Abstract
Whereas structurally dissimilar D1 antagonists competing for [3H]-SCH23390 binding recognize primarily one site in striatum, two distinct affinity states are observed in both amygdala and hippocampus. The binding profile of SCH23390 is similar in both of these regions, with the high affinity site (KD ~0.4 nM) consistent with D1/D5 receptors. The appearance of the low affinity site (KD ~ 300 nM) is dependent upon the absence of MgCl2, but independent of D1 expression (i.e., still present in D1 knockout mice). Although the density of high affinity state receptor is lower in hippocampus or amygdala of D1 knockout mice, some residual binding remains, consistent with the known expression of D5 receptors in these regions. Remarkably, in hippocampus, the affinity of the low affinity site is shifted rightward in the presence of the D2 antagonist domperidone and is largely absent in the hippocampus of D2 knockout animals. Additionally, this site is also shifted rightward in the presence of the A2A ligands SCH58261, CSC, or NECA, or in the absence of A2A receptors. The affinity of SCH23390 for this low affinity site is greater than seen for SCH23390 binding to D2 receptors in heterologous expression systems, consistent with the hypothesis that both D2 and A2A receptors are involved in the low affinity binding site. Therefore, we suggest that the heteromerization of D2 and A2A receptors reported previously in vitro also may occur in the brain of both rats and mice.
Keywords: Dopamine receptor, Adenosine receptor, Knockout mice, Heteromerization, Radioreceptor binding
1. Introduction
Dopamine receptors comprise a subfamily of G protein-coupled receptors (GPCRs) encoded by five distinct genes (D1, D2, D3, D4, D5). Functionally, D1-like receptors (D1 and D5) are characterized by their ability to stimulate adenylate cyclase (AC) (Garau et al., 1978; Kebabian and Calne, 1979). Radio-receptor binding studies, autoradiographic, immunohistochemical, and in situ data clearly show that D1 receptors are present in the amygdala (Dawson et al., 1988; Huang et al., 1992; Hurd et al., 2001; Mansour et al., 1992; Savasta et al., 1986; Sunahara et al., 1990), yet D1-like receptors in the amygdala do not couple to activation of AC (Kilts et al., 1988; Leonard et al., 2003a,b; Mailman et al., 1986). Thus, the mechanisms by which D1 receptors signal in this region remain unknown.
The current work was sparked by the surprising observation that, in the amygdala, but not in the striatum, the D1/D5 antagonist SCH23390 recognizes two clearly different affinity states (Leonard et al., 2003b). SCH23390 has proven very useful in ascribing functions and/or behaviors to D1-like receptor activation due to its >500 fold D1:D2 selectivity and low affinity for most other neuroreceptors (see http://pdsp.cwru.edu/pdsp.asp). SCH23390 cannot, however, distinguish between D1 and D5 receptors. In the current work, we compare SCH23390 binding in the amygdala, striatum, and hippocampus to determine the nature of this unexpected low affinity SCH23390 binding site.
Of these three regions, the density of D1 receptors is highest in the striatum, followed by the amygdala, and then hippocampus, whereas the hippocampus contains the highest density of D5 receptors (Boyson et al., 1986; Montague et al., 2001). D1-like receptors are believed to perform diverse physiological roles in these regions. For example, in the striatum, D1-like receptors play a role in posture and the initiation of movement (Wang et al., 1998), whereas in the amygdala they modulate drug-reward and fear responses (Callahan et al., 1995; Greba and Kokkinidis, 2000). Hippocampal D1-like receptors participate in learning and memory, likely through modulation of cAMP synthesis (Matthies et al., 1997; Otmakhova and Lisman, 1996).
Recent work has shown that many GPCRs, including the dopamine receptors, may evoke physiological responses through interactions with other GPCRs. D1 receptors have been shown to interact with A1 adenosine and NMDA receptors (Franco et al., 2000; Lee et al., 2002), whereas D2 receptors may interact with somatostatin 2 (SST2) and A2A adenosine receptors (Franco et al., 2000; Rocheville et al., 2000). We hypothesized that such an interaction may be responsible for the low affinity binding of SCH23390 in the brain.
Dopamine D2 and adenosine A2A receptors have been shown to interact in an opposing manner on several levels. Behaviorally, antagonism of A2A receptors enhances D2 receptor-mediated locomotor activity (Latini et al., 1996). Biochemically, D2 receptor activation inhibits A2A agonist-activated cAMP accumulation (Hillion et al., 2002). The A2A agonist CGS21680 also decreases the affinity of D2 receptors for dopamine, although dopaminergic agonists have not been shown to have any effect on A2A ligand–receptor interactions (Diaz-Cabiale et al., 2001; Ferre et al., 1991). Recent evidence shows that D2 and A2A receptors colocalize within the same cells, and upon agonist treatment, coaggregate and cointernalize, suggesting the presence of D2/A2A heteromers (Hillion et al., 2002). BRET and FRET studies support this hypothesis, and in concert with chimeric receptor experiments, indicate that specific regions of the D2 receptor are critical for heteromerization (Canals et al., 2003). It appears that the heteromerization of A2A and D2 receptors may be responsible, at least in part, for the molecular and functional interplay of these two receptors. It is expected, however, that only a portion of the D2 or A2A receptor populations are heteromerized at any given time since both receptors perform a variety of functions when expressed independently in cells, or in mice in which one, or both, of the receptors was ablated (Chen et al., 2001).
D2 and A2A receptors colocalize in several brain regions, and both are highly enriched in the striatum (~600–900 fmol D2/mg protein). D2 receptors are expressed at moderate levels in the amygdala (~200–300 fmol/mg protein), and low levels in the hippocampus (~100 fmol/mg protein), whereas the A2A receptors are expressed at low levels in both regions. We now report that both D2 and A2A receptors may play a role in the low affinity binding of SCH23390 in the hippocampus, but not in the amygdala, and suggest that this binding may result from the physical interaction of D2 and A2A receptors.
2. Results
2.1. The absence of MgCl2 reveals a significant second affinity state in hippocampus and amygdala, but not striatum
Competition radioreceptor binding assays were performed in which unlabeled SCH23390 was used to compete for [3H]-SCH23390 binding sites (classically termed a “cold” saturation assay). Data from striatal tissue (Fig. 1A) were consistent with single-site kinetics reported by many laboratories including our own (Schulz et al., 1985). Conducting this experiment in the absence of added MgCl2 led to an increase in apparent receptor density (Fig. 1A) without a significant change in the affinity or kinetics of this antagonist (Fig. 1A and Table 1).
Fig. 1.
