Most of the experiments described in genetics laboratory manuals present only one-time or short-term experiments for high school biology or college genetics courses. In order to deepen conceptual understanding of important genetics concepts, students need to be exposed to a real research environment. Furthermore, the inquiry-based, research-driven laboratory teaching plan is the most effective in nurturing budding scientists through the stimulation of their interest in biology and scientific reasoning. Therefore, the development of simple lab teaching materials and methods in which students have the opportunity to perform genetic analysis within a semester is in much demand. Based on several semesters of teaching an introductory genetics course for undergraduate students and of a Master level genetics course for high school teachers, the author believes that the use of the glabrous1 (gl1) mutants and the homozygous transgenic plants (CA1) in Arabidopsis thaliana is a simple and effective way of teaching genetics laboratory techniques and analytical skills at high school and undergraduate levels. The introduction of Arabidopsis in the laboratory course not only provides an alternative model organism to/or as a complement for Drosophila, but also enables the students to become familiar with modern genetic research in plants. Most importantly, this inquiry-based, research-driven teaching method allows the students to integrate botany and statistic analysis into genetics, and promotes students’ intellectual growth by fostering the interactions between individual students and between students and teachers.
Arabidopsis has quickly emerged as the most widely-used model organism in plant genetic and developmental studies since the 1980s (Meinke et al., 1998). It has several advantages over pea and maize, two organisms frequently mentioned in high school or college genetics courses. Arabidopsis is a tiny weed (Figure 1A) that is a member of the mustard family (Cruciferae or Brassicaceae). It is a self-pollinating species that can also be cross-fertilized. Each plant has about 5,000 seeds, serving as genetic stocks that are easy to manage. It has a very short life cycle of about six to eight weeks for most of the commonly used ecotypes in the laboratory, such as Landsberg erecta (Ler), Columbia (Col) and Wassilewskija (Ws). Arabidopsis has a small genome (125 megabases, five pairs of chromosomes), a size similar to Drosophila but 20–30 folds smaller than pea and maize. The Arabidopsis genome has been almost completely sequenced (115.4 megabases) in 2000, with about 25,000 protein-encoding genes (Arabidopsis Genome Initiative, 2000). Furthermore, genetic transformation in Arabidopsis is rather easy and has become routine.
Figure 1.
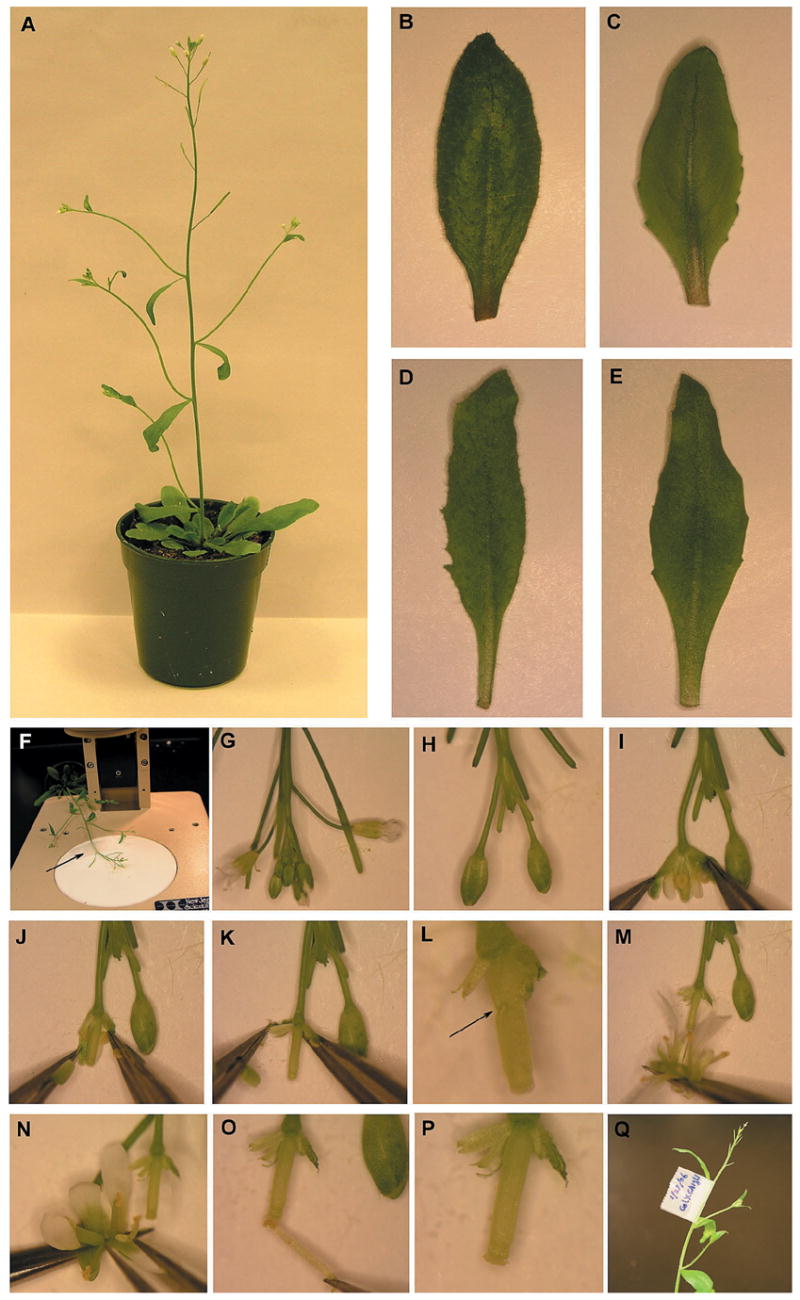
