Abstract
Inflamed tissue is often characterized by the production of NO and superoxide. These radicals react at diffusion-limited rates to form the powerful oxidant peroxynitrite (PN). When protonated, PN decomposes into either nitrate or reactive intermediates capable of mediating tissue damage by oxidation of protein, lipid, and nucleic acid. We recently have identified porphyrin derivatives capable of catalyzing an increase in nitrate formation with a concomitant decrease in the HO·-like and NO2·-like reactivity of PN. Here, we present evidence for the efficacy of these PN decomposition catalysts both in vitro and in vivo. Cells in culture were protected from exogenously added PN by the catalyst 5,10,15,20-tetrakis(2,4,6-trimethyl-3,5-disulfonatophenyl)porphyrinato iron (III), whereas free iron and the structurally related compound without iron 5,10,15,20-tetrakis(2,4,6-trimethyl-3,5-disulfonatophenyl)porphyrin did not protect. Cytoprotection correlated well with a reduction in the nitrotyrosine content of released cytosolic proteins, a biochemical marker for PN formation. Carrageenan-induced paw edema is a model of acute inflammation in which PN may play a major role. When tested in this system, both 5,10,15,20-tetrakis(2,4,6-trimethyl-3,5-disulfonatophenyl)porphyrinato iron (III) and 5,10,15,20-tetrakis(N-methyl-4′-pyridyl)porphyrinato iron (III) caused a dose-dependent reduction in swelling and lactate dehydrogenase release as well as a detectable shift to nitrate formation in paw tissue. In addition, the catalysts did not elevate mean arterial pressure, suggesting a lack of interaction with NO. Taken together, our data provide compelling evidence supporting the therapeutic value of manipulating PN pharmacologically. Thus, PN decomposition catalysts may represent a unique class of anti-inflammatory agents.
Peroxynitrite [ONOO−(PN)] is a highly reactive oxidant produced by the combination of NO and superoxide anion at rates approaching the diffusion limit (1–4). A compelling body of evidence has begun to emerge that suggests that PN forms in significant concentrations in vivo. In addition, it has now been demonstrated clearly that ONOO− is capable of oxidizing lipid membranes (5, 6) and sulfhydryl moieties (7), as well as hydroxylating and nitrating aromatics (8, 9). Furthermore, there are indications that ONOO− may contribute to cell death and tissue injury in a number of human diseases, including arthritis (10), sepsis (11), inflammatory bowel disease (12), and stroke (13). Endothelial cells, neutrophils, and macrophages have been shown to be capable of producing both NO and superoxide. These findings are consistent with the hypothesis that PN may be ultimately responsible for the pathophysiology previously attributed to NO alone. Thus, the ability to intercept and decompose PN may represent a novel and critical point of therapeutic intervention in diseases associated with the overproduction of NO and superoxide.
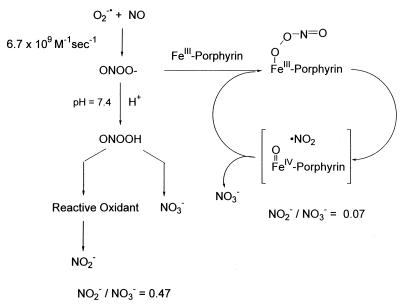
PN is known to undergo acid-catalyzed decomposition by two distinct pathways (14, 15). Isomerization to nitrate is the major decay route, but a significant portion of the decomposition produces a species with reactivity akin to that of a hydroxyl radical (16, 17). We recently have reported that certain water-soluble iron (III) porphyrins are highly active ONOO− decomposition catalysts and that they function by catalyzing the isomerization of ONOO− almost exclusively to nitrate (18). Catalysis is proposed to proceed via an oxo-Fe(IV) intermediate generated from the metal-promoted cleavage of the O–O bond. Subsequent recombination with NO2 regenerates the Fe(III) state and produces nitrate. These catalysts thus dramatically increase the rate of ONOO− isomerization, preempting the formation of oxidizing radical species and generating the harmless nitrate anion. This mode of catalysis manifests itself by dramatic shifts in the resulting nitrite-to-nitrate ratio when compared with the proton-catalyzed decomposition (Fig. 1).
