Abstract
The localized delivery of exogenous, angiogenic growth factors has become a promising alternative treatment of peripheral artery disease (PAD) and critical limb ischemia. In the present study, we describe the development of a novel controlled release vehicle to promote angiogenesis in a murine critical limb ischemic model. Ionic, gelatin-based hydrogels were prepared by the carbodiimide-mediated amidation reaction between the carboxyl groups of gelatin or poly-L-glutamic acid molecules and the amine groups of poly-L-lysine or gelatin molecules, respectively. The degree of swelling of the synthesized hydrogels was assessed as a function of EDC/NHS ratios and the pH of the equilibrating medium, while the release kinetic profile of basic fibroblast growth factor (FGF-2) was evaluated in human fibroblast cultures. The degree of swelling (DS) decreased from 26.5 ±1.7 to 18.5 ±2.4 as the EDC concentration varied from 0.75 to 2.5 mg/ml. 80% of the FGF-2 was released at controlled rates from gelatin-polylysine (gelatin-PLL) and gelatin-polyglutamic acid (gelatin-PLG) hydrogel scaffolds over a period of 28 days. Cell adhesion studies revealed that the negatively charged surface of the gelatin-PLG hydrogels exhibited superior adhesion capabilities in comparison to gelatin-PLL and control gelatin surfaces. Laser Doppler Perfusion Imaging as well as CD31+ capillary immunostaining demonstrated that the controlled release of FGF-2 from ionic gelatin-based hydrogels is superior in promoting angiogenesis in comparison to the bolus administration of the growth factor. Over four weeks, FGF-2 releasing gelatin-PLG hydrogels exhibited marked reperfusion with a Doppler ratio of 0.889 (± 0.04) which was 69.3% higher than in the control groups.
Keywords: angiogenesis, hydrogels, FGF-2, controlled delivery, critical limb ischemia
1. Introduction
Peripheral artery disease (PAD) currently afflicts 27 million people in the western world [1]. Strategies to enhance peripheral blood flow in patients afflicted by PAD are an area of ongoing research [2–4]. Endoarterectomy is the most widely used method to treat patients with critical limb ischemia and PAD, however this treatment is highly invasive [5]. Non-invasive therapies for the treatment of PAD such as the localized delivery of therapeutic growth factors, or autologous stem cell transplantation have become promising alternative options for patients [1,6–10]. The administration of angiogenic peptides like vascular endothelial growth factor (VEGF) and FGF-2 have shown beneficial effects in reversing ischemia and improve regional blood flow in underperfused regions of the heart or extremities in animal as well as controlled clinical studies [9–14]. However persistent concerns relating to growth factor’s long term efficacy, dose selection, growth factor stability and optimal release profiles have prevented exogenous growth factor therapy from becoming a common practice in treating PAD.
FGF-2 is a single chain peptide (MW=16,000) composed of 146 amino acids, which promotes proliferation, differentiation, and numerous other cellular functions in cells derived from the mesoderm and neuroectoderm. It is a potent angiogenic factor in vivo and in vitro, and is mitogenic and chemotactic for both fibroblasts and endothelial cells [15]. Additionally, FGF-2 drives the alignment of smooth muscle cells in the basement membrane of newly formed capillaries [16–18]. A number of clinical studies using FGF-2 have demonstrated significant reperfusion and angiogenesis in patients with myocardial infarction, critical limb ischemia, and cerebrovascular diseases [19–22]. Unfortunately, the exact dosage of FGF-2 or VEGF required to induce the formation of mature vasculature without the occurrence of adverse effects is not yet certain. It is known that excessive dosage of either of these growth factors can have negative consequences. For example, the angiogenic effect commonly associated with FGF-2 administration is rather limited due to its rapid clearance when it’s administered in its free form and can cause immature and leaky blood vessel formation. Furthermore, fibromatosis could be a concern with the prolonged delivery of high doses of FGF-2 [23].
A number of biomaterials including alginate, heparin-sulfate, dextran, glycosaminoglycans, and poly(ethylene) glycol have been employed to develop controlled delivery vehicles for FGF-2 that facilitate a sustained, localized and effective dose response [24–29]. Gelatin, a readily available natural biomaterial derived from the denaturation of collagen, has been widely used as an additive in pharmaceutical formulations, drug delivery and tissue engineering applications. A number of studies describe the stabilizing effect of gelatin on FGF-2 via poly-ion complexation [6,10], as well as its efficiency in maintaining a release profile for an extended period of time [19,28]. Herein, we investigate the effectiveness of the controlled delivery of FGF-2 from highly ionic gelatin-based hydrogel scaffolds to reperfuse ischemic tissues in a murine hindlimb ischemic model. Ionic hydrogels were prepared under mild conditions by covalently crosslinking gelatin with either poly-L-lysine (PLL) or poly-L-glutamic acid (PLG). The cytotoxicity and adhesion characteristics of the hydrogels were tested in human neo-natal fibroblast cell culture and the release kinetics of FGF-2 was assessed in vitro as a function of hydrogel properties. Laser Doppler Perfusion Imaging (LDPI) was used to quantify the extent of angiogenesis that has occurred over an eight-week period for each treatment group in a murine hindlimb ischemic model, and validated by CD31+ immunohistological staining for capillaries in the quadriceps muscle.
2. Materials and Methods
2.1 Materials
Gelatin type A, alkaline wash from porcine skin (~ 225 bloom; IEP = 5.0), gelatin B, acidic wash from bovine bone (~ 300 bloom; IEP = 9.0), poly-L-glutamic acid (PLG; MW = 75,000 − 120,000 kDa), poly-L-lysine (PLL; MW = 90,000 − 140,000 kDa), 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide (EDC), and N-hydroxysuccinimide (NHS) were obtained from Sigma Aldrich (St. Louis, USA) and were used as indicated by the manufacturer. All other chemicals and organic solvents were purchased at their highest purity from Sigma Aldrich.