Leaf morphology phenotypes of the gl1, CA1 and CA1; gl1 double mutant plants, and the illustration of the crossing procedure. (A) A 5-week-old Col wild-type plant. (B–E) Developing leaves for various genotypes. (B) A normal leaf from Col. (C) A leaf from gl1 showing the absence of trichomes on the leaf surface but with a similar shape as Col. (D) A serrated leaf from CA1 that has a narrow shape but contains trichomes. (E) A leaf from the CA1; gl1plant that exhibits phenotypes of both gl1 and CA1. (F) Fastening the plant on the microscope base by the tape (indicated by the arrow). (G–H) Removing siliques, flowers, and young flower buds. (I–K) Removing anthers. (L) A damaged pistil (indicated by the arrow). (M–O) Pollinating the stigma. (P) A pollinated stigma. (Q) Labeling the cross.
The gl1 mutant (Oppenheimer et al., 1991) and the CA1 transgenic plants that express the constitutively active form of ROP2 small GTPase transgene (designated CA-rop2; Li et al., 2001) have different phenotypes in leaf morphology. Importantly, their leaf morphology phenotypes are easy for the students to score, although CA1 has other phenotypes (Li et al., 2001). In contrast to the hairy (with trichomes) leaves with regular shapes of the wild-type (WT), Col (Figure 1B), gl1 has no trichomes (hairless, or smooth) on the leaf surface (Figure 1C) but still has trichomes on the leaf edge, and the CA1 leaves are serrated and narrower and have longer petioles (Figure 1D). GL1 is located on Chromosome 3 and encodes a member of the large family of MYB transcription factors (Oppenheimer et al., 1991). CA-rop2 was constructed by placing the G12V mutant form of ROP2 small GTPase gene under the control of the constitutively active CaMV35S promoter and this cassette was then transformed into the Arabidopsis Col plants (Li et al., 2001). The G12V mutation locks ROP2 GTPase in the GTP bound form, thus causing it to become constitutively active and consequently activate the downstream signaling events.
In this semester-long laboratory exercise, Col, the gl1-1 allele (abbreviated as gl1 in this report) and the CA1 transgenic plant are used for pair-wise crossing and subsequent genetic analysis. The resulting F1 generations would give indications regarding the dominance and recessiveness of the respective phenotypes. The F2 generations are then used to confirm the inheritance patterns and determine the number of genes involved using Chi-square (χ2)analysis. The cross of gl1 and CA1 results in the double mutants, CA1 gl1 (Figure 1E). Interestingly, the F2 segregation from the cross of gl1 and CA1 indicates that the GL1 locus and the CA-rop2 transgene integration site are linked. Therefore, these materials provide the students an excellent opportunity to learn how to infer the linkage and calculate the genetic distance between these two loci.