Figure 1.
Schematic representation of proposed chemical mechanism for PN decomposition catalysts. Porphyrin denotes TMPS or TMPyP.
The objective of this work was to demonstrate the in vitro and in vivo efficacies of these catalysts, thus supporting the notion that ONOO− may indeed be an important pathophysiological agent in a variety of diseases associated with NO production. Accordingly, we report here the results of studies using two Fe porphyrin catalysts, 5,10,15,20-tetrakis(2,4,6-trimethyl-3,3-disulfonatophenyl)porphyrinato iron (III) (FeTMPS) and 5,10,15,20-tetrakis(N-methyl-4′-pyridyl)porphyrinato iron (III) (FeTMPyP), which we previously have shown to be effective ONOO− decomposition catalysts.
MATERIALS AND METHODS
Cellular Assay.
RAW 264.7 cells were plated onto a 96-well plate at ≈2 × 105 cells per well in Earle’s MEM (GIBCO/BRL) without phenol red and containing 10% fetal calf serum. Before the addition of PN to each well, the plate was washed free of serum-containing medium with Dulbecco’s PBS. Cell injury was initiated by the addition of PN to each well of cells containing 200 μl of Dulbecco’s PBS with or without compound. After 15 min, cells were washed once with growth medium, and then 200 μl/well 10% Alamar blue (in growth medium) (Alamar, Sacramento, CA) was added for 1–2 h at 37°C and 5% CO2. At the end of the experiment, 100 μl was removed from each well and the fluorescence was measured using a Pandex fluorescent plate reader (Idexx Laboratories, Westbrook, MA) at an excitation of 545 nm and an emission wavelength of 575 nm (1% gain setting). The mitochondrial generation of the fluorescent metabolite of Alamar blue was linear over this period and correlated well with cellular viability and number (data not shown). Viability is expressed as the percentage of the untreated control after subtraction of the background of nonmetabolized dye.
Western Analysis.
Western blot analyses were performed as described with the following modifications (19). A 15-μl aliquot was removed from each well of cells that had been treated with 200 μM PN in the presence or absence of a catalyst. If PN-mediated lysis occurred, then cellular proteins containing nitrotyrosine were released into the medium and detected by the Western analysis. Rabbit polyclonal antisera to nitrotyrosine was incubated with blots at a dilution of 1:500 overnight at 4°C, followed by exposure to reagents using chemiluminescent detection (19). All labeling was eliminated when the primary antibody was incubated with 10 mM nitrotyrosine.
Carrageenan Paw Edema.
Male Sprague–Dawley rats (175–200 g, Harlan–Sprague–Dawley) were housed and cared for under the guidelines of the Institutional Animal Care and Use Committee. Edema was produced by an intraplantar injection of carrageenan (0.1 ml of a 1% suspension in 0.85% saline) into the right hind paw. Paw volume was measured using a plethysmometer (Ugo Basile, Varese, Italy) immediately before the injection of carrageenan and for 10 h thereafter at hourly intervals. Edema was expressed as the increase in paw volume (milliliters) after carrageenan injection relative to the preinjection value for each animal. Unless specified, drugs were administered i.v. in a volume of 2.5 ml/kg either 1 h before or 1 h after carrageenan injection.
Measurement of Nitrite, Nitrate, and Lactate Dehydrogenase (LDH) from Carrageenan-Injected Rat Paws.
