
Participants' abilities to conceptualize the specific maximal pain scale anchors (i.e., our first research question) were examined by descriptive statistics of the maximal nature of participants' original self-selected events. In addition, within the group of participants who changed their self-selected events after the cold pressor, the painfulness of their original and revised self-selected events was compared by conducting paired-samples t-test. Then, in order to evaluate the effects of variability in events on pain ratings (i.e., our second research question), the differences in cold pressor tolerance ratings when using self-selected and investigator-provided events were analyzed with paired sample t-tests. Also mixed model analyses of variance (ANOVAs) with cold pressure tolerance trial (i.e., first or second) as a repeated measure and group (i.e., changed self-selected events or did not change self-selected events) were run. Finally, the influence of previous pain experiences on the type of pain events selected by participants (i.e., our third research question) was determined by comparing the proportions of temperature-related events for maximal anchors before and after the cold pressor task.
Statistical significance was set at an alpha level of .05. Eta (η2) squared values of .01, .06, and .14 corresponded to small, medium, and large effect sizes, respectively and Cohen's d values of .20, .50, and .80 corresponded to small, medium, and large effect sizes, respectively.7 Analyses were conducted using SPSS 13.0 (SPSS, Inc.).
Results
Common events selected to represent maximal pain scale anchors included such things as “third degree burns all over,” “surgery without anesthesia,” and “breaking most of the bones in your body in a car accident.” The events were different for the maximal anchors of pain intensity and pain unpleasantness 68.1% of the time. The events were largely rated as maximal for pain intensity (M = 97.62, SD = 4.60) and pain unpleasantness (M = 95.47, SD = 6.51). When given the opportunity to revise their self-selected events after the first cold pressor trial, 41.2% of participants changed the event/s. For example, participants switched to events such as “freezing to death” and “falling into a frozen lake.” (See Table 1.) However, within the group of participants who changed their self-selected events after the first cold pressor task, the painfulness of the original and revised self-selected events were not significantly different (ds = .10 & .34). (See Table 2.) Thus, it appears that variability in the actual events used to represent the anchors may occur across time, but the painfulness of the events themselves remains maximal.
Table 1.
Frequencies and percentages of change in self-selected events to represent maximal pain scale anchors.
| Frequency | Percentage | |
|---|---|---|
| No change in event/s | 40 | 58.8 |
| New events for both pain dimensions | 11 | 16.2 |
| New event only for pain intensity | 8 | 11.8 |
| New event only for pain unpleasantness | 9 | 13.2 |
Table 2.
Descriptive statistics for pain ratings of previous events, self-selected events to represent maximal pain scale anchors, and cold pressor pain tolerance.
| Pain Intensity | Pain Unpleasantness | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Previously most painful physical event | 75.76 | 15.63 | 78.59 | 18.71 |
| Ratings of self-selected events | ||||
| Pre-cold pressor task | 97.62 | 4.60 | 95.47 | 6.51 |
| Post-cold pressor task* | 97.65 | 4.16 | 95.59 | 5.61 |
| Ratings of first cold pressor tolerance trial | ||||
| Self-selected event/s | 80.13 | 19.30 | 80.84 | 19.07 |
| Investigator-provided event | 60.81 | 27.45 | 59.07 | 27.53 |
Note. These ratings are only from the participants who changed their self-selected events (n = 22).
For the sample as a whole, no significant trial by group interactions were detected for actual ratings of cold pressor pain intensity (F 1, 63 = 0.02, p = .90, η 2 < .01) or pain unpleasantness (F 1, 63 = 1.31, p = .26, η 2 = .02) and no group effects were observed for pain intensity (F 1, 63 = 1.65, p = .20, η 2 = .03) or pain unpleasantness (F 1, 63 = 0.59, p = .45, η 2 = .01). Therefore, it does not appear that changing self-selected events affects pain ratings. However, ratings of both cold pressor pain intensity (F 1, 63 = 12.97, p < .01, η 2 = .17) and pain unpleasantness (F 1, 63 = 10.96, p < .01, η 2 = .15) decreased from the first to the second cold pressor tolerance trial as if habituation occurred. (See Table 3.)
Table 3.