2.2 Synthesis and characterization of ionic gelatin hydrogels
In a typical procedure a 10 wt/vol % gelatin solution was prepared by adding 100 mg of gelatin A or B to 1 mL of phosphate buffered saline (PBS; pH = 7.4) and heating the mixture to 40 °C for 5 minutes to allow complete dissolution. Following gelatin solubilization, 10 mg of PLL or PLG was added to the mixture, upon stirring. EDC/NHS was added into the mixture to activate the carboxyl groups of gelatin or PLG during the amidation reaction. EDC/NHS solutions were prepared at NHS concentrations of 0.22 mg/ml, 0.44 mg/ml, and 0.75 mg/ml, and EDC was added in 1:1, 2:1, and 4:1 molar ratios in phosphate buffered solution (PBS; pH = 7.4) and stored at 4 °C until use. 1 mL of EDC/NHS solution was added to the heated gelatin-PLL or gelatin-PLG solution and the reaction mixture was allowed to polymerize for 10 minutes at 4 °C (Figure 1). Following crosslinking, the ionic hydrogels were immersed in 40 mL of PBS for 30 minutes to equilibrate and allow the removal of the unreacted by-products and the urea derivative of EDC. The hydrogels were then swollen in buffer solutions of pH 4.5, 7.4, and 10.0 for 2 hours. Following equilibration, the hydrogel disks were removed from the buffer solution and their swollen weight was recorded. The swollen gel was then placed in an oven at 65 °C overnight to allow complete water evaporation. The dry weight of the gels was recorded, and the degree of swelling was then calculated by Equation 1.
Figure 1. Reaction scheme for the synthesis of ionic, gelatin-based hydrogels.
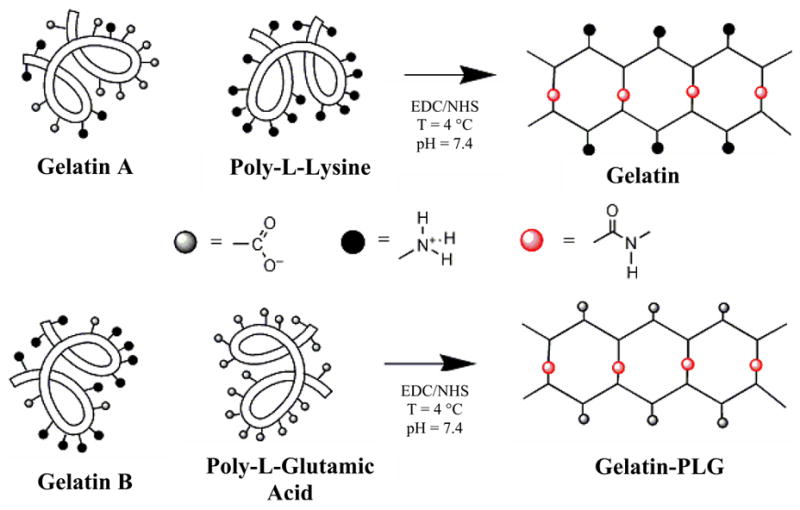
Gelatin A and Gelatin B were co-crosslink with PLL (cationic gels) and PLG (anionic gels) respectively via an EDC/NHS coupling reaction.
| (1) |
where φ is the degree of swelling, MW is the weight of the swollen gel, and MD is the dry weight of the cross-linked hydrogel.
2.3 Cytotoxicity of ionic hydrogels
In 6-well culture plates, ionic gelatin hydrogel films (300 μL) were prepared from filter-sterilized (0.2 μm) gelatin solutions according to the procedures described previously. All experiments were conducted in triplicates and the following groups were tested to assess the cytotoxicity of the hydrogels as well as of the EDC/NHS concentration: gelatin solutions, gelatin hydrogels alone (no PLL or PLG), hydrogels without being washed, and gelatin-PLL and gelatin-PLG hydrogels washed in PBS. The groups that required rinsing were placed immediately after cross-linking in 5 mL of deionized water for 40 minutes and the soluble by-products were aspirated. The gels were further sterilized in a biological fume hood upon exposure to 254 nm irradiation for 2 hours.
The cytotoxic nature of the cross-linked hydrogels was examined using a 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazo-lium hydroxide (XTT) assay (Roche Applied Sciences; Penzberg, Germany). Human neonatal fibroblast (HNF) cells (passage 4–7; ATCC; Manassas, VA) were grown to confluence in a 75 cm2 cell culture flask in 10 ml of 90% Iscove Modified Dulbecco Medium (IMDM) supplemented with 10% fetal bovine serum (FBS) and a 5 U/ml penicillin-streptomycin solution. Fibroblast cells were incubated at 5% CO2 and 37 °C to promote cell growth. Medium was replaced every two to three days allowing the cells to grow to confluence. Once they reached confluence the cells were trypsinized, counted, and 60,000 cells were added into culture plates containing the hydrogels. The cells-hydrogel mixtures were incubated in a 5% CO2/37 °C atmosphere for 96 hours and the cytotoxicity of the gels was assessed at the end of the incubation period with a viability assay. Optical density readings were measured on an ELISA reader (Bio-Rad Laboratories; Philadelphia, PA) at 490 nm with reference wavelength of 655 nm.
2.4 Cellular adhesion on ionic gelatin-based hydrogels
Cell adhesion on gelatin-based hydrogel films was assayed according to methods described previously [30,31]. Briefly, 300 μL of gelatin solution were poured into a 24-well plate and crosslinked with EDC and NHS at 4°C for 10 min as described previously. Following polymerization, the gelatin sheets were UV-sterilized by irradiation at 254 nm for 2 hours.
60,000 cells/well were seeded in the wells containing the experimental groups and allowed to adhere for either 1 or 24 hours at 37 °C and 5% CO2 atmosphere. After incubation, loosely adherent or unbound cells were removed by aspiration, the wells were washed twice with PBS, and the remaining bound cells were fixed with 10% formalin in PBS for 15 min. The fixative was aspirated, the wells were washed twice with PBS, and the attached cells were stained with hematoxylin and eosin. The wells were gently washed 3 times with double distilled water and cell adhesion was confirmed using a LEICA Microsystems AF6000LX brightfield/fluorescence microscope and Q Imaging 5.0 software immediately after the staining procedure. Only cells that exhibited a flattened, polygonal shape with whip-like projections were considered to be viably adherent cells onto gelatin films.
2.5 FGF-2 in vitro release
250 ng of FGF-2 was reconstituted in 0.1% bovine serum albumin (BSA) and incorporated into the gelatin solutions prior to crosslinking. Plastic cylinders (h = 4.0 mm; D = 6.5 mm; V = 135.0 mm3) were sealed to the bottom of a 6-well culture plate using silicon glue (Dow Corning). 150 μL of gelatin solutions with or without PLL or PLG (10 wt/vol % gelatin; 1 wt/vol % poly-amino group) containing FGF-2 and 150 μL of EDC (1.3 mg/mL) and NHS (0.44 mg/mL) solutions were placed in the cylinders and immediately cooled to 4 °C for 10 minutes to facilitate cross-linking. Release experiments were conducted in an orbital shaker (MaxQ 4000 Bench Top Orbital Shaker - Barnstead International; Dubuque, IA) at 37 C with constant agitation at 100 rpm. PBS (5 mL) was added to each well, and the wells were covered with a parafilm sheet to avoid medium evaporation. Medium was changed at predetermined times (ranging from 2 hours up to 28 days) and the collected medium was placed in a 10 mL vial and frozen at −20 °C until the ELISA analysis was performed. The amount of FGF-2 released from the hydrogels was determined using a FGF-2 ELISA assay (EMD Biosciences; San Diego, CA). The optical density was determined at a wavelength of 450 nm with reference of 570 nm.