At specified time points after the intraplantar injection of carrageenan, rats were killed, and each paw was cut at the level of the calcaneus bone. Paws were centrifuged gently at 250 × g for 20 min to recover a sample of the edematous fluid. The volume of fluid that was recovered from each paw was measured. To remove the hemoglobin arising from red blood cell contamination and hemolysis, paw exudates were filtered through a 10,000-MW cutoff filter (Millipore). Nitrite and nitrate (NO2− and NO3−, respectively) concentrations were measured using the diaminonaphthalene assay as described (20). To determine total nitrite and nitrate in the paw exudates, nitrate in the paw fluid samples (10 μl) was converted to nitrite by the incubation with nitrate reductase (14 mU) and the reduced form of nicotinamide adenine dinucleotide phosphate (1 nmol) for 10 min at room temperature. Total nitrite in the exudate was measured in the absence of nitrate reductase. Sodium nitrite was used as the standard. The reaction was terminated by the dilution with water and the addition of the diaminonaphthalene reagent. NO2− was then determined fluorometrically (20). Total (T) NO2− and NO3− present in the entire edematous fluid of each paw was calculated as follows: T = pmol nitrite and nitrate in the sample × paw fluid volume (ml)/sample volume (ml). Results are expressed as nmol of NO2− and NO3−. The ratio of NO2− to NO3− for each sample in the absence and presence of the PN decomposition catalysts was calculated. Levels of LDH in the paw exudates (5 μl of a 1:10-fold dilution of each sample in saline) were used as an indicator of cellular toxicity and death and were measured spectrophotometrically (at an absorbance of 340 nm) 6 h after carrageenan administration by using a Sigma LDH assay kit.
In Vivo Measurement of Hemodynamic Changes.
Male Sprague–Dawley rats (175–200 g) were purchased from Charles River Breeding Laboratories and used throughout these studies. All animal protocols were approved by the Institutional Animal Care and Use Committee. Animals were anesthetized with methoxyflurane before surgical preparation. Polyethylene catheters (PE50) were inserted into a femoral artery and vein. The animals were placed into individual restraining cages and allowed to regain consciousness. Mean arterial blood pressure and heart rate were measured continuously via the femoral artery by using a pressure transducer (type 041-500-503, Cobe Laboratories, Lakewood, CO) connected to a Grass Model 7E polygraph (Grass Instruments, Quincy, MA). After a 30-min period of equilibration, rats received a bolus i.v. injection of the highest dose of PN decomposition catalysts (30 mg/kg) or an equivalent volume of saline (1.6 ml/kg). Mean arterial pressure and heart rate were recorded at 5-min intervals for a total period of 6 h on a computerized data acquisition system.
Materials.
Male Sprague–Dawley rats were purchased from Harlan–Sprague–Dawley or Charles River Breeding Laboratories and were housed and cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee and in accordance with National Institutes of Health guidelines on laboratory animal welfare. 2,3-Diaminonaphthalene was purchased from Aldrich. All other chemicals and reagents were obtained from Sigma.
Statistical Analysis.
Results are expressed as mean ± SEM for (n) rats. The results were analyzed by Student’s unpaired t test to determine the significant differences between means or by a two-way ANOVA followed by a least significant procedure. A P value of <0.05 was considered to be statistically significant.
RESULTS
In Vitro Efficacy of PN Decomposition Catalysts.