Descriptive statistics for cold pressor pain tolerance ratings of the first and second trials using self-selected events by groups who did change their self-selected event/s (n = 28) and who did not change their self-selected event/s (n = 40).
| Pain Intensity | Pain Unpleasantness | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| First cold pressor tolerance trial | ||||
| Changed self-selected event/s | 83.54 | 17.82 | 81.46 | 21.14 |
| No change in self-selected event/s | 77.18 | 20.08 | 79.90 | 17.70 |
| Second cold pressor tolerance trial | ||||
| Changed self-selected event/s | 73.33 | 25.28 | 75.00 | 25.04 |
| No change in self-selected event/s | 67.58 | 22.22 | 68.16 | 24.14 |
In contrast, there was a difference in ratings of the first cold pressor pain tolerance when using self-selected events than when using investigator-provided event, which was rated as 7.38 (SD = 1.50) on the SPQ's 1-10 scale. The first cold pressor pain tolerance ratings were higher for intensity (t67 = 5.06, p < .01, d = .83) and unpleasantness (t67 = 5.67, p < .01, d = .93) when rated based on self-selected event/s than the investigator-provided event. (See Table 2.) However, the participants' ratings from the first cold pressor tolerance trial using self-selected and investigator-provided events were not significantly correlated (rs = .11 − .13, ps > .05) so the participants' ratings did not rank order similarly when using the self-selected and investigator-provided events.
Regardless, the proportion of revised (i.e., post-cold pressor task) self-selected events involving temperature was higher (53.6%) than the original self-selected events (32.4%) and this difference in proportions approached statistical significance (z = −2.00, p = .05). However, participants' recalled ratings of their previously most painful events were not significantly correlated to cold pressor tolerance ratings (rs = .04− (− .17), ps > .05). Thus, previous pain experiences may influence events used to represent maximal pain anchors, but previous pain experiences did not appear to influence actual pain ratings within this study beyond the occurrence of habituation to the cold pressor.
Discussion
In the present study, we found that participants were able to conceptualize our maximal pain scale anchors of “most intense pain sensation imaginable” and “most unpleasant imaginable” by generating specific events that were maximally rated. Importantly, no difference was detected in the painfulness of the originally selected events and revised events after the first cold pressor trial. Thus, despite fluctuations in the types of self-selected events, the maximal pain scale anchors were appropriately extreme for respondents to conceptualize them.
The cold pressor pain ratings decreased when participants used an investigator-provided event to represent maximal anchors, which might support that using a submaximally painful event resulted in narrowing the width of the scales as if the scales were elastic. Bartoshuk and colleagues2 have suggested that the width of 0-100 pain scales may be variable based on a study of attendees of an international conference about sensation. In their study, the participants rated the “strongest pain of any kind experienced” using a 0-100 scale with a maximal anchor of “strongest imaginable sensation of any kind.” The participants also rated the “brightest light ever seen” among other types of sensations. When the pain ratings were weighted by the light ratings, men's ratings of experienced pain were 81% as intense as women's ratings. This finding was interpreted as evidence of the numerical scale's elasticity and potential systematic differences in the use of pain scales, but there may have been sex differences in the “brightest light” anchor as well, which is a critical test not reported in the study.
However, before concluding that the present study supports elasticity of numerical pain scales, it is important to consider additional results of this investigation. For example, the finding that the cold pressor pain tolerance ratings did not differentially change from one trial to the next trial between those who changed their self-selected events and those who did not change their self-selected events does not support elasticity of numerical scales. This result is probably because the painfulness of the revised self-selected events remained equally extreme.
Actual pain ratings may only be affected when the new events used to represent maximal scale anchors are not rated maximally. For example, consider generating a rating for pain from being burned over 65% of one's body using a rating scale with the maximal anchor being conceptualized as the most painful event previously experienced. If you had previously experienced an event that was more painful than this burn, then you could probably rate the recent burn pain. However, if you had no previous experience with an event that was more painful than the burn, then your rating of the burn pain would be “off the scale.” Indeed, the low correlations between the first cold pressor tolerance trial pain ratings using self-selected and investigator-provided events suggests that decreasing the extreme nature of the event used to represent the maximal pain scale anchors may have decreased the participants ability to reliably use the scale. Furthermore, the use of a non-extreme event to represent the maximal pain scale anchor may not be ecologically valid because, when participants chose to change their self-selected events, there was no significant change in the events' painfulness; the ratings of the original and revised events remained maximal. Thus, the actual event used to represent the maximal anchor may matter less than the painfulness of the event itself and the width of the pain scales is likely to be stable as long as the events used to represent the maximal pain scale anchor remain maximally painful.