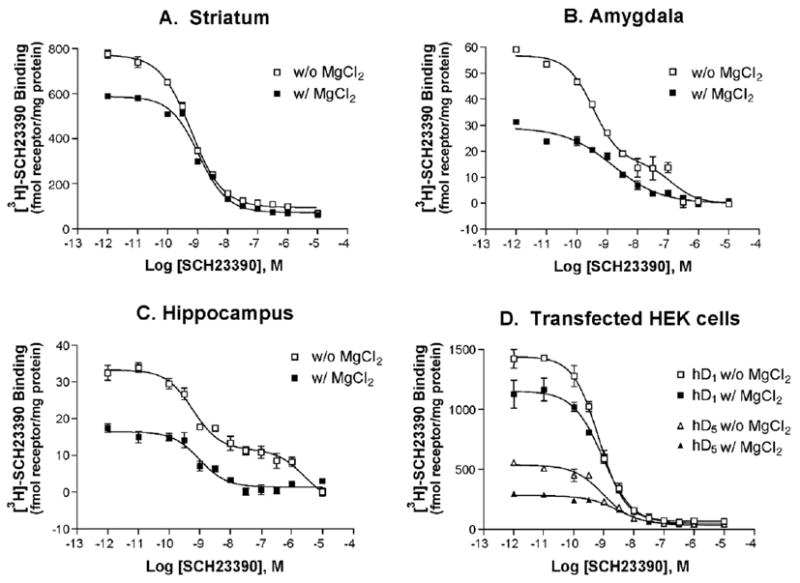
MgCl2 has dramatically different effects on affinity of SCH23390 binding in rat striatum and hD1 or hD5 transfected HEK cells versus rat amygdala or hippocampus. Panels A–D show representative SCH23390 vs. [3H]-SCH23390 competition binding experiments in the indicated tissues in the absence (open symbols) or presence (filled symbols) of 4 mM MgCl2. These data represent two (amygdala), three (hippocampus) or four (striatum) separate experiments with triplicate data. See Table 1 for quantification.
Table 1.
Competition binding of SCH23390 against [3H]-SCH23390 in the absence of MgCl2 demonstrates the presence of a low affinity binding site present in different amounts in different brain regions
| KH | RH | KL | n | ||
|---|---|---|---|---|---|
| Striatum | with MgCl2 | 0.28±0.09 | 90±2 | 45±26 | 4 |
| w/o MgCl2 | 0.22±0.03 | 92±3 | 135±56 | 4 | |
| Amygdala | with MgCl2 | 0.27±0.08 | 68±15 | 35±6 | 2 |
| w/o MgCl2 | 0.42±0.04 | 75±3 | 132±41 | 9 | |
| Hippocampus | with MgCl2 | 1.02±0.48 | 91±6 | 16±13 | 5 |
| w/o MgCl2 | 0.37±0.08 | 59±2 | 343±93 | 12 |
Data are presented as the mean± SEM. n is the number of experiments.
Abbreviations: KHKH—affinity constant for high affinity binding, RH—percentage of binding in the high affinity state (relative to the total binding), KL—affinity constant for low affinity binding.
Similar results were observed in amygdala and hippocampal tissue, but the appearance of complex binding kinetics in membranes prepared from these brain regions suggests multiple (or interactive) binding sites (Figs. 1B and C, and Table 1). MgCl2 in a concentration range of 2–10 mM is commonly used in pharmacological assays because it increases the affinity of agonists for GPCRs and is required for many functional effects. In the presence of 4 mM MgCl2, competition binding experiments in tissue from both the amygdala and hippocampus revealed shallow binding curves with Hill slopes much less than 1, suggestive of more than one binding site. In the absence of added MgCl2, the difference between the affinities of the two sites is amplified and the data clearly demonstrate the presence of an additional low affinity binding component.
In order to determine if either the greater apparent density of binding sites or the appearance of the low affinity binding site (s), in the absence of MgCl2, is characteristic of D1 or D5 receptors alone, we examined the binding of SCH23390 in HEK cells transiently transfected with D1 or D5 cDNA. As was observed in brain tissue, the apparent receptor density was markedly higher in the absence of MgCl2. Conversely, the affinity of SCH23390 at D1 and D5 receptors was unaffected by MgCl2 (Fig. 1D).
2.2. The effects of MgCl2 on apparent receptor density vary by brain region
We next compared the effects of MgCl2 quantitatively since the striatum, hippocampus, and amygdala contain different total and relative expression levels of D1 and D5 receptors. As shown in Fig. 2, the absence of added MgCl2 caused a greater increase in SCH23390 binding in hippocampus (122%) than in amygdala (68%) or striatum (33%). In D1 and D5 expressing cell lines, there was a 25% increase in hD1 binding, whereas there was a 97% increase in hD5 binding in the absence of MgCl2. This effect is not specific to MgCl2 as 4 mM MgSO4 or CaCl2 had the same effect on total specific binding. MgSO4 and CaCl2 decreased the percentage of sites in the low affinity state, without completely precluding the appearance of the low affinity site in hippocampus, unlike the effect observed with the addition of MgCl2 (data not shown).
Fig. 2.
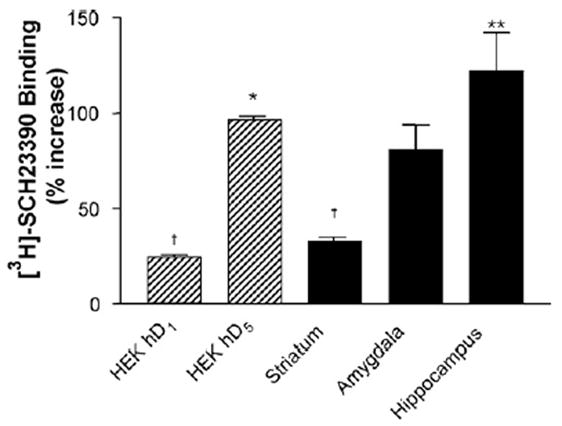
D1 and D5 receptors appear to be affected differently by the absence of MgCl2. Data reflect the average increase of maximal [3H]-SCH23390 binding in the absence compared to in the presence of 4 mM MgCl2 of n =2 (amygdala, HEK hD1, HEK hD5), n =3 (hippocampus), or n =4 (striatum) replicates. * Indicates significant difference vs. HEK hD1, p <0.05, **p<0.01; † indicates significant difference vs. HEK hD5, p<0.05, as determined by one-way ANOVA and Dunnett post hoc analysis.
2.3. Structurally diverse D1 antagonists recognize multiple affinity sites in the amygdala
As we have previously reported, the two-site binding is not unique to SCH23390, but also is seen with a number of other D1 antagonists (Leonard et al., 2003a,b). As shown in Fig. 3 and Table 2, competition binding experiments with a number of antagonists versus [3H]-SCH23390 yielded shallow binding curves that could be fitted well by a two-site model. These compounds were chosen because they are all D1 receptor antagonists, yet bind to other neuroreceptors with markedly different affinities (e.g., see http://kidb.bioc.cwru.edu/pdsp.php). Detailed binding statistics are shown in Table 2. There was no significant difference in the percentage of receptor in the high affinity state (RH) with each of the competing ligands (p =0.176), and these sites were not guanine nucleotide sensitive. The affinity of each of the ligands in striatum was consistent with that of the high affinity state in the amygdala, except in the case of clozapine. Whereas the affinity of clozapine in striatum was in agreement with published reports, the affinity in amygdala was much higher, but we were unable to reconcile this observation with what is known about the affinity of SCH23390 and clozapine for other receptors.
Fig. 3.