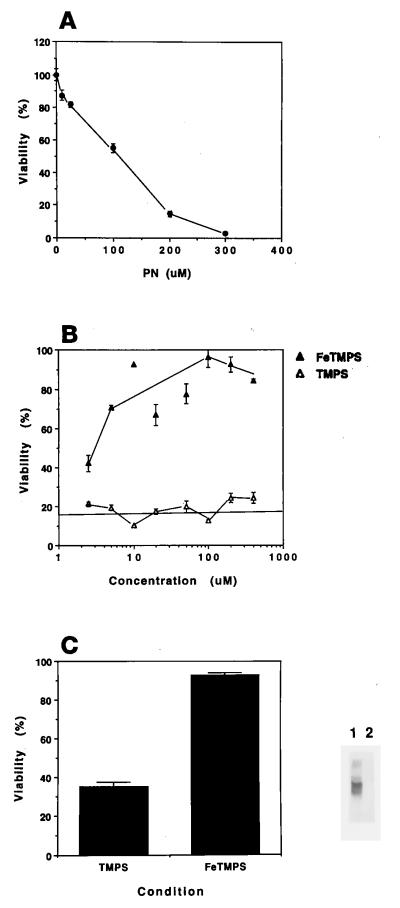
To assess the capacity of PN decomposition catalysts to protect cells against PN insult, the size of the bolus needed to produce maximal injury was determined first. A typical concentration–response curve titrating the effect of PN on cellular viability assessed by measuring the mitochondrial activity of RAW 264.7 cells is shown in Fig. 2A. Cellular damage resulting from the exogenous addition of synthetic PN was prevented in a concentration-dependent manner by the presence of either 5,10,15,20-tetrakis(N-methyl-4′-pyridyl)porphyrinato iron (III) (FeTMPyP) (data not shown) or its analog 5,10,15,20-tetrakis(2,4,6-trimethyl-3,5-disulfonatophenyl)porphyrinato iron (III) (FeTMPS) (Fig. 2B). Treatment with a PN pulse, either alone (not shown) or in the presence of the inactive analog TMPS, resulted in an intense nitrotyrosine staining pattern for released cellular constituents. In contrast, no labeled proteins from RAW cells were detected with a 200- μM PN bolus in the presence of the active catalyst FeTMPS (100 μM), demonstrating that an increase in culture viability correlated with a reduction in the formation of nitrotyrosine moieties. When the free ligand TMPS (Fig. 2B), FeCl3, or the inactive zinc compound ZnTMPyP was administered, no protection was observed (data not shown). In addition, neither the vehicle nor the previously reacted PN produced any detectable damage.
Figure 2.
PN-mediated cell death is prevented by PN decomposition catalysts. Cells were treated as described in Materials and Methods. (A) Titration of cellular injury with increasing pulses of PN. (B) FeTMPS protected RAW 264.7 cells from a 200-μM PN challenge (closed triangles). In contrast, the addition of TMPS did not protect the cells (open triangles). The solid line indicates the percentage of viability of PN-treated cells in the absence of compound. (C) Western blot for nitrotyrosine content of released cellular proteins after PN (200 μM) treatment in the presence of 100 μM FeTMPS (lane 2) or TMPS (lane 1). Cellular viability is depicted in the bar graph. Each point represents the mean of four individual wells ± SEM. Data are representative of at least three independent experiments.
Important to note, when the addition of catalyst followed the challenge with PN, no protection was observed. The EC50 for the catalysts was ≈1/50 of the final concentration of PN used, a result consistent with their primary mechanism of action as catalysts. These data contrast sharply with the characteristics of a free radical scavenger such as ascorbate (data not shown), which protects fully at a 2:1 molar ratio of ascorbate to PN (400:200 μM). In comparison, 10 μM FeTMPS showed essentially complete cellular protection.
Antiinflammatory Effects of PN Decomposition Catalysts.
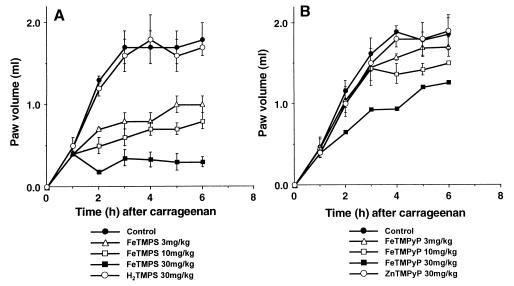
The intraplantar injection of carrageenan in rats resulted in a time-dependent increase in paw volume that was maximal after 3–6 h (Fig. 3). The active PN catalysts FeTMPS or FeTMPyP (3–30 mg/kg), administered i.v. 1 h after carrageenan (n = 6), inhibited paw edema at all subsequent time points with FeTMPyP being much less potent (Fig. 3 A and B).
Figure 3.
Effects of active (FeTMPS, FeTMPyP) and inactive (H2TMPS, ZnTMPyP) PN decomposition catalysts on carrageenan-induced paw edema. The injection of carrageenan caused a time-dependent increase in paw volume that reached a maximum within 3–6 h. When given 1 h after carrageenan, FeTMPS or FeTMPyP (3–30 mg/kg) inhibited carrageenan-induced paw edema. H2TMPS or ZnTMPyP at 30 mg/kg lacked anti-inflammatory activity. Results are expressed as the increase in paw volume (milliliters). Each point is the mean ± SEM for n = 6.