2.6 In vivo assessment of angiogenesis induced by FGF-2-releasing gelatin hydrogels
Balb/C mice (Charles River Laboratories; Boston, MA) of age 39–49 days, weighing 25–35 g, were cared for and operated following IUCAC guidelines at the University of Miami Miller School of Medicine. Prior to surgery, mice were anesthetized using a ketamine/xylazine/aceprozamine cocktail injection of 40 mg/kg, 8 mg/kg, and 10 mg/kg respectively. The hair was sheared from the left leg of the mouse (dorsal), and an incision was made, exposing the femoral artery and vein. A 7-0 grade silk suture was used to ligate the femoral artery and vein in three locations (above the saphenous branches, along the deep femoral artery, and below the external iliac artery) as seen in Figure 2. The femoral artery and vein were excised and the ends were cauterized to prevent leaking.
Figure 2.
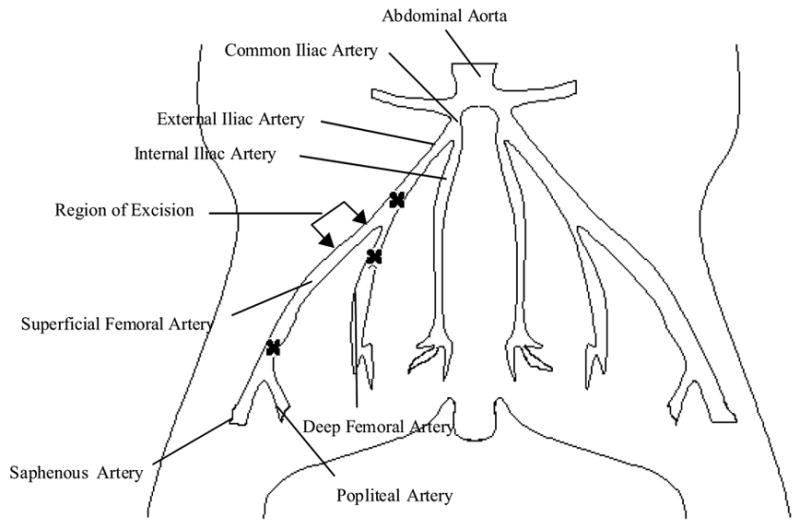
Schematic representation for the induction of critical hindlimb ischemia in a murine model.
Following surgery, the mice were randomized to the following treatment groups (N=5 per time point per group): phosphate buffered saline (PBS; 300 μL), gelatin-PLL (300 μL), gelatin-PLG (300 μL), gelatin A with FGF-2 (300 μL; 250 ng FGF-2), gelatin B with FGF-2 (300 μL; 250 ng FGF-2), gelatin-PLL with FGF-2 (300 μL; 250 ng FGF-2), gelatin-PLG with FGF-2 (300 μL; 250 ng FGF-2), FGF-2 bolus injection (300 μL; 250 ng FGF-2). The treatment groups were placed on the quadriceps muscle adjacent to the femoral artery excision site and the wound was closed with sutures.
2.6.1 Laser Doppler Perfusion Imaging
Hindlimb perfusion was assessed using laser Doppler perfusion imaging (LDPI; Perimed Medical Systems New York, NY). LDPI provides a non-invasive measurement of blood flow by determining the Doppler frequency shift due to light reflecting off the moving red blood cells. Data acquisition was performed in a method similar to Rivard et al. [32]. Briefly, the sedated mice were secured on a monochromatic surface and an area of 2070 mm2 was scanned from the lower abdomen to the end of the toes. Color images were obtained and hindlimb perfusion ratios were determined by comparing the perfusion of the hindlimb prior to surgery to that at specified time points: post-surgery, one, two, three, four, and eight weeks. An ischemic Doppler ratio was established for all groups and compared.
2.6.2 Tissue Preparation
The mice were sacrificed at predetermined time points (two, four, and eight weeks) with an overdose of halothane inhalation. Quadriceps muscle tissue was removed and immediately placed in 10% formalin in PBS overnight. After fixation, tissue was embedded in paraffin and 5-μm tissue sections were cut and mounted on positively charged slides. The tissue’s antigenicity was recovered using a warmed proteinase K solution. The tissue samples were then treated with a diluted primary antibody (1:200), mouse anti-CD31 monoclonal antibody (BD Pharmingen, Minneapolis, MN), and incubated for 2 hrs. They were then rinsed, incubated with a secondary biotinylated antibody, goat anti-mouse (Vector Labs; Burlingame, CA) and treated with ABC and DAB chromogen reagent and counterstained with hematoxylin. Slides were then viewed using light microscopy at 40X using a LEICA Microsystems AF6000LX brightfield/fluorescence microscope (Bannockburn, IL), and images were captured using Zeiss Axiovision 4.1 Software. CD31+ cells were counted in five separate fields and all treatment groups were compared by determining capillaries per square millimeter.
3. Results and discussion
3.1 Characterization of ionic gelatin-based hydrogels
In the present study, ionic hydrogels were prepared under mild conditions by the direct crosslinking of gelatin molecules with either linear polylysine or polyglytamic acid polymers. Figure 1 describes the EDC/NHS mediated crosslinking reaction via covalent amide bond formation. The degree of swelling (provides a direct relationship to the crosslinking density) of the gelatin-based hydrogels was determined as a function of EDC/NHS concentration and pH. In the case of gelatin-PLL hydrogels, the degree of swelling did not significantly changed at EDC/NHS molar ratios from 1:1 to 4:1 with EDC concentrations ranging from 0.75 to 2.5 mg/ml (Fig. 3 A). Alternatively, the DS for the gelatin-PLG hydrogels depended on the amount of the EDC/NHS catalyst used. As seen in Figure 3B, there was a significant change in the degree of swelling (p < 0.01) from 26.5 ±1.7 to 18.5 ±2.4 at a 1:1 ratio and EDC concentration increase from 0.75 to 1.3 mg/mL. There is a similar effect for the other EDC/NHS ratios of 2:1 and 4:1. This is attributed to the increased number of free carboxyl groups capable of being activated on the PLG molecule (2.2 mol/g catalyst) than on the gelatin A molecule (~8 mmol/g catalyst) which in turn require more EDC/NHS for optimal activation. As the EDC concentration was increased more carboxyl groups were activated, which led to the formation of gelatin-PLG hydrogels with higher crosslinking density and lower degree of swelling values.