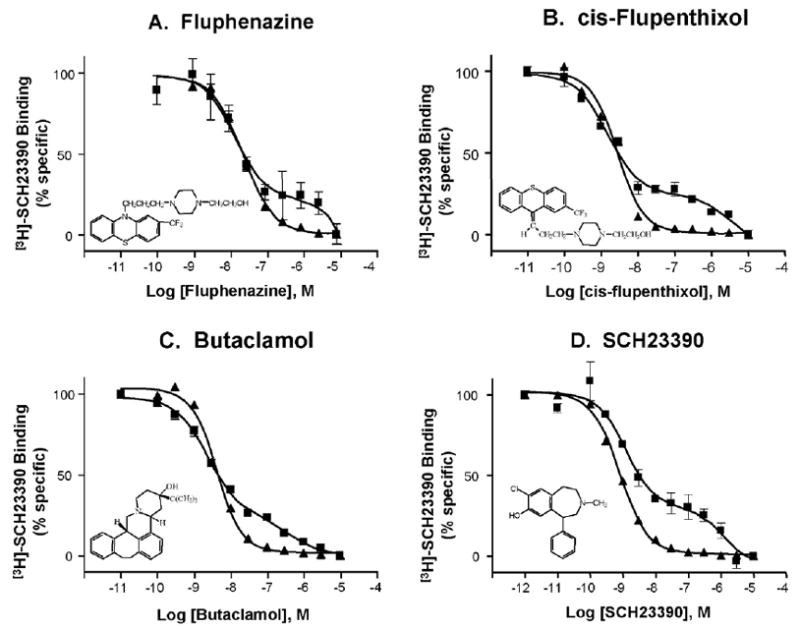
Competition binding of structurally diverse D1 antagonists versus [3H]-SCH23390 in the absence of MgCl2 reveals a low affinity binding site in amygdala (squares) but not in the striatum (triangles). Representative data are shown here, and Table 2 contains complete binding statistics. In each panel, the solid triangles (steeper curve) always represent striatum, and solid squares (shallow, biphasic curve) always represent amygdala.
Table 2.
Competition binding with several D1 receptor antagonists against [3H]-SCH23390 in the absence of MgCl2 reveals a low affinity binding site in amygdala but not in the striatum
| Amygdala
|
Striatum
|
|||
|---|---|---|---|---|
| KH | RH | KL | KD | |
| Butaclamol | 1.6±0.5 (4) | 81±3 | 329±93 | 3.3±0.9 (5) |
| Clozapine | 8.3±5.0 (3) | 44±11 | 538±267 | 114±38 (2) |
| Chlorpromazine | 8.6±4.2 (3) | 54±23 | 427±349 | 19.7±1.9 (3) |
| Fluphenazine | 11.6±4.1 (7) | 71±8 | 1600±440 | 14.8±2.2 (7) |
| cis-Flupenthixol | 1.1±0.4 (4) | 71±2 | 874±251 | 1.7±0.6 (4) |
| Thioridazine | 27.5±3.3 (6) | 72±6 | 947±374 | 43.3±7.2 (6) |
Affinity values for each ligand were determined with one- (striatum) or two-site (amygdala) binding analysis in Prism v. 3.0. Data are presented as the mean±SEM of n experiments. RH values were not significantly different between competing ligands (p=0.176).
2.4. D1 and D5 receptors are not required for the low affinity binding site
We tested the hypothesis that these low affinity binding sites in the hippocampus and amygdala were not dependent upon the D1 receptor by conducting competition binding experiments in mice lacking D1 receptors. These experiments indicated that the density of receptors in the high affinity state was lower than in wildtype mice, consistent with the absence of D1 receptors (Fig. 4, left panels), but that SCH23390 still binds to the hippocampus and amygdala of D1 knockout mice at two different affinities (Fig. 4, right panels). The quantification of these data is shown in Table 3.
Fig. 4.
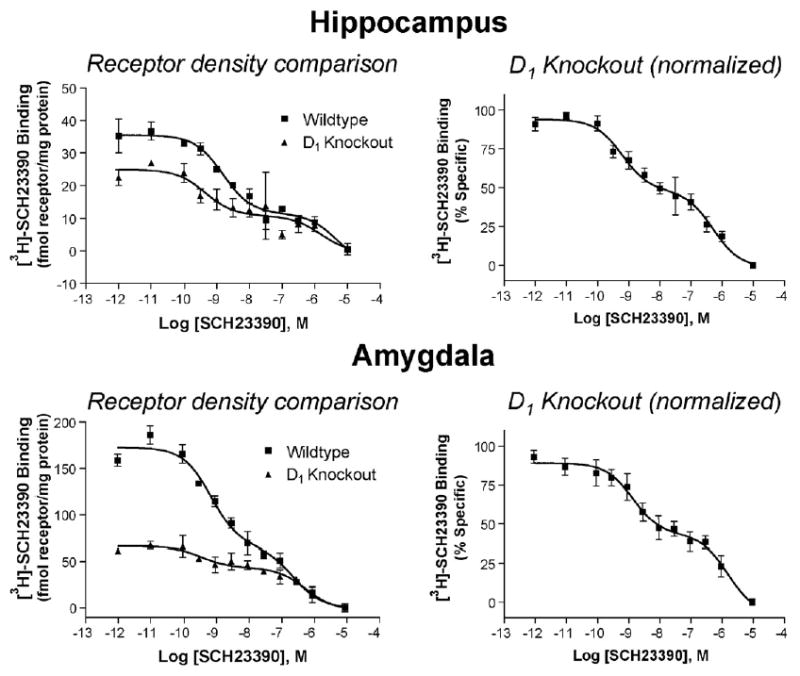
Two affinity sites are still apparent in competition binding of SCH23390 vs. [3H]-SCH23390 in hippocampus and amygdala of D1 knockout mice. Left: representative data presented in fmol receptor per mg total protein for comparison of receptor density. Right: normalized binding from three separate experiments run in triplicate. See Table 3 for binding affinities.
Table 3.
Competition binding of SCH23390 vs. [3H]-SCH23390 in hippocampus and amygdala of mice not expressing D1, D2, or D5 receptors
| Hippocampus
|
Amygdala
|
|||||
|---|---|---|---|---|---|---|
| KH | RH | KL | KH | RH | KL | |
| WT | 0.59±0.08 (6) | 64±4 | 451±90 | 0.30±0.03 (3) | 71±5 | 200±70 |
| D1KO | 0.39±0.13 (4) | 47±6 | 269±90 | 0.45±0.30 (3) | 48±9 | 411±120 |
| D2KO | 0.32±0.04 (3) | 75±14 | >10,000* | 0.18±0.04 (3) | 71±3 | 115±50 |
| D5KO | 0.32±0.07 (3) | 41±2 | 302±75 | N.D. | N.D. | N.D. |
Data are presented as the mean±SEM of n experiments.
Indicates significant difference as compared to WT in one-way ANOVA with Dunnett post hoc analysis, no other values were found to be significantly different from WT.
To determine if the low affinity site required the presence of D5 receptors, similar experiments were conducted in the hippocampus of D5 knockout mice. Interestingly, not only were two sites still seen, but the percentage of low affinity sites labeled by SCH23390 was actually increased in the absence of D5 receptors (Fig. 5). These data suggested that the low affinity site was not the D1 or D5 receptor itself, yet is a site that can bind D1-like ligands.