In contrast, the inactive PN decomposition catalysts H2TMPS or ZnTMPyP (30 mg/kg, n = 6) failed to inhibit carrageenan-induced paw edema (Fig. 3 A and B). The amount of iron present in the highest dose (30 mg/kg) of the active PN decomposition catalyst FeTMPS was 5 mg/kg. To test whether free Fe may have anti-inflammatory activity, FeCl3 was tested. An i.v. bolus injection of 5 mg/kg FeCl3 failed to inhibit carrageenan-induced paw edema (n = 6; data not shown).
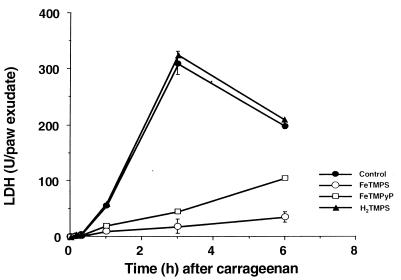
The anti-inflammatory effects observed with FeTMPS or FeTMPyP (30 mg/kg) were associated with a marked time-dependent reduction in the release of LDH (Fig. 4). This cytoprotective effect was not seen with the inactive PN decomposition catalyst TMPS (Fig. 4). Similarly, the inactive PN decomposition catalysts ZnTMPyP (30 mg/kg, n = 6) or FeCL3 (5 mg/kg, n = 6) failed to inhibit LDH release (at 6 h after carrageenan administration, the levels of LDH in paw exudates were 200 ± 7 unit/paw for carrageenan alone and 210 ± 5 and 200 ± 18 unit/paw for carrageenan plus ZnTMPyP and carrageenan plus FeCL3, respectively, n = 6).
Figure 4.
Effects of active (FeTMPS, FeTMPyP) and inactive (H2TMPS) PN decomposition catalysts on the release of LDH in paw exudates. The injection of carrageenan caused a time-dependent increase in LDH release in paw exudate. When given 1 h after carrageenan at 30 mg/kg, FeTMPS (open circle) or FeTMPyP (open square) inhibited carrageenan-induced LDH release at all time points (closed square), whereas H2TMPS (closed triangle) was ineffective. Each point is the mean ± SEM for n = 6.
Mechanism of Antiinflammatory Action of PN Decomposition Catalysts: Effects on Nitrite/Nitrate and Blood Pressure.
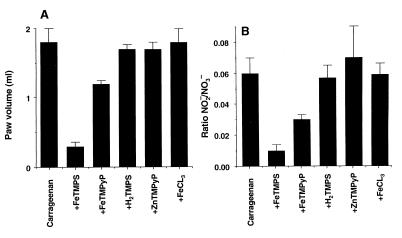
As reported earlier (18), ion chromatographic evaluations of NO3− and NO2− ratios after decomposition of PN allowed us to determine that active but not inactive PN decomposition catalysts function as PN isomerases shifting the NO2−-to-NO3− ratio toward the production of NO3−. To determine whether the anti-inflammatory effect of the PN catalysts in vivo also was associated with a shift to greater levels of NO3−, both nitrite and nitrate were measured in paw exudates. The inhibition of paw edema by FeTMPS and FeTMPyP (Fig. 5A) at 6 h after carrageenan administration correlated with a shift from nitrite to nitrate as evidenced by a reduction in the nitrite-to-nitrate ratio in the presence of the active catalyst FeTMPS (Fig. 5B). In contrast, the inactive compounds H2TMPS, ZnTMPyP, and FeCL3 decreased neither swelling nor the ratio of nitrite-to-nitrate (Fig. 5 A and B).
Figure 5.