Materials
Arabidopsis plants
Col
gl1
CA1
*Note: Upon request, the seeds of Col, gl1, CA1, and the double mutant CA1 gl1 are freely distributed for teaching purposes.
Equipment
low magnification stereo microscopes
a pair of extra fine-pointed tweezers (Item No. 14099, World Precision Instruments, Inc., FL) for each group
scissors
tapes
-
labels
growth chambers or greenhouse (16 hours light and 8 hours dark).
Other Materials
70% ethanol
growing trays and pots
soilless media
Experimental Procedures
The following are the essential steps for performing this experiment (with timeframes in the parentheses).
-
Preparation of plant materials (five to six weeks before the lab)
Seeds are sowed on the soilless media, cold-treated (4°C) for two days and transferred to the long-day growth chamber or greenhouse for germination and seedling development. In two weeks, individual seedlings are transplanted to individual pots, one seedling per pot. After an additional two weeks, plants begin to bolt and in another one to two weeks, plants flower and are ready for crossing (Figure 1A).
-
Phenotypic analysis (Week 1)
Students are asked to characterize the phenotypes of Col, gl1 and CA1 plants. It is important to let students familiarize themselves with the plant morphology so that they will be able to determine the phenotypes of the F1 and F2 plants. A sample table is provided to students for their phenotypic characterization (Table 1). Note that a slight (two to four days) flowering delay can also be noticed by some students and this can be recorded in the “Others” column.
-
Crossing Arabidopsis plants (Week 1)
This step is crucial and, based on the past experience, only about 50–60% of students succeed in more than two crosses. In this case, students can be scheduled for another time of crossing. In addition, the instructor should also make several crosses so that the students have some F1 seeds to proceed with in case their crossing fails. For more details in the crossing procedure, refer to the protocol described elsewhere (Koornneef et al., 1998).
Select the parents for the cross. Two students work in cooporative or lab groups. Some can use pollens from Col or CA1 plants (male) to fertilize gl1 stigmas (female). Some may use pollens of CA1 to fertilize Col or gl1.
Fasten gently with transparent tape the female plants on the microscope base (Figure 1F). Remove all siliques, young flower buds and flowers with petals until two to four large buds (white petals not yet visible) are left for each inflorescence branch (Figures 1G & 1H).
Hold one side of the bud with one tweezer, and carefully open the bud with the other tweezer under the low magnification of a stereo microscope. Remove all six anthers one by one without damaging the pistil (Figures 1I–K). Make sure that no anthers are left to prevent any self fertilization (Figure 1K). Removing petals and sepals is not a problem, but damaging the pistil (Figure 1L) will result in the failure of growth and thus the failure of obtaining any seeds.
Take a fresh fully-open flower from the male parent by holding the base of the flower. This will allow students to observe the viable pollens (yellow powders) on the anther. Then “brush” the anthers of this flower onto the stigma of the female plant that has been emasculated as above (Figure 1M) until the pollen is clearly visible on the stigma. Students may also take the individual anther with the tweezer to pollinate the stigma (Figures 1N–O). Make sure to transfer an overdose of pollen to prevent any cross-pollination by open flowers from the same or surrounding plants (Figure 1P). When making several crosses of different genotypes, make sure to sterilize the tweezers with 70% ethanol.
Mark the flower buds with transparent tape attached to the lower part of the stem (Figure 1Q). Clearly label the type of crosses (female and male plants) and date on the tape. Also label the pots for each group.
-
Harvesting F1 seeds (Week 5) and phenotypically characterizing F1 plants (Weeks 8–9)
Two to three days later, the pistil should have developed as a young, elongating silique. This indicates that the cross has been successful. If the crossing fails, students can attempt another crossing. In about three to four weeks, the cross-fertilized flower buds develop from green to yellow to brown siliques and they are ready to harvest. Germinate the F1 seeds and grow the F1 plants on the soilless media as described above. In about three weeks, F1 seedlings are ready for phenotypic characterization.