Fig. 5.
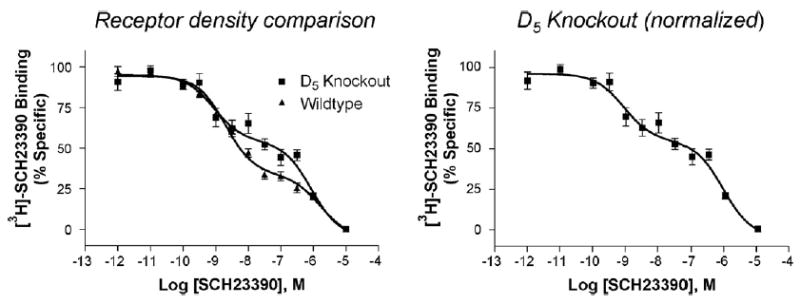
Competition binding of SCH23390 vs. [3H]-SCH23390 in hippocampus of D5 knockout mice reveals a statistically significant increase in the proportion of receptor in the low affinity state. Significance determined by two-tailed t test, p=0.006. Left: comparison of binding in WT (n=6) vs. D5 knockout (n =3) mice. Total specific binding was not significantly different between WT and D5 knockout. Right: normalized binding from duplicate samples in three separate experiments in D5 knockout mice. Curves represent approximately 50–60 fmol receptor/mg protein. See Table 3 for binding affinities.
2.5. The role of 5-HT, D2, and A2A receptors in the low affinity binding site
In order to investigate the identity of the low affinity site, we masked candidate receptors with an appropriate high affinity ligand and then conducted competition binding as done previously. SCH23390, although a relatively selective ligand, has affinity for several serotonin receptors. The presence of serotonin (50 nM), however, did not affect either the affinity, or relative quantity, of receptor in the low affinity population (data not shown). In addition, the presence of ketanserin (100 nM) or mianserin (100 nM) did not have any effect on the low affinity site seen in the SCH23390 competition binding, although the presence of these 5-HT2A&C receptor antagonists did reduce the amount of binding in the high affinity state (Fig. 6A). This observation is consistent with reports that both ketanserin and mianserin have moderate affinity for D1-like receptors and that a portion of the high affinity SCH23390 binding in hippocampus may include binding to 5-HT2C receptors (Nicklaus et al., 1996).
Fig. 6.
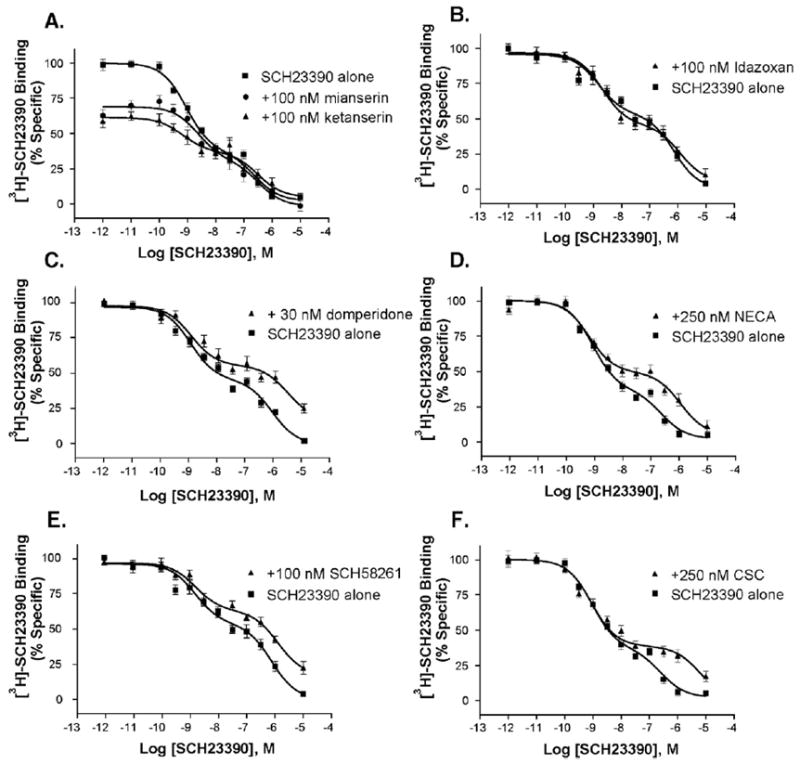
In rat hippocampus, the low affinity binding of SCH23390 is not disrupted by the presence of ketanserin or mianserin (A), and neither site is affected by the α2 adrenergic receptor antagonist idazoxan (B). In contrast the low, but not the high, affinity binding of SCH23390 is modulated by the presence of the D2 antagonist domperidone (C), the A2A receptor agonist NECA (D), and the A2A antagonists SCH58261 and CSC (E and F). The indicated concentration of drug was incubated in each tube simultaneously with the SCH23390 competition binding reaction, and each was run in triplicate, three times.
Another candidate we investigated was the D2 receptor, which has micromolar affinity for SCH23390, and whose affinity can be affected by modulating proteins. In particular, adenosine A2A receptors can interact with D2 receptors (Franco et al., 2000), and agonist binding to A2A receptors affects the affinity of D2 receptors for dopamine (Diaz-Cabiale et al., 2001; Ferre et al., 1991). We thus sought to test the hypothesis that D2 or A2A receptor occupation would modulate SCH23390 binding to the low affinity state. In these experiments, we conducted SCH23390 competition binding in the presence of the high affinity D2 antagonist domperidone (30 nM), the high affinity A2A antagonist SCH58261 (100 nM), the lower affinity A2A antagonist CSC (250 nM), or the A2A agonist NECA (250 nM). These compounds were compared to a control compound, idazoxan (100 nM), that is a high affinity α2A adrenergic antagonist. As is shown in Fig. 6, there was a decrease in affinity of the low affinity state when either D2 or A2A receptors were occupied. Conversely, idazoxan had no effect. SCH58261 did not compete for [3H]-SCH23390 labeled HEK hD1 receptors or for [3H]-spiperone labeled CHO-D2L receptors with significant affinity, suggesting that this high affinity A2A antagonist was not directly modulating D1 or D2 receptors (Fig. 7).
Fig. 7.
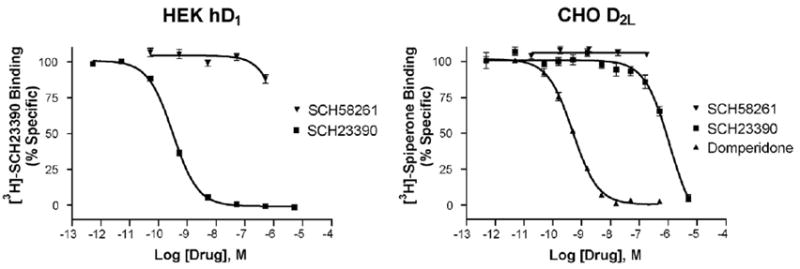
SCH58261 has negligible affinity for D1 and D2 receptors as demonstrated by competition binding with SCH23390 and SCH58261 vs. [3H]-SCH23390 in HEK hD1 membranes (left) or domperidone, SCH23390, and SCH58261 vs. [3H]-spiperone in CHO-D2L membranes (right); n =3. Affinities in nM: D1: SCH23390 0.29±0.01, SCH58261 3050±700; D2: domperidone 0.51±0.03, SCH23390 920±170, SCH58261>10,000.