Effects of active and inactive PN decomposition catalysts on carrageenan-induced edema (A) and nitrite-to-nitrate ratio (B). Inhibition of paw edema by FeTMPS or FeTMPyP (30 mg/kg) observed 6 h after carrageenan administration was associated with a shift in the ratio of NO2−-to-NO3− toward NO3− (B). H2TMPS or ZnTMPyP (30 mg/kg) or FeCl3 (5 mg/kg) had no anti-inflammatory effects (A) and did not change the NO2−-to-NO3− ratio (B). Each point is the mean ± SEM for n = 6.
At the highest dose tested, FeTMPS and FeTMPyP did not cause significant changes in mean arterial pressure. The mean arterial pressure was 120 ± 3 mmHg (1 mmHg = 133 Pa) for control rats, 118 ± 4 mmHg for rats treated with FeTMPS and 123 ± 5 mmHg for rats treated with FeTMPyP (n = 4). These values remained at the same level for the entire observation period (6 h after i.v injection of PN decomposition catalyst or equivalent volume of saline).
DISCUSSION
The role of NO in physiological and pathophysiological processes is well documented (21, 22). NO originally was thought to be the primary mediator of cellular and tissue injury. However, there is now mounting evidence that supports a role for PN-mediated damage at inflammatory sites (1, 23, 24). In fact, NO may at times be cytoprotective, acting through mechanisms such as free radical chain termination or by increasing blood flow after an ischemic insult (6, 23, 25, 26). In contrast, PN, the kinetically favored product of the reaction of superoxide with NO, is a powerful oxidant whose cellular targets include lipids, nucleic acids, cytosolic thiol pools, and key metabolic enzymes such as cytochrome oxidase of the mitochondrial electron transport chain (2, 23, 27–31). Because of its high reactivity under physiological conditions, direct measurement of PN in vivo is not achieved easily. However, the nitration of tyrosyl residues has been shown to be a stable biochemical marker for the production of PN both in vitro and in vivo (32, 33).
Pathological changes produced by the formation of PN should be amenable to pharmacological intervention at either the reactant (NO, superoxide) or the product (PN) stage of PN chemistry. In fact, use of superoxide dismutase and/or NO synthase inhibition has been effective in attenuating both acute and chronic inflammatory responses in animal models of human disease (23, 34, 35). Recently, a novel class of anti-inflammatory agents, PN decomposition catalysts, has been identified (18). The proposed mechanism for the PN catalyst is that of a PN isomerase that increases nitrate formation, the predominant pathway for PN decomposition (18, 27, 28), while decreasing tyrosine nitration and hydroxyl radical-like peroxidation.
Here, we present evidence for the efficacy of PN decomposition catalysts in vitro and in vivo. At the cellular level, the catalyst FeTMPS was cytoprotective against exogenously added PN with an EC50 of ≈3.5 μM, a concentration almost 1/60 of the PN added. This protection correlated well with a reduction in the nitrotyrosine content of cellular proteins released after PN insult, an observation consistent with the proposed mechanism of action for these compounds. Moreover, we report that neither TMPS, an inactive structurally related compound, nor free Fe was cytoprotective.
Despite increasing evidence suggesting a pathophysiological role for PN in a variety of diseases, questions still remain regarding its role in cytotoxicity (36). Our data provide support for the direct involvement of PN in the inflammatory response in vivo by direct pharmacological intervention with a unique catalyst specifically designed to decompose PN to nitrate. Carrageenan-induced paw edema, a widely accepted rodent model of acute inflammation, is amenable to pharmacological manipulation with superoxide dismutase and inhibitors of NO synthase (35) and thus represents an ideal model to study PN decomposition catalysts in vivo. Immunostaining for nitrotyrosine has been detected in inflamed paw tissue, thus implicating PN as a potential mediator of tissue damage (35). Administration of the PN decomposition catalysts FeTMPS and FeTMPyP produced a marked, dose-dependent reduction of paw swelling in carrageenan-treated animals. This protective effect was specific for active catalyst. Neither the inactive compounds (TMPS, TMPyP, or ZnTMPyP) nor free Fe was effective. Tissue damage, assessed by release of lactate dehydrogenase from injured and dying cells, was reduced significantly in animals treated with active catalyst as compared with untreated animals or those treated with inactive compounds. In addition, the paw exudate was analyzed for nitrite and nitrate. A detectable shift to nitrate formation occurred in animals treated with catalyst. This result is consistent with the proposed chemical mechanism for these compounds that have been shown to shift significantly the NO2−-to-NO3− ratio to NO3− during catalysis. Important to note, there was no increase in mean arterial blood pressure, a well documented vascular response to inhibition of NO production by endothelial NO synthase (3, 4, 6), after administration of the highest doses of catalyst used in our studies. From these observations, we conclude that the chemical action of the catalyst favors interaction with PN over NO, supporting the proposed mechanism of action at sites of inflammation and the lack of elevation in blood pressure.