It is still noteworthy that almost half of the participants (41.2%) changed the event/s used to represent the maximal pain scale anchors from pre- to post-cold pressor task and these revised self-selected events were more likely to involve temperature than the original self-selected events. Such change in events may have been related to the participants' lack of previous experience with the cold pressor task, but the change in events did not affect pain ratings. In addition, participants' recalled ratings of their most painful event experienced were not related to cold pressor pain tolerance times or ratings. Thus, it seems possible that change in events representing maximal pain scale anchors may occur within longitudinal examinations and previous pain experiences may influence the type of events used to represent maximal pain anchors, but our data suggests that previous pain experience in healthy samples does not influence actual pain ratings beyond habituation.
In summary, this investigation is the first report of change in self-selected events representing maximal pain scale anchors and decreased pain ratings when a non-extreme event was used to present maximal pain scale anchors. However, change in self-selected events did not cause significant change in pain ratings and the use of a non-extreme event from the investigator may have negatively impacted the reliability and ecological validity of the pain scales. Therefore, this study supports the stability of the numerical pain scales we administered as long as the events used to represent maximal pain scale anchors are appropriately extreme. We propose that it may be beneficial for instructional sets to routinely ensure the maximal nature of events used by respondents to represent maximal pain scale anchors. For example, at each meeting, respondents may be asked with an open question to provide physical event/s that they believe could represent the pain scales' maximal anchor (e.g., “most intense pain imaginable”) and to rate the painfulness of the provided event using the same pain scales in order to ensure respondents' understanding of a truly maximal anchor. Such instructional sets may reduce the discordance between actual change in pain ratings throughout treatment and retrospective ratings of pain relief that is observed in the clinic.5,8,16,17,26,30
Certainly, our results may have been influenced in a reactive manner by having participants describe and rate the painfulness of events they used to represent maximal pain scale anchors. Studies have detected that pain ratings are different between groups that self-monitor and groups that use other coping strategies (e.g., distraction),12,21,29,36,42 but examinations of momentary pain assessments have observed stable average pain ratings over time as if such self-monitoring was not reactive1,33,40 and several studies have not detected differences in recalled pain between groups that rated pain throughout stimulus administration and groups that did not rate pain.28,32,43 Regardless, additional studies comparing instructional sets that request self-monitoring and do not request self-monitoring are needed. Furthermore, investigations of the occurrence and effects of change in the events representing pain scales' maximal anchors across time in divergent samples such as older adults and clinical samples are warranted.
Acknowledgements
Support for this research was provided from Grant 5F32 (AR08623-01) and 1KO1 (AR050146-01A1) to Dr. Erin A Dannecker from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aaron LA, Turner JA, Mancl L, Brister H, Sawchuk CN. Electronic diary assessment of pain-related variables: is reactivity a problem? J Pain. 2005;6:107–115. doi: 10.1016/j.jpain.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Bartoshuk LM, Duffy VB, Chapo AK, Fast K, Yiee JH, Hoffman HJ, Ko WC, Snyder DJ. From psychophysics to the clinic: missteps and advances. Food Qual Preference. 2004;15:617–632. [Google Scholar]