To explore further the role of D2 receptors in this phenomenon, we examined the binding of SCH23390 in D2 knockout mice. Strikingly, there was a sharp reduction in the percentage of SCH23390 binding to the low affinity state in the hippocampus of D2-null mice (Fig. 8A). In contrast, SCH23390 binding was not altered in the amygdala of D2 knockout mice (Fig. 8B). In similar experiments with hippocampus tissue from A2A knockout mice, the affinity of the low affinity site was shifted rightward considerably, whereas the high affinity site was unchanged as compared to their wildtype littermates (Fig. 9). Ligand occupation of A2A receptors, or the absence of A2A receptors in A2A knockout mice, resulted in an affinity for the low affinity site consistent with that of SCH23390 for D2 receptors in transfected cells.
Fig. 8.
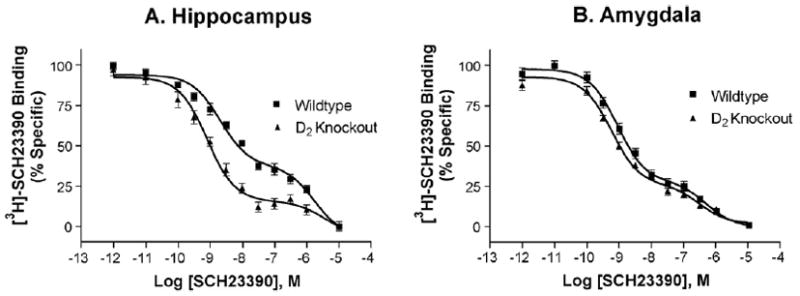
The low affinity binding of SCH23390 is almost completely lost in D2 knockout mice but is unaffected by the lack of D2 receptors in amygdala of D2 knockout mice. (A) Normalized binding from three separate experiments run in triplicate with hippocampus from D2 knockout mice. (B) Normalized binding from three separate experiments run in duplicate with amygdala from D2 knockout mice.
Fig. 9.
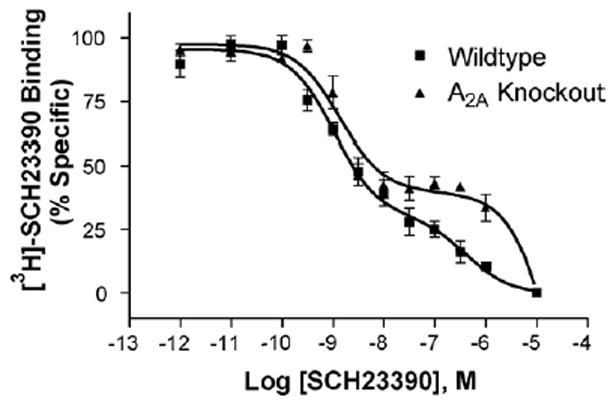
In the hippocampus of A2A receptor knockout mice, the high affinity SCH23390 binding site is unchanged whereas the low affinity site is shifted rightward dramatically. Wildtype KH 0.34±0.10, KL 162±42, RH 67±8; A2A knockout KH 0.42±0.01 KL 1322±255*, RH 50±4 (n =3). * Indicates p<0.005 as compared to WT in two-tailed unpaired t test.
3. Discussion
3.1. MgCl2 decreases maximal D1-like receptor binding
Whereas studies have found that Mg2+ increases agonist affinity for GPCRs (and is required for functional activation), it has not been reported to affect either the total binding or affinity of antagonists. In contrast to the finding of Schetz and Sibley (1997) that 4 mM MgCl2 had no effect on D1 receptor binding density in transfected CHO cells, we found an increase in receptor density in the absence of added MgCl2 in all three brain regions we studied, as well as in D1 or D5 transfected HEK cells. As a percentage of total binding, the increase we observed was much greater for hD5 than for hD1 receptors, indicating that D5 receptors may be more sensitive to MgCl2, at least in this cell background. Accordingly, brain regions with a greater ratio of D5:D1 receptors showed greater increases in binding, such that the hippocampus showed the greatest percent increase, followed by amygdala, and finally striatum (Ciliax et al., 2000; Khan et al., 2000; Montague et al., 2001). An alternate interpretation of these data is that the increase in binding in the absence of MgCl2 is related to the absolute amount of D1-like receptor in the region, instead of different D1 or D5 receptor sensitivities. This interpretation is also consistent with our data in that the actual increase in binding was highest in the striatum followed by the amygdala and then the hippocampus (i.e., paralleling the rank order of D1 and D5 receptor expression in these brain regions).
MgCl2 has the greatest effect in reducing the percentage of receptors in the low affinity state, although the other salts tested (MgSO4, CaCl2) reduced the percentage of receptors in the low affinity state without completely eliminating the latter (data not shown). This suggests that both magnesium and chloride ions may influence binding to the low affinity site.
3.2. Multiple SCH23390 binding sites in the hippocampus and amygdala
The shapes of the competition curves of a number of antagonists against [3H]-SCH23390 are significantly different between striatum and amygdala. As expected, the competition curves in striatum had kinetics consistent with interaction primarily at a single population of binding sites. Conversely, in amygdala, competition of several antagonists consistently gave concentration–response curves that were “shallow” (i.e., nH <1) relative to those in striatum. When these competition binding experiments were performed in the amygdala in the absence of MgCl2, analysis of the data using non-linear regression clearly indicated that a model with two sites fit the data better than a one-site model.
The absence of MgCl2 allowed for the separation of a low affinity SCH23390 binding site from the well-described high affinity D1 receptor site in amygdala and hippocampus, but not in D1 or D5 expression systems. Upon careful examination, a small portion of low affinity binding was also noted in striatum. As noted, GPCR–ligand binding experiments are typically conducted in the presence of ≥1 mM MgCl2 because Mg2+ increases agonist affinity. In blood and cerebrospinal fluid, the free concentration of Mg2+ is 0.5–1.0 mM and may be higher intracellularly or in the local region of the receptor (Blair et al., 1989; Jeong et al., 2006). Our remaining experiments were conducted in the absence of MgCl2 because the low affinity state we observed was easily distinguished from the high affinity site under these conditions.
3.3. Low affinity binding of SCH23390 does not require D1 or D5 receptors
Whereas SCH23390 is widely known as a selective D1-like receptor antagonist, the differences we have demonstrated between SCH23390 binding sites in the amygdala and hippocampus versus the striatum do not implicate the D1 or D5 receptor or complexes involving either of these receptors in the low affinity binding site. Indeed, we have shown that this site is not due to an additional mode of binding at D1 receptors since the presence of D1 receptors is not required for SCH23390 binding to this low affinity site. The remaining high affinity binding in the hippocampus and amygdala of D1 knockout mice is probably due to binding to the D5 receptor and is proportional to the known expression of D5 receptors in these regions. Furthermore, the low affinity SCH23390 binding site remains in the hippocampus of D5 knockout mice. The independence of the low affinity state from D1 and D5 receptors could be determined conclusively with similar experiments conducted in tissue from D1/D5 double knockout animals, though these are not yet available.