A better understanding of the molecular mechanisms underlying the inflammatory response is essential to the discovery of therapeutics capable of modifying the progression of human disease. The involvement of PN-mediated tissue injury in human diseases such as Alzheimer’s disease, multiple sclerosis, atherosclerosis and amyotrophic lateral sclerosis is suggested by the localization of nitrotyrosine-like immunoreactivity at sites of tissue damage (37–43). However, the overall contribution of PN in producing the pathological changes in disease states remains to be elucidated. Whether PN is a key player in the pathogenesis of these diseases or part of a panoply of inflammatory mediators remains to be determined. Our studies demonstrate that pharmacological intervention at the level of PN may represent a useful therapeutic strategy for disease modification.
Acknowledgments
We thank Linda Branson for the blood pressure measurements and Dr. Pamela T. Manning for her critical evaluation of the manuscript.
ABBREVIATIONS
- PN or ONOO−
peroxynitrite
- FeTMPS
[5,10,15,20-tetrakis(2,4,6-trimethyl-3, 5-disulfonatophenyl)porphyrinato iron III]
- FeTMPyP
[5,10,15,20-tetrakis(N-methyl-4′-pyridyl)porphyrinato iron III]
- LDH
lactic dehydrogenase
References
- 1.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman J S, Crow J P. Biochem Soc Transact. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 3.Ischiropoulos H, Zhu L, Beckman J S. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 4.Huie R E, Padmaja S. Free Radical Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 5.Radi R, Beckman J S, Bush K M, Freeman B A. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 6.Rubbo H, Radi R, Trujillo M, Teller R, Kalyanaraman B, Barnes S, Kirk M, Freeman B A. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 7.Radi R, Beckman J S, Bush K M, Freeman B A. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 8.van der Vliet A, O’Neill C A, Halliwell B, Cross C E, Kaur H. FEBS Lett. 1994;339:89–92. doi: 10.1016/0014-5793(94)80391-9. [DOI] [PubMed] [Google Scholar]
- 9.van der Vliet A, Eiserich J P, O’Neill C A, Halliwell B, Cross C E. Arch Biochem Biophys. 1995;319:341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]
- 10.Kaur H, Halliwell B. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 11.Szabo C, Salzman A L, Ischiropoulos H. FEBS Lett. 1995;363:235–238. doi: 10.1016/0014-5793(95)00322-z. [DOI] [PubMed] [Google Scholar]
- 12.Rachmilewitz D, Stamler J S, Karmeli F, Mullins M E, Singel D J, Loscoalzo J, Xavier R J, Podolsky D K. Gastroenterology. 1993;105:1681–1688. doi: 10.1016/0016-5085(93)91063-n. [DOI] [PubMed] [Google Scholar]
- 13.Oury T D, Piantadosi C L, Crapo J D. J Biol Chem. 1993;268:15394–15398. [PubMed] [Google Scholar]
- 14.Keith W G, Powell R E. J Chem Soc Dalton Trans. 1969;1969:90. [Google Scholar]
- 15.Hughs M N, Nicklin H G. J Chem Soc Dalton Trans. 1968;1968:450–451. [Google Scholar]
- 16.Tsai J-H M, Harrison J G, Martin J C, Hamilton T P, van der Woerd M, Jablonsky M J, Beckman J S. J Am Chem Soc. 1994;116:4115–4116. [Google Scholar]
- 17.Crow J P, Spruell C, Chen J, Gunn C, Ischiropoulos H, Tsai M, Smith C D, Radi R, Koppenol W H, Beckman J S. Free Radical Biol Med. 1994;16:331–338. doi: 10.1016/0891-5849(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 18.Stern M K, Jensen M P, Kramer K. J Am Chem Soc. 1996;118:8735–8736. [Google Scholar]