-
Harvesting F2 seeds (Week 12) and segregation analysis of F2 plants (Weeks 14–15)
About three to four more weeks later, F2 seeds are ready for harvest and germination as described above. When to characterize the F2 phenotypes depends on the time remained for the semester. If less than two weeks are left, use the very young seedlings at the two-true-leaf stage (about 10 days after cold-treatment). In this case, compare the cotyledon shapes (which will be narrower than Col) for the CA1-related phenotype (Li et al., 2001) and the presence of trichomes on the emerging young true leaves for the gl1-related phenotype.
Table 1.
Phenotypic characterization of various genotypes.
| GENOTYPES | PHENOTYPES:
|
||
|---|---|---|---|
| Leaf Shape | Leaf Surface | Others | |
| Col | |||
| gl1 | |||
| CA1 | |||
| CA1gl1 | |||
Data Collection & Analysis
In order to better record and organize the data, a sample data sheet (Table 2) is provided. This will also help students to analyze their data. Several representative F2 data examples from the student experiments are given in Table 3 for demonstration purposes of data analysis. For the first and the second crosses, Col X CA1 and gl1 X Col, the χ2-test shows that the observed phenotypic segregations are consistent with the expected ratio of 3:1. This confirms that the CA-rop2 transgene is dominant and likely has only one insertion site and that gl1 is a monogenic, recessive mutation.
Table 2.
Data of the genetic of analysis GL1 and CA1 in leaf morphogenesis.
| Type of crosses (♀ X ♂) | No. Flower Buds Crossed | No. Siliques Obtained | No. F1 Seeds Obtained | F1Seedlings Phenotypes | F2 Seedlings Phenotypes |
|---|---|---|---|---|---|
| Col X CA1 | |||||
Table 3.
The χ2-test analysis of the sample crossing data. The χ2-value is defined as SUMMATION of [(Observed-Expected)2/Expected], and the critical χ2-value for α=0.05 is 3.841 (df=1) or 7.815 (df=3).
| Types of Crosses(♀ X ♂) | F1 Seedlings Phenotypes | F2 Seedlings Phenotypes | Expected Ratios | χ2-test Results(α=0.05) |
|---|---|---|---|---|
| Col X CA1 | All had CA1 leaf shape. | 53 (CA1)
22 (Col) |
3:1 | χ2-value=0.751
p-value >0.05 (df=1) |
| gl1 X Col | All had Col leaf surface. | 69 (Col)
26 (gl1) |
3:1 | χ2-value=0.284
p-value >0.05 (df=1) |
| Col X gl1 | All had Col leaf surface. | 72 (Col)
0 (gl1) |
3:1 | χ2-value=24.0
p-value <0.05 (df=1) |
| gl1 X CA1 | All had Col leaf surface and CA1 leaf shape. | 85 (CA1)
15 (Col) 11 (CA1 gl1) 17 (gl1) |
9:3:3:1 | χ2-value=22.889
p-value <0.05 (df=3) |
The third cross, Col X gl1, is an example showing the importance of picking the recessive mutant as the female parent. From the F1 result, it is not clear whether the cross has been successful because the progenies from self-fertilization are phenotypically undistinguishable from the F1 that is resulted from the successful cross, gl1 X Col. However, the F2 data suggest that this cross is very likely due to the self-cross. Although failing to get positive data, the students can still discuss their potential problems, such as that the flower has already self-fertilized before the crossing, or that the pollen coming from an old flower might have a viability problem and thus the flower bud might be self-pollinated from the flowers on the same plant, or even that the anther might be accidentally picked from another Col plant.
The data from the fourth cross, gl1 X CA1, are very interesting. The χ2-test for the 9:3:3:1 ratio does not support that gl1 and the CA-rop2 integration site are independently assorted. Instead, these two loci are likely linked. The potential linkage has not been revealed because the integration site for the CA-rop2 transgene is unknown. Based on the percentage of homozygous gl1 on the F2 progenies (17/128=13.3%), which is larger than 1/16=6.25% if the two loci are unlinked, it can be inferred that the percentage of the parental gamete genotype WT (no transgene); gl1 would be 36.4%, and accordingly the other parental gamete genotype CA-rop2; GL1 would also be 36.4%. Therefore, the recombination frequency (RF) would be 27.2% (i.e., 100% − 2 × 36.4%). According to the Kosambi’s mapping function:
where r is the RF, the CA-rop2 insertion site and the GL1 locus are about 30.5 cM apart. To verify whether this calculation result fits the observed 85:15:11:17 phenotypic segregation, the χ2-test is performed. Based on the 27.2% RF, the expected phenotypic classes can be calculated and the results are 81 (CA1):15 (Col):15 (CA1 gl1):17 (gl1). The χ2-test analysis shows that the observed phenotypic segregation data are very consistent with the expected results (χ2=1.264, p<0.05).