Figure 3. Degree of swelling (DS) measurements for crosslinked hydrogels at various pHs.
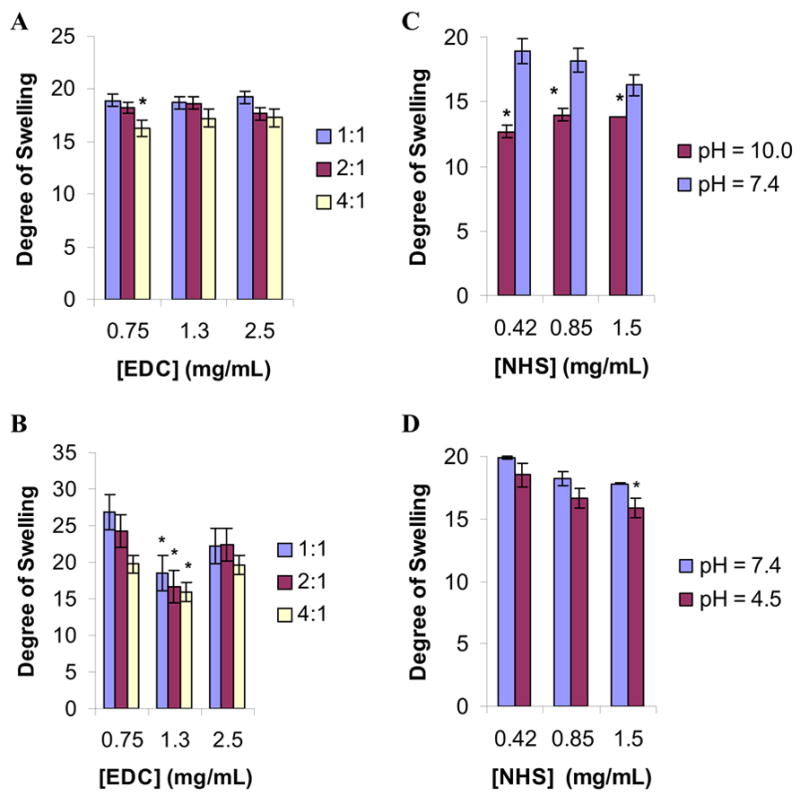
The DS of gelatin-PLL (A) and gelatin-PLG (B) hydrogels was determined as a function of EDC/NHS molar ratios at pH 7.4 (n= 3 per group, [gelatin] = 0.1 g/mL, [PLL] = 0.01 g/mL, [PLG] = 0.01 g/mL). (C): DS of gelatin-PLL at pHs of 7.4 and 10.0 (pKa of lysine = 10.4). (D): DS of gelatin-PLG hydrogels at pHs of 7.4 and 4.5 (pKa of glutamic acid = 4.5). Statistical significance was determined at p < 0.05 (denoted by *; n=3 per group).
Due to the ionic nature of the hydrogels, pH significantly affected the degree of swelling of the prepared gelatin hydrogels. Aqueous buffers of pH 4.5 and 10.0 were selected because these values approach the pKa for glutamic acid and lysine respectively. The degree of swelling dependency on pH for gelatin-PLL and gelatin-PLG is shown in Figure 3C and Figure 3D respectively at various NHS concentrations while the EDC concentration was held constant at 1.3 mg/mL. In the case of gelatin-PLL as the pH increased from 7.4 to 10 (the pKa of the lysine group) the DS was decreased due to the deprotonation of the lysine’s amine groups and the decrease of the electrostatic repulsion (positively charged ammonium cations) among the polylysine chains of the hydrogel. Similarly, in the case of gelatin-PLG the DS was decreased as the pH of the equilibrating buffer solution was changed from 7.4 to 4.5 due to protonation of the carboxyl groups and the reduction of the electrostatic repulsion between the carboxylate groups of the polyglutamic chains of the hydrogels.
3.2 Adhesion and cytotoxicity
The cytotoxicity and the cell adhesion behavior of the gelatin-based hydrogels were assessed in neo-natal fibroblast cell cultures. Figure 4(A) depicts the percent adherence of HNF cells on hydrogel films at 1 and 24 hours of cell incubation. The ionic nature of the gels had a profound effect on cell adhesion, specifically the negative charged surfaces which demonstrated the highest cell adhesion. The amount of HNF cells positively stained with hematoxylin were counted in five separate fields (triplicate experiments) and compared to control. Gelatin-PLG films demonstrated the greatest adhesion over a 24 hour incubation period with 42,409 ±2547 cells/mm2 compared to control which adhered 33,827 ±392 cells/mm2. One hour incubation studies revealed no discernable adhesion characteristics on gelatin surfaces compared to control.
Figure 4. Adhesion and cytotoxicity studies of the gelatin-based hydrogels in human neonatal fibroblast cell cultures.