- 19.Misko T P, Trotter J L, Cross A H. J Neuroimmunol. 1995;61:195–204. doi: 10.1016/0165-5728(95)00091-f. [DOI] [PubMed] [Google Scholar]
- 20.Misko T P, Schilling R J, Salvemini D, Moore W M, Currie M G. Anal Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- 21.Moncada S, Higgs A. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 22.Nathan C. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 23.Dawson V L. Clin Exp Pharmacol Physiol. 1995;22:305–308. doi: 10.1111/j.1440-1681.1995.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 24.Shigenaga M K, Lee H H, Blount B C, Christen S, Shigeno E T, Yip H, Ames B N. Proc Natl Acad Sci USA. 1997;94:3211–3216. doi: 10.1073/pnas.94.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wink D A, Ingeborg H, Krishna M C, DeGraff W, Gamson J, Mitchell J B. Proc Natl Acad Sci USA. 1993;90:9813–17. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipton S A, Choi Y-B, Pan Z-H, Lei S Z, Chen H-S, Sucher N J, Loscalzo J, Singel D J, Stamler J S. Nature (London) 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 27.Beckman J S, Koppenol W H. Am J Physiol. 1996;40:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 28.Pryor W A, Squadrito G L. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 29.Radi R, Rodriguez M, Castro L, Telleri R. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 30.Castro L, Rodriguez M, Radi R. J Biol Chem. 1994;269:29409–29415. [PubMed] [Google Scholar]
- 31.Hausladen A, Fridovich I. J Biol Chem. 1994;269:29405–29408. [PubMed] [Google Scholar]
- 32.Beckman J S, Chen J, Ischiropoulos H, Crow J P. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 33.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin J C, Smith C D, Beckman J S. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 34.Chan P H, Epstein C J, Li Y, Huang T T, Carlson E, Kinouchi H, Yang G, Kamii H, Mikawa S, Kondo T, Copin J-C, Chen S F, Chan T, Gafni J, Gobbel G, Reola E. J Neurotrama. 1995;12:815–824. doi: 10.1089/neu.1995.12.815. [DOI] [PubMed] [Google Scholar]
- 35.Salvemini D, Wang Z-Q, Wyatt P S, Bourdon D M, Marino M H, Manning P T, Currie M G. Br J Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuto J M, Ignarro L J. Acc Chem Res. 1997;30:149–152. [Google Scholar]
- 37.Good P F, Werner P, Hsu A, Olanow C W, Perl D P. Am J Pathol. 1996;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- 38.Smith M A, Richey Harris P L, Sayre L M, Beckman J S, Perry G. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hooper D C, Ohnishi S H, Kean R, Numagami Y, Dietzschold B, Koprowski H. Proc Natl Acad Sci USA. 1995;92:5312–5316. doi: 10.1073/pnas.92.12.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckman J S, Carson M, Smith C D, Koppenol W H. Nature (London) 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 41.Beckman J S, Ye Y Z, Anderson P, Chen J, Accavetti M A, Tarpey M M, White C R. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 42.Beckman J S, Chen J, Crow J P, Ye Y Z. Prog Brain Res. 1994;103:371–380. doi: 10.1016/s0079-6123(08)61151-6. [DOI] [PubMed] [Google Scholar]
- 43.Abe K, Pan L-H, Watanabe M, Kato T, Itoyama Y. Neurosci Lett. 1995;199:152–154. doi: 10.1016/0304-3940(95)12039-7. [DOI] [PubMed] [Google Scholar]