To further support that the CA-rop2 transgene is located on Chromosome 3 and linked to GL1, a molecular mapping with the Simple Sequence Length Polymorphism (SSLP) markers has been performed. Genotyping with the three SSLP markers, NGA162, CIW11 and CIW4, all on Chromosome 3, suggests 2.7%, 27.6% and 45% RF, respectively, with the CA-rop2 insertion site (data not shown here). This indicates that the CA-rop2 insertion site is approximately 2.7 and 31.1 cM apart from NGA162 and CIW11, respectively, and is unlinked to CIW4. Because CIW11 is approximately 2 cM closer than GL1 to NGA162 based on the Arabidopsis genome map (http://www.arabidopsis.org), this indicates a discrepancy between the molecular mapping and genetic mapping data for the distance of CA-rop2 to CIW11 (31.1 cM) and to GL1 (30.5 cM). This discrepancy might be caused by the experimental variations due to the sample sizes used in the mapping (40 plants were used for the molecular mapping versus 128 for the genetic mapping). Nevertheless, a rough map of the CA-rop2 insertion site and the GL1 locus can be constructed (Figure 2). This map can be used for the students to compare their own genetic mapping data with this molecular mapping data and to explain the discrepancy they may observe.
Figure 2.
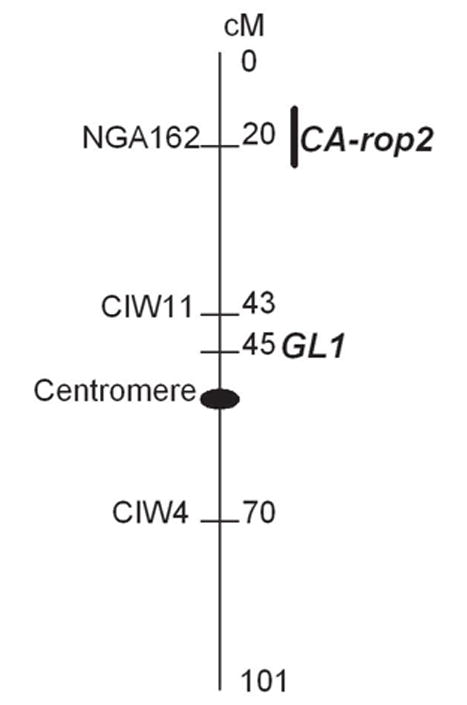
Linkage mapCA-rop2 of and GL1 on Arabidopsis Chromosome 3.
Discussion
In the biology laboratory that involves genetics at high school or college level, one key objective is to develop the students’ critical skills in analyzing the patterns of inheritance and inferring the linkage of genetic loci. Three years ago when the author just started to develop the course, an online quest for the most suitable materials for teaching purpose was posted. Interestingly, the Arabidopsis flower patterning mutants, hormone response mutants, or even the GUS reporter transgenic lines were suggested. However, after thoroughly considering these options and based on the author’s own research projects, the leaf morphology mutants and transegnic plants, such as gl1 and CA1 described here, turned out to be the best choice for several reasons. First, the leaf morphology phenotypes are easily characterized. Unlike flower patterning mutants that need to wait until the flowering stage for phenotypic characterization and most of hormone response mutants that require certain bioassays such as root growth on the hormone-containing medium, these two genotypes are readily characterized at the very early stage (cotyledons and very young true leaves). This allows the students to complete the crossing and the characterization of F1 and F2 generations perfectly within a semester. Second, the unexpected linkage of gl1 and the CA-rop2 transgene integration site revealed through this laboratory exercise makes the cross of gl1 with CA1 a perfect choice for inferring the linkage and mapping of their positions on the chromosome. Third, the use of one recessive mutant and one dominant transgenic plant gives students the best opportunity to study dominance relationships. Fourth, the transgenic materials provide an opportunity for the discussion of genetically modified organisms.