3.4. Low affinity binding of SCH23390 appears to involve both D2 and A2A receptors
The percentage of binding sites in each affinity state was found to be dependent on the brain region and the competing drug. When SCH23390 was used to compete against [3H]-SCH23390 in rat hippocampus, amygdala, or striatum, 59, 74, or 92% of the binding, respectively, was in the high affinity state. Although the percentage of sites in the low affinity state is very low in the striatum, a two-site analysis was warranted because of the presence of this site in both the amygdala and hippocampus. We note the general guideline, however, that at least 20% of the sites should be in each of the affinity states in order to resolve accurately two sites. Nonetheless, the actual estimated amount of receptor in the low affinity state is directly proportional to D2-like receptor density in these regions (Boyson et al., 1986). In addition, we have shown that the presence of the low affinity state in hippocampus appears to require the presence of available D2 receptors. In the hippocampus, either a D2 antagonist or the absence of D2 receptors reduced the low affinity SCH23390 binding site. In contrast, in the amygdala, the binding of SCH23390 is unaltered by the absence of D2 receptors, and thus the identity of this site remains to be determined. It is plausible, however, that D3 receptors compensate for the lack of D2 receptors in the amygdala of D2 knockout mice. In fact, a recent report demonstrated that D3 and A2A receptors interact in a similar manner as do D2 and A2A receptors (Torvinen et al., 2005).
Despite the apparent dependence of the low affinity site on the presence of D2 receptors in hippocampus, this is not simply a “free-standing” D2 receptor. Whereas D2-like receptors appear to be involved, the reported affinity of SCH23390 for D2 receptors, as well as those determined in our experiments in hD2L expressing CHO cells, is much lower (~1 μM) than the affinity we observed in brain. These data suggest that the affinity of SCH23390 for D2-like receptors in amygdala and hippocampus may be altered by a D2-receptor associated protein. Several proteins have been discovered recently that bind to and modify the function of D2-like receptors and could be responsible for this change (Binda et al., 2002; Griffon et al., 2003; Jeanneteau et al., 2004). Additionally, D2 and A2A receptors have been shown to interact behaviorally, biochemically, and physically. One consequence of this interaction is manifest in the observation that CGS21680, an A2A receptor agonist, can decrease the affinity of dopamine for D2 receptors (Diaz-Cabiale et al., 2001; Ferre et al., 1991).
Our observation that ligands of both D2 and A2A receptors alter the low affinity SCH23390 binding site is in accordance with these previous observations and suggests a mode of interaction whereby the binding of SCH23390 to D2 receptors is modulated by physical interaction with A2A receptors. The A2A–D2 interaction could result in a modified D2 receptor binding site with greatly increased affinity for SCH23390. This hypothesis is supported by the almost complete absence of the low affinity site in the hippocampus of mice lacking the D2 receptor and by the striking shift in affinity in A2A knockout mice.
If this site is truly due to a modulation of D2 receptor binding by interaction with A2A receptors, it represents the largest dimerization-influenced change in pharmacology in native tissue to date and provides a method whereby it may be possible to quantify the amount of heteromerized A2A–D2 receptors in relation to their total respective expression levels. Though we are unable to provide an accurate estimate of the amount of receptors bound by SCH23390 at the low affinity site, the relative amount of binding in the low affinity site suggests that only a small percentage of D2 receptors are heteromerized, and therefore modified by A2A receptors, at any given time. In striatum, where both A2A and D2 receptors are enriched, we found that less than 10% of the total bound receptor was in this state. Despite the fact that this reflects the relative affinities of the two sites, as well as the relative abundance of the two sites, this estimate supports the recent assertion of Chen et al. (2001) that A2A receptors function independently of D2 receptors although they also modulate D2 receptor signals.
Our data provide insight into the A2A receptor-mediated allosteric modulation of D2 receptor binding. Others have investigated the regions of the two receptors (D2 and A2A) that interact to modulate the pharmacology of the D2 receptor through chimeric approaches (Canals et al., 2003; Torvinen et al., 2004). Together, these approaches may lay the foundation for modeling of the A2A-modified D2 receptor pharmacophore.
3.5. Implications
These findings have clear implications for ongoing research of dopamine receptor pharmacology. In the majority of D1-like receptor studies, SCH23390 inhibition of function is considered evidence that the function is D1-like receptor-mediated (Bourne, 2001; Caine et al., 1995). As we have shown, however, SCH23390 is binding to at least one site in addition to D1/5 receptors. Classic D1-like agonists (dopamine, SKF-38393) have been demonstrated to activate IP3 turnover through Gαq even in D1 knockout animals (Friedman et al., 1997; Jin et al., 1998, 2001; Undie and Friedman, 1992). This function has been attributed to an as-of-yet unidentified D1-like receptor based on data that indicate that the site is blocked by high concentrations of SCH23390. It is clear that the low affinity binding of SCH23390 demonstrated here is independent of D1 receptors, however, it is intriguing to hypothesize that the low affinity SCH23390 binding may provide an alternate explanation for the previously unidentified receptor linked to activation of PI hydrolysis. Whether or not this is true, our findings present clear caution that pharmacological tools are at best selective, but never specific, over a broad concentration range.
4. Experimental procedures
4.1. Materials
Chlorpromazine and fluphenazine (GlaxoSmithKline, Philadelphia, PA); clozapine and thioridazine (Novartis Inc., East Hanover NJ); and piflutixol (Lundbeck GmbH & Co., Hamburg Germany) were received as gifts from the original manufacturers. CSC and NECA were a gift from Dr. Kenneth A Jacobson (NIDDK, NIH, Bethesda, MD). The following drugs were purchased from RBI/Sigma Inc. (St. Louis, MO): butaclamol; SCH23390; idazoxan; ketanserin; mianserin; and SCH58261. HEPES (N-2-hydroxy-ethylpiperazine-N-2-ethylsulfonic acid) was obtained from Research Organics Inc. (Cleveland, OH). Magnesium chloride hexahydrate, calcium chloride dihydrate, and magnesium sulfate were purchased from Mallinckrodt (Mallinckrodt Laboratory Chemicals, Phillipsburg, NJ). [3H]-SCH23390 was synthesized by a previously published method (Wyrick and Mailman, 1985). All other compounds and reagents were purchased from standard commercial sources.