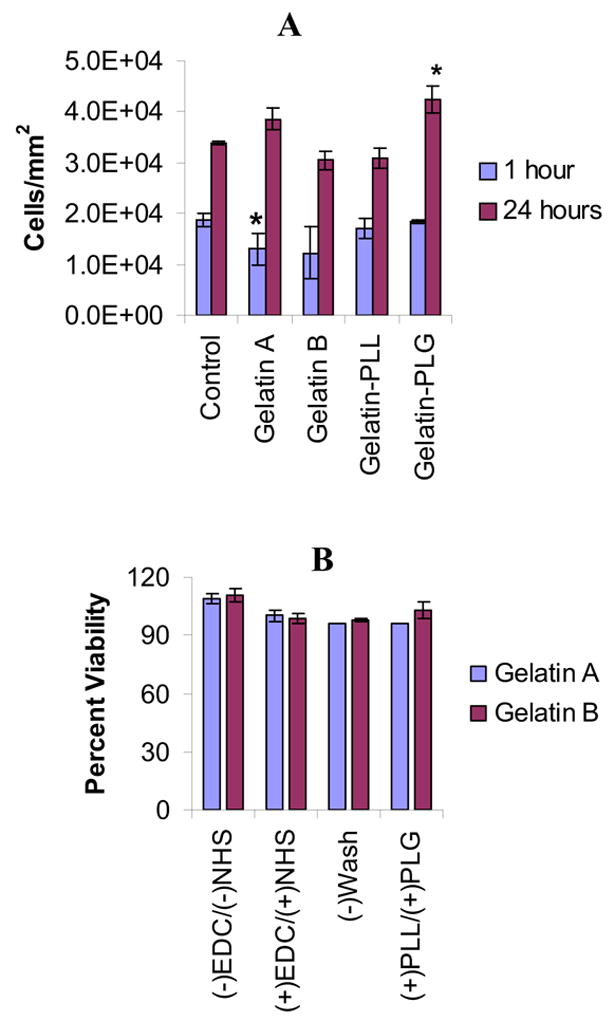
(A): Percent adhesion of HNF cells on the hydrogel surfaces was assessed by counting the cells that showed whip-like projections and stained their cytoplasm (H&E staining, n=3 per time point per group, p < 0.05 denoted by *). (B): Cell viability in the presence of ionic gelatin hydrogels was determined with and without EDC and NHS as well as in the presence of the urea byproduct derivative. [EDC]=1.3 mg/mL, [NHS] = 0.44 mg/mL.
Based on the XTT viability assay, all of the synthesized gels demonstrated no in vitro cytotoxicity. In all groups the percent viability of the gelatin cross-linked complexes utilizing NHS and EDC was greater than 97% compared to the polystyrene control (Fig. 4B). The percent viability was derived as function of the optical density and compared to that of the control group (polystyrene plates). Our results demonstrated that the EDC and NHS at the reported concentrations used to prepare the hydrogel scaffolds had no significant effect on cell viability.
3.3 FGF-2 In Vitro Release
The need for a sustained growth factor exposure at the ischemic site for optimal collaterization has led to the employment of delivery vehicles that facilitate a therapeutic, localized dose, provide a stabilizing matrix for the growth factor, and minimize toxic side effects usually associated with systemic administration. In the present study the effect of the ionic characteristics of the gelatin-based hydrogels on the release kinetics of FGF-2 was determined in temperature-controlled (37 ºC) conditions. The gels were swollen within the cylinder and FGF-2 was released (top surface, one dimension) into the surrounding medium. Within 5 days, gelatin-PLG released 20.2% ±5.4% of the growth factor whereas the alternate anionic hydrogel, gelatin A, released 36.3% ±4.9% of the FGF-2. For the gelatin A and gelatin B vehicles, 100 % of the growth factor was released within 28 days, whereas in the case of gelatin-PLL and gelatin-PLG hydrogels 81.3% ±2.2%, and 78.9% ±4.8% of the FGF-2 was released over the same period. The presence of either PLL or PLG significantly affected the rate of FGF-2 release compared to the gelatin gels without PLL or PLG. Gelatin-PLL and gelatin-PLG hydrogels demonstrated a sustained release profile of the FGF-2 in comparison to gelatin hydrogels that exhibit pronounced burst release profiles within the first 4 days. On average, gelatin-PLL and gelatin-PLG released 17.2% ±3.6% less of the growth factor over the first 5 days compared to gelatin A and gelatin B. There was a minimal difference on the release profile of FGF-2 from gelatin-PLL and gelatin-PLG over 28 days; contrary to what has been previously reported in the literature that positively charged scaffolds (gelatin-PLL) tend to release FGF-2 in a burst manner [6]. The release profile of FGF-2 could be potentially modified to meet the requirements of natural angiogenesis by altering the PLL/PLG content or the EDC concentration. Previous studies have shown that using a smaller amount of catalyst, less than 0.5 mg/mL for EDC, a total of 64 ±5% of FGF-2 was released over 5 days [28]. In addition, longer crosslinking times (12 hrs at 4 °C) led to prolonged release profiles over a period of 12 days with [EDC] concentrations of 0.25 mg/mL [33].
3.4 Laser Doppler Perfusion Imaging
To determine the rate of reperfusion in the ischemic murine model with respect to FGF-2 releasing gelatin hydrogels, Doppler analysis was performed pre-surgery and compared to, one hour post surgery, and then after at 1, 2, 3, 4, and 8 weeks. In all treatment groups, a significant decrease in the perfusion ratio occurred within one hour, confirming the excision of the femoral artery and induction of hindlimb ischemia (Fig. 6). Significant angiogenic effects can be seen within two weeks between treatment groups (p < 0.01). Total limb loss occurred within one week in groups without FGF-2. Additionally, the PBS, gelatin-PLL, and gelatin-PLG groups demonstrated no significant change on their LPDI ratios over a period of eight weeks (Fig 6). The gelatin A and gelatin B hydrogels with FGF-2 exhibit a minimal angiogenic response compared to cross-linked, ionic hydrogels with FGF-2 over the first four weeks. The FGF-2 releasing cationic hydrogels (gelatin-PLL) elicited a significant angiogenic response within two weeks of treatment but maximal angiogenic activity occurred at four weeks with the FGF-2 releasing gelatin-PLG group with a Doppler ratio of 0.889 (±0.04) which was slightly greater than gelatin-PLL with FGF-2 with a Doppler ratio of 0.760 ±0.05. Substantial angiogenesis was observed as early as one week and continued over four weeks. Significant capillary regrowth was still present after eight weeks, although the Doppler ratio had decreased noticeably since the previous time point. Interestingly, between four and eight weeks both FGF-2 releasing gelatin-PLG and gelatin-PLL hydrogels began to lose their angiogenic capacity probably due to growth factor exhaustion, and scaffold biodegradation. However, no limb loss was observed in any of the groups that were treated with FGF-2 releasing gel scaffolds during the eight week period. It is likely that due to deep femoral artery removal, revascularization to pre-surgery levels would require continuous and/or multiple cytokine therapy for an extended period of time.
Figure 6. Laser Doppler Perfusion Imaging intensity ratios of various treatments in a murine hindlimb ischemic model.