Several semesters’ teaching experience have provided four additional tips that are helpful in the improvement of teaching. First, to make sure that the students have materials to proceed with in this semester-long exercise, the instructor not only should emphasize the importance of successful crosses to the students, but also prepare the F1 seeds for every type of cross. This is critical, given that 15–40% of the students did not succeed in the cross in the past. In case the students obtain the seeds from self-fertilization instead of cross-fertilization, they should be encouraged to continue through F2 data analysis, which can verify the failure of crossing. However, in the meantime, they should be provided with the F1 seeds prepared by the instructor so that they can compare these two F2 datasets.
Second, the instructor should try to stimulate the discussions by students. Interactions among individuals and groups in the classroom are vital in advancing the understanding of genetics concepts and the nature of scientific endeavors. Several occasions are particularly worthy of mentioning. The students might observe contrasting phenotypes of the F1 plants from the same type of crosses but done by different groups. This is most likely due to self-fertilization. In addition, there are always some students/groups who obtain the F2 data completely different from others. Consequently, this does not lead to the conclusions that the traits are segregating as 3:1 or that GL1 and CA-rop2 transgene insertion site are linked. The instructor should encourage the debate and discussion. This can be best achieved through the PowerPoint presentations. Presenting their results using the PowerPoint format in the classroom is most beneficial to the students. Each group is assigned to present for eight to ten minutes and answer questions for two to three minutes, which fosters the discussion. At the end, the instructor should summarize the diverse datasets and then ask the students to evaluate their peers’ performance.
Third, students are asked to write a six or seven page research report, by following the journal format. This final synthesis stage is particularly helpful because after the presentations, students can learn how to discuss their experiments/data and make modifications of their conclusions.
Fourth, after the first semester of teaching, the instructor will obtain additional materials, the CA1 gl1 double mutants. Because CA-rop2 is dominant, the students will need to determine the genotypes of the double mutants which are completely unknown to both students and instructors. Additionally, CA1 plants can also be used as a molecular mapping laboratory exercise in a higher level college genetics course or to those highly-motivated students.
In summary, this laboratory exercise, when it is combined with other genetics labs during the semester (such as maize kernel segregation analysis, meiosis, Drosophila three-point testcross, literature search and bioinformatic tools) provides students with a real research environment by integrating knowledge of biology, experimental skills, and statistical analysis to address the fundamental concepts of genetics. Such an almost cost-free laboratory exercise could stimulate the students’ interest in biology and science in general and thus help to achieve the goal of nurturing budding scientists that has been actively pursued for decades in high school and college education in the U.S.
Acknowledgments
The study in the Zheng Laboratory at Lehman College, City University of New York, is partly funded through the National Institutes of Health (S06GM008225, Subproject 12) and the United States Department of Agriculture (2004–35304–14911) grants. I thank Dr. Guohua Yang in my laboratory for providing the CA-rop2 transgene molecular mapping data. I am grateful to Prof. Joseph Rachlin (Lehman College, City University of New York) for his critical reading of the manuscript. I also thank the Editor and anonymous reviewers for their constructive comments.
References
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Stam P. Genetic analysis. In: Martinez-Zapater JM, Salinas J, editors. Arabidopsis Protocol. NJ: Humana Press Inc; 1998. pp. 105–117. [Google Scholar]
- Li H, Shen J, Zheng ZL, Lin Y, Yang Z. The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiology. 2001;126:670–684. doi: 10.1104/pp.126.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke DW, Cherry JM, Dean C, Rounsley SD, Koornneef M. Arabidopsis thaliana: A model plant for genome analysis. Science. 1998;282:679–682. doi: 10.1126/science.282.5389.662. [DOI] [PubMed] [Google Scholar]
- Oppenheimer DG, Herman PL, Sivakumaran S, Esch J, Marks MD. A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell. 1991;67 (3):483–493. doi: 10.1016/0092-8674(91)90523-2. [DOI] [PubMed] [Google Scholar]