4.2. Cell transfection and culture
Human D1 and D5 receptors were cloned from a HeLA cDNA library. The constructs were amplified by PCR and ligated into the pcDNA3 vector containing a hemagglutinin tag (YPYDVP-DYA). The receptor DNAs were expressed in HEK-293 cells according to the Lipofectamine protocol (GibcoBRL-Life Technologies) using 0.5 μg (hD1) or 0.75 μg (hD5) DNA per 100 mm culture dish of HEK cells at 80% confluency. Transfected plates of HEK cells were harvested 48 h after transfection. Cells were rinsed in ice-cold PBS and lysed at 4 °C for 10 min using 3 mL of hypo-osmotic buffer (10 mM HEPES, pH 7.4 with KOH). Cells were lifted with a cell scraper, and the resulting membranes were washed three times before being resuspended in storage buffer (20 mM HEPES, 250 mM sucrose, pH 7.4 with KOH). One milliliter aliquots were flash frozen and stored at −80 °C until use in binding assays. Stably transfected CHO hD2 cells were grown and harvested as previously described (Gay et al., 2004).
4.3. Knockout animals
Breeding stocks of both D1 (Drago et al., 1994) and D2 (Jung et al., 1999) knockout mice were obtained and were bred in a C57/ BLJ6 background while housed in an IACUC approved facility. All procedures were reviewed and approved by the UNC-CH Institutional Animal Care and Use Committee. Both male and female adults were sacrificed by cervical dislocation, and brains were removed for immediate dissection. Whole brains from D5 knockout mice (Holmes et al., 2001) were a gift from Dr. David Sibley at NINDS, Bethesda, Maryland. Hippocampus from A2A knockout mice (Chen et al., 1999) and wildtype littermates were the kind gift of Dr. Jiang-Fan Chen and Michael Schwarzschild of the Boston University School of Medicine, Boston, MA.
4.4. Brain dissection
Frozen rat brains (Pel-Freeze Biologicals, Rogers, AR) were thawed in saline at 4 °C, and the amygdala, striatum, and hippocampus were removed as described below. No differences were detected when frozen brain tissue was compared to fresh tissue from adult male Sprague–Dawley rats obtained from Charles River Laboratories (Wilmington, MA, USA). Brains were sliced in a 0.8 mm brain block (Heffner et al., 1980). Whole amygdala was microdissected with a 1.5 mm punch (Leonard et al., 2003a,b; Palkovits and Brownstein, 1983). Striatum and hippocampus regions were removed from slices according to the atlas of Paxinos and Watson (1986). Tissue was used immediately or stored at −80 °C until the day of the assay.
4.5. Radioreceptor competition assays
The competition assays were performed in 50 mM HEPES buffer, pH adjusted to 7.4 with KOH. Briefly, tissue was homogenized in buffer using a Teflon-glass homogenizer (eight manual strokes) and centrifuged for 15 min at 27,000×g. The tissue pellet was resuspended in ice-cold buffer at a final concentration of 10 mg/ mL wet weight. Non-specific binding of [3H]-SCH23390 (ca. 0.85 nM) was defined using unlabeled SCH23390 (10 μM). Assay tubes contained a total volume of 1 mL; 50 μL labeled ligand, 100 μL competing ligand or buffer; 100 μL tissue homogenate (0.45–0.65 mg protein/mL as measured by the BCA method (Pierce, Rockford, IL)); and 750 μL buffer. Assay tubes were incubated for 15 min at 37 °C, and the binding was terminated by filtering with 15 mL of ice-cold buffer on a cell harvester (Skatron, Inc., Sterling, VA). Radioactivity was quantified using an LKB-1219 liquid scintillation counter. In experiments comparing the effects of MgCl2, MgSO4, or CaCl2, 4 mM of the indicated salt was included in the binding buffer.
4.6. Data analysis
Radioreceptor data were analyzed by non-linear regression using Prism Version 3.02 (GraphPad Inc., San Diego CA). Initial analysis used a sigmoidal model with variable slope. Curves were concluded to represent one population of sites when the data were consistent with normal steepness (i.e., 0.85<nH <1.15), and when there was no evidence by visual inspection of a second affinity site of low density. In the remaining cases, the data were resolved using a two-site model, although it was recognized that even more complex models could not be excluded. In all cases, the analysis was not accepted as valid if the plotted curves of the regressed lines did not incorporate essentially all of the data points. Significant differences between group means were analyzed as appropriate with InStat version 3.05 (GraphPad Inc., San Diego CA), and the particular methods are specified in the individual figure legends. KD values were calculated from the IC50 values in the radioreceptor experiments using the Cheng and Prusoff (1973) equation for a single populations of competitive sites, , where R* is the concentration of radioligand used in each experiment, and KD is equal to 0.4 nM (the historical value for this laboratory). It should be noted that Prism supposes a single KD in its calculation of KL from resolved IC50s. In the present work, it is likely that the KD of SCH23390 actually is higher than 0.4 nM, and this would result in a slight underestimation of the true KL.
Acknowledgments
The authors would like to thank Daniel Mooney and John M. Pettito for making the initial observations in this work (Ann. N.Y. Acad Sci., 985: 1–4, 2003), Dr. David Sibley (NINDS, Bethesda, MD) for providing D5 receptor knockout tissue, and Dr. Michael Schwarzschild (Massachusetts General Hospital, Charlestown, MA) and Dr. Jiang-Fan Chen (Boston University School of Medicine, Boston, MA) for providing A2A receptor knockout tissue. This work was supported by NIH grant MH40537 and Training grant GM07040.