- 3.Broderick JE, Stone AA, Calvanese P, Schwartz JE. Recalled pain ratings: a complex and poorly defined task. J Pain. 2006;7:142–149. doi: 10.1016/j.jpain.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Bryant RA. Memory for pain and affect in chronic pain patients. Pain. 1993;54:347–351. doi: 10.1016/0304-3959(93)90036-O. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of visual analogue scale. Pain. 1983;16:87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 6.Clark WC, Yang JC. Applications of sensory decision theory to problems in laboratory and clinical pain. In: Melzack R, editor. Pain: measurement and assessment. Raven Press; New York: 1983. pp. 15–25. [Google Scholar]
- 7.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 8.Dalton JA, Toomey TC, Workman MR. Pain relief for cancer patients. Cancer Nurs. 1988;11:322–328. doi: 10.1097/00002820-198812000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Dannenbring D, Stevens M, House A. Predictors of childbirth pain and maternal satisfaction. J Behav Med. 1997;20:127–143. doi: 10.1023/a:1025526610524. [DOI] [PubMed] [Google Scholar]
- 10.Davenport-Slack B, Boylan LH. Psychological correlates of childbirth pain. Psychosom Med. 1974;36:215–223. doi: 10.1097/00006842-197405000-00004. [DOI] [PubMed] [Google Scholar]
- 11.de C. Williams AC, Davies HTO, Chadury Y. Simple pain rating scales side complex idiosyncratic meanings. Pain. 2000;85:457–463. doi: 10.1016/S0304-3959(99)00299-7. [DOI] [PubMed] [Google Scholar]
- 12.Dowman R. Distraction produces an increase in pain-evoked anterior cingulate activity. Psychophysiology. 2004;41:613–624. doi: 10.1111/1469-8986.00186.x. [DOI] [PubMed] [Google Scholar]
- 13.Dunbar AH, Price DD, Newton RA. An assessment of pain responses to thermal stimuli during stages of pregnancy. Pain. 1988;35:265–269. doi: 10.1016/0304-3959(88)90136-4. [DOI] [PubMed] [Google Scholar]
- 14.Eich E, Reeves JL, Jaeger B, Graff-Radford SB. Memory for Pain: relation between past and present pain intensity. Pain. 1985;22:375–379. doi: 10.1016/0304-3959(85)90007-7. [DOI] [PubMed] [Google Scholar]
- 15.Erskine A, Morley S, Pearce S. Memory for pain: a review. Pain. 1990;41:255–265. doi: 10.1016/0304-3959(90)90002-U. [DOI] [PubMed] [Google Scholar]
- 16.Feine JS, Lavigne GJ, Dao TTT, Morin C, Lund JP. Memories for chronic pain and perceptions of relief. Pain. 1998;77:137–141. doi: 10.1016/S0304-3959(98)00089-X. [DOI] [PubMed] [Google Scholar]
- 17.Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient's view of change as a clinical outcome measure. JAMA. 1999;282:1157–1162. doi: 10.1001/jama.282.12.1157. [DOI] [PubMed] [Google Scholar]
- 18.Freyd M. The graphic rating scale. J Educ Psychol. 1923;43:83–102. [Google Scholar]
- 19.Haas M, Nyiendo J, Aickin M. One-year trend in pain and disability relief recall in acute and chronic ambulatory low back pain patients. Pain. 2002;95:83–91. doi: 10.1016/s0304-3959(01)00377-3. [DOI] [PubMed] [Google Scholar]
- 20.Holroyd KA, France JL, Nash JM, Hursey KG. Pain state as artifact in the psychological assessment of recurrent headache sufferers. Pain. 1993;53:229–235. doi: 10.1016/0304-3959(93)90085-4. [DOI] [PubMed] [Google Scholar]
- 21.James JE, Hardardottir D. Influence of attention focus and trait anxiety on tolerance of acute pain. Br J Health Psychol. 2002;7:149–162. doi: 10.1348/135910702169411. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi: 10.1016/0304-3959(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 23.Jensen MP, Karoly P, O'Riordan EF, Bland F, Burns RS. The subjective experience of acute pain: an assessment of the utility of 10 indices. Clin J Pain. 1989;5:153–159. doi: 10.1097/00002508-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55:195–203. doi: 10.1016/0304-3959(93)90148-I. [DOI] [PubMed] [Google Scholar]
- 25.Manning EL, Kee WG. Assigning meaning to commonly used numerical pain rating scales. J Pain. 2001;2:8. [Google Scholar]
- 26.Matera D, Morelli M, La Grua M, Sassu B, Santagostino G, Prioreschi G. Memory distortion during acute and chronic pain recalling. Minerva Anestesiol. 2003;69:775–783. [PubMed] [Google Scholar]
- 27.Melzack R, Taenzer P, Feldman P, Kinck RA. Labour is still painful after prepared childbirth training. Can Med Assoc J. 1981;125:357–363. [PMC free article] [PubMed] [Google Scholar]