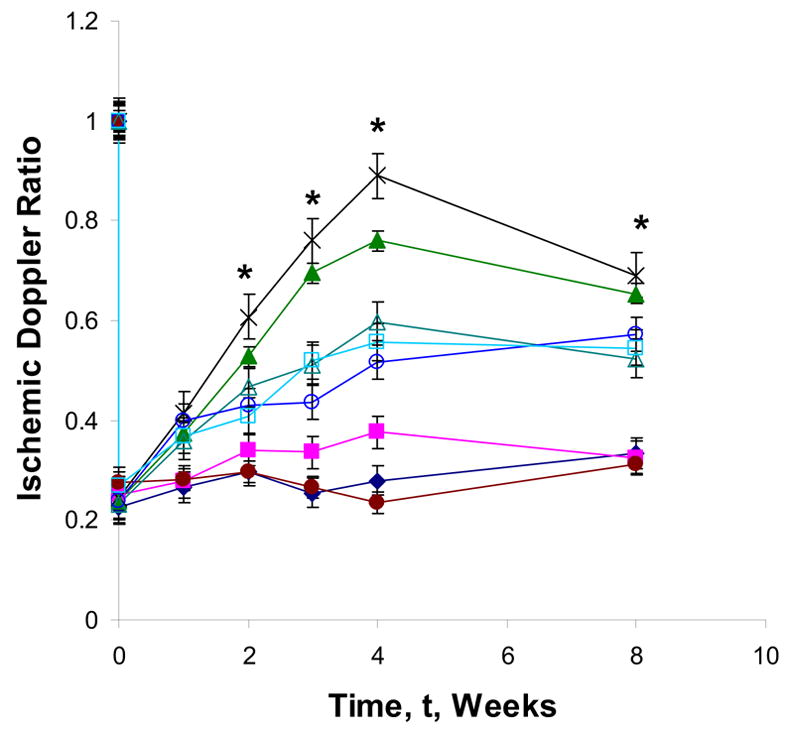
Paw blood flow velocity (n = 5 mice per time point per group) was determined at specified time points for 8 weeks. Representative Doppler ratios of PBS injection (●), gelatin-PLL (◆), gelatin-PLG (■) gelatin A with FGF-2 (△), gelatin B with FGF-2 (○), FGF-2 bolus injection (□), gelatin-PLL with FGF-2 (▲), and gelatin-PLG with FGF-2 (×). Statistical significance was determined at p < 0.01 for all treatment groups (denoted by *). Concentrations of constituents: [gelatin]=0.1 g/mL; [PLL or PLG]=0.01 g/mL; [FGF-2]=250 ng; [EDC]=1.3 mg/mL; [NHS]=0.44 mg/mL.
The bolus injection of FGF-2 produced an initial angiogenic response that lasted for four weeks but it was substantially lower than the effect seen with the delivery vehicles probably due to growth factor’s rapid diffusion to the surrounding tissue and biodegradation (Fig. 6). Marginal perfusion was still present after four weeks and no further increase in angiogenesis was observed at eight weeks. Conversely, the LDPI ratios for gelatin-PLG with FGF-2 were significantly higher at eight weeks demonstrating the effectiveness of the controlled release of FGF-2 to maintain angiogenic activity.
3.5 Immunohistochemistry
In order to validate the use of Laser Doppler Perfusion Imaging data as a sound method for assessing angiogenesis, we quantified the capillary density using immunohistochemistry. Tissue sections were stained for CD31+, which specifically stains cells present in capillaries and small arterioles, and their density was determined using brightfield microscopy at a 40 X magnification (Figure 7). Capillary density was quantified using Image J 6.0 software to count positively stained capillaries in five separate fields (Figure 8).
Figure 7. Representative immunohistological staining (CD31+) images of ischemic hindlimb quadriceps muscle at 8 weeks.
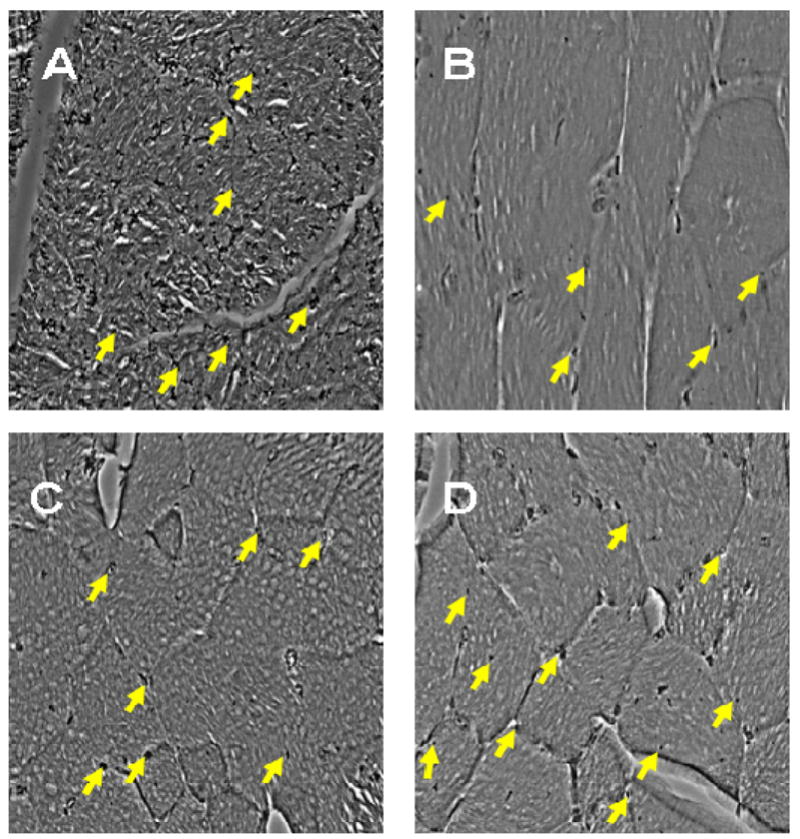
(A): CD31+ staining of a non-ischemic limb; (B): Gelatin-PLG treatment; (C): Bolus injection of FGF-2; (D): Gelatin-PLG releasing FGF-2. Concentrations of constituents: [gelatin]=0.1 g/mL; [PLL or PLG]=0.01 g/mL; 250 ng of FGF-2; [EDC]=1.3 mg/mL; [NHS]=0.44 mg/mL.
Figure 8. CD31+ capillary density measurements.
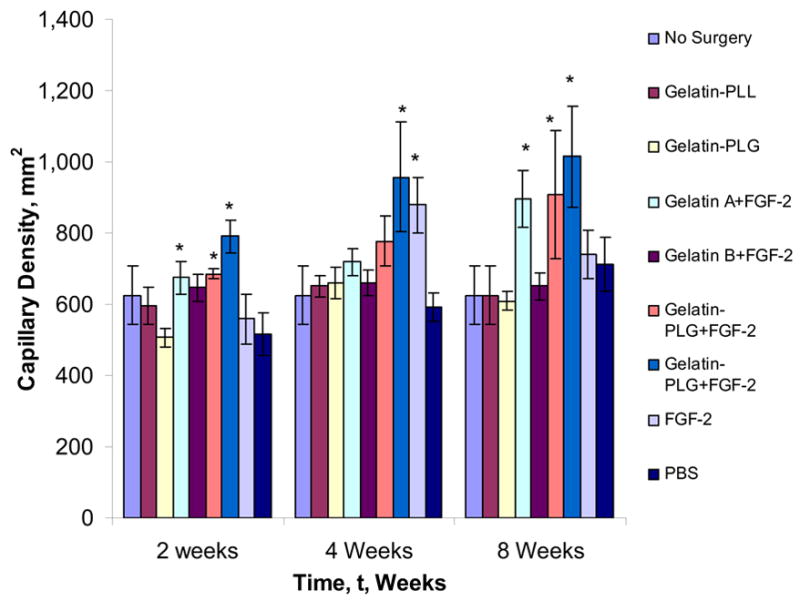
At two, four, and eight weeks, positively stained capillaries were counted in five fields per slide in triplicates by a blinded observer and the treatment groups compared to the controls. Statistical significance was determined at p < 0.05 and denoted by *.
As conferred with the LDPI data, the efficacy of slow releasing FGF-2 from ionic gelatin hydrogels was validated from the histological studies. After four weeks, an increased capillary density, greater than 900 capillaries per square millimeter was observed in both FGF-2 containing gelatin-PLL and gelatin-PLG networks respectively (Figure 8). In addition, in the FGF-2 group (bolus injection) the capillary density was significantly lower than the FGF-2 containing gelatin-PLG group at eight weeks, suggesting a potential growth factor degradation and/or clearance in the case of the bolus administration from the ischemic site thus compromising its angiogenic effect. A number of studies have demonstrated a similar loss in angiogenic activity of bolus FGF-2 administration highlighting the need of a continuous exposure of the ischemic site to FGF-2 to achieve a therapeutic response [11,23]. Furthermore, it is evident based on our own preliminary studies and work by others that in order to achieve a mature vasculature more than one cytokine would need to be administered at the ischemic site in a pattern that mimics the natural progression of angiogenesis [23,26].
5. Conclusions
Tunable ionic gelatin-based hydrogel were prepared under mild conditions for the controlled delivery of FGF-2 in an experimental critical limb ischemic model. Due to the stabilization of the FGF-2 via ionic complexation, the negatively charged gelatin-PLG gels were the best carrier for the FGF-2 by inducing the most pronounced angiogenic effect in comparison to other gel formulations and bolus control groups. The rate of FGF-2 release was controlled by altering the ratios of EDC/NHS and/or the gelatin and polyamino acid composition. Studies are underway in evaluating these hydrogel scaffolds as potential vehicles for multiple and stage cytokine delivery in the area of therapeutic angiogenesis.
Figure 5. Release profile of FGF-2 from gelatin-based hydrogels.
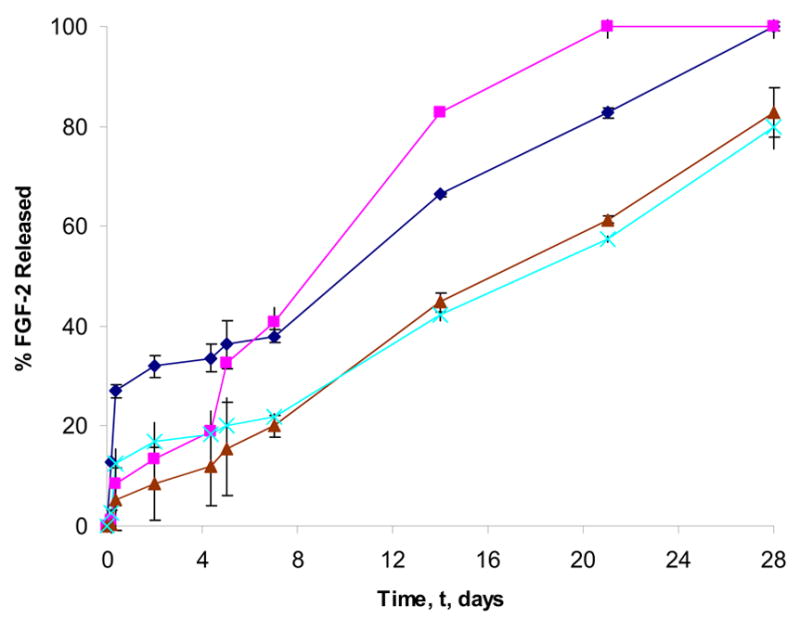
The overall release rate from gelatin A (
 ), gelatin B (
), gelatin B (
 ), gelatin-PLL (
), gelatin-PLL (
 ), gelatin-PLG (
), gelatin-PLG (
 ) hydrogels was monitored over 28 days (n=3 per time point per group). [gelatin] = 0.1 mg/mL; [PLL or PLG] = 0.01 mg/mL; FGF-2 = 250 ng; [EDC] = 1.3 mg/mL; [NHS] = 0.44 mg/mL.
) hydrogels was monitored over 28 days (n=3 per time point per group). [gelatin] = 0.1 mg/mL; [PLL or PLG] = 0.01 mg/mL; FGF-2 = 250 ng; [EDC] = 1.3 mg/mL; [NHS] = 0.44 mg/mL.