- 28.Mikail R, VanDeursen J, von Baeyer CL. Rating pain or rating serenity: effects on cold pressor pain tolerance. Can J Behav Sci. 1986;18:126–132. [Google Scholar]
- 29.Nouwen A, Cloutier C, Kappas A, Warbrick T, Sheffield D. Effects of focusing and distraction on cold pressor-induced pain in chronic back pain patients and control subjects. J Pain. 2006;7:62–71. doi: 10.1016/j.jpain.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Ohnhaus EE, Adler R. Methodological problem in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379–384. doi: 10.1016/0304-3959(75)90075-5. [DOI] [PubMed] [Google Scholar]
- 31.Papageorgiou AC, Croft PR, Thomas E, Ferry S, Jaysin MI, Silman AJ. Influence of previous pain experience on the episode incidence of low back pain: results from the South Manchester Back Pain Study. Pain. 1996;66:181–185. doi: 10.1016/0304-3959(96)03022-9. [DOI] [PubMed] [Google Scholar]
- 32.Peckerman A, Saab PG, Llabre MM, Hurwitz BE, McCabe PM, Schneiderman N. Cardiovascular and perceptual effects of reporting pain during the foot and forehead cold pressor tests. Int J Behav Med. 1998;5:106–117. doi: 10.1207/s15327558ijbm0502_2. [DOI] [PubMed] [Google Scholar]
- 33.Peters ML, Sorbi MJ, Kruise DA, Kerssens JJ, Verhaak PFM, Bensing JM. Electronic diary assessment of pain, disability and psychological adaptation in patients differing in duration of pain. Pain. 2000;84:181–192. doi: 10.1016/s0304-3959(99)00206-7. [DOI] [PubMed] [Google Scholar]
- 34.Poyhia R, Da Costa D, Fitzcharles MA. Previous pain experience in women with fibromyalgia and inflammatory arthritis and nonpainful controls. J Rheumatol. 2001;28:1888–1891. [PubMed] [Google Scholar]
- 35.Robinson ME, George SZ, Dannecker EA, Jump RL, Hirsh AT, Gagnon C, Brown JL. Sex differences in pain anchors revisited: further investigation of “most intense” and common pain events. Eur J Pain. 2004;8:299–305. doi: 10.1016/j.ejpain.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Roelofs J, Peters ML, van der Zijden M, Vlaeyen JW. Does fear of pain moderate the effects of sensory focusing and distraction on cold pressor pain in pain-free individuals? J Pain. 2004;5:250–256. doi: 10.1016/j.jpain.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Seymour RA, Simpson JM, Charlton JE, Philips ME. An evaluation of length and end-phrase of visual analogue scales in dental pain. Pain. 1985;21:177–185. doi: 10.1016/0304-3959(85)90287-8. [DOI] [PubMed] [Google Scholar]
- 38.Smith WB, Safer MA. Effects of present pain level on recall of chronic pain and medication use. Pain. 1993;55:355–361. doi: 10.1016/0304-3959(93)90011-D. [DOI] [PubMed] [Google Scholar]
- 39.Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G. Studies with different types of visual analog scales for measurement of pain. Clin Pharmacol Ther. 1983;34:234–239. doi: 10.1038/clpt.1983.159. [DOI] [PubMed] [Google Scholar]
- 40.Stone AA, Broderick JE, Schwartz JE, Shiffman S, Litcher-Kelly L, Calvanese P. Intensive momentary reporting of pain with an electronic diary: reactivity, compliance, and patient satisfaction. Pain. 2003;104:343–351. doi: 10.1016/s0304-3959(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 41.Tasmuth T, Estlanderb A, Kalso E. Effect of present pain and mood on the memory of past postoperative pain in women treated surgically for breast cancer. Pain. 1996;68:343–347. doi: 10.1016/s0304-3959(96)03219-8. [DOI] [PubMed] [Google Scholar]
- 42.Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, Matthews PM. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Baeyer CL. Reactive effects of measurement of pain. Clin J Pain. 1994;10:18–21. doi: 10.1097/00002508-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Whipple B, Josimovich JB, Komisaruk BR. Sensory thresholds during the antepartum, intrapartum and postpartum periods. Int J Nurs Stud. 1990;27:213–221. doi: 10.1016/0020-7489(90)90036-i. [DOI] [PubMed] [Google Scholar]
- 45.Williams A C de C, Davies HTO, Chadury Y. Simple pain rating scales side complex idiosyncratic meanings. Pain. 2000;85:457–463. doi: 10.1016/S0304-3959(99)00299-7. [DOI] [PubMed] [Google Scholar]