Acknowledgments
We acknowledge the support of NIH-NIAMS (1RO3-AR50518 to F.M.A), and the Departments of Biomedical Engineering and Surgery at the University of Miami.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hackam DG, Goodman SG, Anand SS. Management of risk in peripheral artery disease: recent therapeutic advances. Am Heart J. 2005;150:35–40. doi: 10.1016/j.ahj.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Aranow WS. Management of peripheral artery disease. Cardiol Rev. 2005;13:61–68. doi: 10.1097/01.crd.0000126082.86717.12. [DOI] [PubMed] [Google Scholar]
- 3.Dashwood MR, Tusi JC. The effect of acute ischemia on ET-1 and its receptors in patients with underlying chronic ischemia of the lower limb. Exp Biol Med. 2006;231:802–805. [PubMed] [Google Scholar]
- 4.Parsson H, Holmberg A, Siegbahn A, Berggvist D. Activation of coagulation and fibrinolytic systems in patients with CLI is not normalized after surgical revascularization. Eur J Vasc Surg. 2004;27:186–192. doi: 10.1016/j.ejvs.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Comerota AJ. Endovascular and surgical revascularization for patients with intermittent claudication. Am J Cardiol. 2001;87(A):34D–43D. doi: 10.1016/s0002-9149(01)01674-5. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen HS, Rasmussen CS, Macko J. VEGF gene therapy for coronary artery disease and peripheral vascular disease. Cardiovasc Radiat Med. 2003;3:114–117. doi: 10.1016/s1522-1865(02)00158-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, Bi YY, Zan ZB, Ren H, Fang ZH, Yu XF, Poon MC, Han ZC. G-CSF-mobilized peripheral blood mononuclear cells from diabetic patients augment neovascularization in ischemic limbs but with impaired capability. J Thromb Haemost. 2006;4:993–1002. doi: 10.1111/j.1538-7836.2006.01906.x. [DOI] [PubMed] [Google Scholar]
- 8.Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2006;29:478–479. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 9.Tabata Y, Ikada Y. Vascularization effect of basic fibroblast growth factor released from gelatin hydrogels with different biodegradabilities. Biomaterials. 1999;20:2169–2175. doi: 10.1016/s0142-9612(99)00121-0. [DOI] [PubMed] [Google Scholar]
- 10.Gounis MJ, Spiga MG, Graham RM, Wilson A, Haliko S, Lieber BB, Wakhloo AK, Webster KA. Angiogenesis is confined to the transient period of VEGF expression that follows adenoviral gene delivery to ischemic muscle. Gene Ther. 2006;9:762–771. doi: 10.1038/sj.gt.3302481. [DOI] [PubMed] [Google Scholar]
- 11.Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY, Quyyumi AA. Basic fibroblast growth factor in patients with intermittent claudication: results in phase I trial. J Am Coll Cardiol. 2000;36:1239–44. doi: 10.1016/s0735-1097(00)00882-2. [DOI] [PubMed] [Google Scholar]
- 12.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomized trial. Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary artery disease. Circulation. 1998;97:645–50. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 14.Shyu KG, Chang H, Wang BW, Kuan P. Intramuscular vascular endothelial growth factor gene therapy in patients with chronic critical limb ischemia. AM J Med. 2003;114:85–92. doi: 10.1016/s0002-9343(02)01392-x. [DOI] [PubMed] [Google Scholar]
- 15.Cote MF, Laroche G, Gagnon E, Chevallier P, Doillon CJ. Denatured collagen as support for a FGF-2 delivery system: physicochemical characterizations and in vitro release kinetics and bioactivity. Biomaterials. 2004;25:3761–3772. doi: 10.1016/j.biomaterials.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Tabata Y, Yamada K, Miyamoto S, Nagata I, Kikuchi H, Aoyama I, Tamura M, Ikada Y. Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials. 1999;19:807–815. doi: 10.1016/s0142-9612(98)00233-6. [DOI] [PubMed] [Google Scholar]
- 17.Detillieux KA, Cattini PA, Karadami E. Beyond angiogenesis: the cardioprotective potential of fibroblast growth factor-2. Can J Physiol Pharmacol. 2004;82:1044–1052. doi: 10.1139/y04-126. [DOI] [PubMed] [Google Scholar]
- 18.Nugent MA, Iozzo RV. Fibroblast growth factor-2. Int J Biochem Cell Biol. 2000;32:115–120. doi: 10.1016/s1357-2725(99)00123-5. [DOI] [PubMed] [Google Scholar]
- 19.Iwakura A, Tabata Y, Koyama T, Doi K, Nishimura K, Kataoka K, Fujita M, Komeda M. Gelatin sheet incorporating basic fibroblast growth factor enhances steral healing after harvesting bilateral internal thoracic arteries. J Thorac Cardiovasc Surg. 2003;126:1113–1120. doi: 10.1016/s0022-5223(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 20.Efthimiadou A, Asimakopoulos B, Nikolettos N, Giatromanolaki A, Sivridis E, Papachristou DN, Kontoleon E. Angiogenic effect of intramuscular administration of basic and acidic fibroblast growth factor on skeletal muscles and influence of exercise on muscle angiogenesis. Br J Sports Med. 2006;40:35–39. doi: 10.1136/bjsm.2005.018754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layman H, Li S, Pham SM, Andreopoulos FM. Therapeutic angiogenesis in critical limb ischemia via the localized delivery of bFGF from ionic hydrogels. J Surg Res. 2006;130:265. [Google Scholar]
- 22.Huang NF, Yu Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11:1860–1866. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 23.Post MJ, Laham R, Sellke FW, Simons M. Therapeutic angiogenesis in cardiology using protein formulations. Cardiov Res. 2001;49:522–531. doi: 10.1016/s0008-6363(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 24.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 26.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 27.Raymond J, Metcalfe A, Desfaits AC, Ribourtout E, Salazkin I, Gilmartin K, Embry G, Boock RJ. Alginate for endovascular treatment of aneurysms and local growth factor delivery. AJNR Am J Neuroadiol. 2003;24:1214–1221. [PMC free article] [PubMed] [Google Scholar]
- 28.Pieper JS, Hafmans T, van Wachem PB, van Luyn MJ, Brouwer LA, Veerkamp JH, van Kuppevelt TH. Loading of collagen-heparan sulfate matricies with bFGF promotes angiogenesis and tissue regeneration in rats. J Biomed Mater Res. 2002;62:185–194. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 29.Dogan AK, Gumisderelioglu M, Aksoz E. Controlled release of EGF and bFGF from dextran hydrogels in vitro and in vivo. J Biomed Mater Res B Appl Biomater. 2005;74:504–510. doi: 10.1002/jbm.b.30231. [DOI] [PubMed] [Google Scholar]
- 30.Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterals. 2004;25:1289–1297. doi: 10.1016/j.biomaterials.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 31.Mould AP, Askari JA, Humphries MJ. Molecular basis of ligand recognition by integrin alpha-5 beta-1. I. Specificity of ligand biding is determined by amino acid sequences in the second and third NH2-terminal binding repeats of the alpha subunit. J Biol Chem. 2000;275:20324–20336. doi: 10.1074/jbc.M000572200. [DOI] [PubMed] [Google Scholar]
- 32.Rivard A, Silver M, Chen D, Kearney D, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikada Y, Tabata Y. Protein release from gelatin matricies. Adv Drug Deliv Rev. 1998;31:287–301. doi: 10.1016/s0169-409x(97)00125-7. [DOI] [PubMed] [Google Scholar]