Abbreviations
- AC
adenylate cyclase
- cAMP
cyclic AMP, adenosine 3′ 5′-cyclic monophosphate
- CGS21680
2-[4-(2-carboxyethyl)-phenylethylamino]-5′N-ethyl-carboxamido-adenosine
- CHO
Chinese Hamster Ovary cell-line
- CSC
8-(3-chlorostyryl)caffeine
- GPCR
G protein-coupled receptor
- HEK
Human Embryonic Kidney cell-line
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IC50
concentration inhibiting 50% of total binding
- K0.5
concentration corrected
- IC50
(apparent affinity constant) when nH ≠ 1.0
- KH
affinity constant for high affinity state
- KI
affinity constant (nH =1.0)
- KL
affinity constant for low affinity state
- NECA
5′-N-ethylcarboxamide-adenosine
- nH
Hill coefficient
- RH
relative amount (percentage) of receptor in the high affinity state
- SCH23390
7-chloro-8-hydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- SCH58261
7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3e]-1,2,4-triazolo[1,5c]pyrimidine
References
- Binda AV, Kabbani N, Lin R, Levenson R. D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol. Pharmacol. 2002;62:507–513. doi: 10.1124/mol.62.3.507. [DOI] [PubMed] [Google Scholar]
- Blair JL, Warner DS, Todd MM. Effects of elevated plasma magnesium versus calcium on cerebral ischemic injury in rats. Stroke. 1989;20:507–512. doi: 10.1161/01.str.20.4.507. [DOI] [PubMed] [Google Scholar]
- Bourne JA. SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Rev. 2001;7:399–414. doi: 10.1111/j.1527-3458.2001.tb00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Bryan SK, Cunningham KA. Discriminative stimulus effects of cocaine: antagonism by dopamine D1 receptor blockade in the amygdala. Pharmacol Biochem Behav. 1995;51:759–766. doi: 10.1016/0091-3057(95)00027-t. [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods AS, Ferre S, Lluis C, Bouvier M, Franco R. Adenosine A2A–dopamine D2 receptor–receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem. 2003;278:46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink JS, Low MJ, Ongini E, Schwarzschild MA. The role of the D2 dopamine receptor (D2R) in A2A adenosine receptor (A 2AR)-mediated behavioral and cellular responses as revealed by A2A and D2 receptor knockout mice. Proc Natl Acad Sci U S A. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. Dopamine D5 receptor immunolocalization in rat and monkey brain. Synapse. 2000;37:125–145. doi: 10.1002/1098-2396(200008)37:2<125::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Barone P, Sidhu A, Wamsley JK, Chase TN. The D1 dopamine receptor in the rat brain: quantitative autoradiographic localization using an iodinated ligand. Neuroscience. 1988;26:83–100. doi: 10.1016/0306-4522(88)90129-7. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Hurd Y, Guidolin D, Finnman UB, Zoli M, Agnati LF, Vanderhaeghen JJ, Fuxe K, Ferre S. Adenosine A2A agonist CGS 21680 decreases the affinity of dopamine D2 receptors for dopamine in human striatum. NeuroReport. 2001;12:1831–1834. doi: 10.1097/00001756-200107030-00014. [DOI] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A. 1994;91:12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A. 1991;88:7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Ferre S, Agnati L, Torvinen M, Gines S, Hillion J, Casado V, Lledo PM, Zoli M, Lluis C, Fuxe K. Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacology. 2000;23:S50–S59. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol. 1997;51:6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- Garau L, Govoni S, Stefanini E, Trabucchi M, Spano PF. Dopamine receptors: pharmacological and anatomical evidences indicate that two distinct dopamine receptor populations are present in rat striatum. Life Sci. 1978;23:1745–1750. doi: 10.1016/0024-3205(78)90102-9. [DOI] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol. 2004;66:97–105. doi: 10.1124/mol.66.1.97. [DOI] [PubMed] [Google Scholar]
- Greba Q, Kokkinidis L. Peripheral and intraamygdalar administration of the dopamine D1 receptor antagonist SCH 23390 blocks fear-potentiated startle but not shock reactivity or the shock sensitization of acoustic startle. Behav Neurosci. 2000;114:262–272. doi: 10.1037//0735-7044.114.2.262. [DOI] [PubMed] [Google Scholar]
- Griffon N, Jeanneteau F, Prieur F, Diaz J, Sokoloff P. CLIC6, a member of the intracellular chloride channel family, interacts with dopamine D(2)-like receptors. Brain Res Mol Brain Res. 2003;117:47–57. doi: 10.1016/s0169-328x(03)00283-3. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS. A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav. 1980;13:453–456. doi: 10.1016/0091-3057(80)90254-3. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115:1129–1144. [PubMed] [Google Scholar]
- Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M. Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci U S A. 1992;89:11988–11992. doi: 10.1073/pnas.89.24.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Suzuki M, Sedvall GC. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat. 2001;22:127–137. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Diaz J, Sokoloff P, Griffon N. Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol Biol Cell. 2004;15:696–705. doi: 10.1091/mbc.E03-05-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SM, Hahm KD, Shin JW, Leem JG, Lee C, Han SM. Changes in magnesium concentration in the serum and cerebrospinal fluid of neuropathic rats. Acta Anaesthesiol Scand. 2006;50:211–216. doi: 10.1111/j.1399-6576.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Cai G, Wang HY, Smith C, Friedman E. Characterization of the phosphoinositide-linked dopamine receptor in a mouse hippocampal–neuroblastoma hybrid cell line. J Neurochem. 1998;71:1935–1943. doi: 10.1046/j.1471-4159.1998.71051935.x. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Wang HY, Friedman E. Stimulated D1 dopamine receptors couple to multiple Galpha proteins in different brain regions. J Neurochem. 2001;78:981–990. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis NK, Polites HG, Pintar JE, Schmauss C. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–924. doi: 10.1016/s0306-4522(98)00705-2. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, De La CA. Dopamine D5 receptors of rat and human brain. Neuroscience. 2000;100:689–699. doi: 10.1016/s0306-4522(00)00274-8. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Anderson CM, Ely TD, Mailman RB. The biochemistry and pharmacology of mesoamygdaloid dopamine neurons. Ann N Y Acad Sci. 1988;537:173–187. doi: 10.1111/j.1749-6632.1988.tb42105.x. [DOI] [PubMed] [Google Scholar]
- Latini S, Pazzagli M, Pepeu G, Pedata F. A2 adenosine receptors: their presence and neuromodulatory role in the central nervous system. Gen Pharmacol. 1996;27:925–933. doi: 10.1016/0306-3623(96)00044-4. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein–protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Anderson CM, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB. Amygdaloid D1 receptors are not linked to stimulation of adenylate cyclase. Synapse. 2003a;50:320–333. doi: 10.1002/syn.10272. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Petitto JM, Anderson CM, Mooney DH, Lachowicz JE, Schulz DW, Kilts CD, Mailman RB. D1 dopamine receptors in the amygdala exhibit unique properties. Ann N Y Acad Sci. 2003b;985:536–539. [Google Scholar]
- Mailman RB, Schulz DW, Kilts CD, Lewis MH, Rollema H, Wyrick S. Multiple forms of the D1 dopamine receptor: its linkage to adenylate cyclase and psychopharmacological effects. Psychopharmacol Bull. 1986;22:593–598. [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Zhou Q, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1992;46:959–971. doi: 10.1016/0306-4522(92)90197-a. [DOI] [PubMed] [Google Scholar]
- Matthies H, Becker A, Schroeder H, Kraus J, Hollt V, Krug M. Dopamine D1-deficient mutant mice do not express the late phase of hippocampal long-term potentiation. NeuroReport. 1997;8:3533–3535. doi: 10.1097/00001756-199711100-00023. [DOI] [PubMed] [Google Scholar]
- Montague DM, Striplin CD, Overcash JS, Drago F, Lawler CP, Mailman RB. Quantification of D1B (D5) receptors in dopamine D1A receptor-deficient mice. Synapse. 2001;39:319–322. doi: 10.1002/1098-2396(20010315)39:4<319::AID-SYN1015>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Nicklaus KJ, McGonigle P, Molinoff PB, Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptor activation increases the magnitude of early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 1996;16:7478–7486. doi: 10.1523/JNEUROSCI.16-23-07478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Brownstein M. Microdissection of brain areas by the punch technique. In: Cuello AC, editor. Brain Microdissection Techniques. Wiley; New York: 1983. pp. 1–36. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney: 1986. [Google Scholar]
- Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H] SCH 23390. Brain Res. 1986;375:291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- Schetz JA, Sibley DR. Zinc allosterically modulates antagonist binding to cloned D1 and D2 dopamine receptors. J Neurochem. 1997;68:1990–1997. doi: 10.1046/j.1471-4159.1997.68051990.x. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Stanford EJ, Wyrick SW, Mailman RB. Binding of [3H]SCH23390 in rat brain: regional distribution and effects of assay conditions and GTP suggest interactions at a D1-like dopamine receptor. J Neurochem. 1985;45:1601–1611. doi: 10.1111/j.1471-4159.1985.tb07233.x. [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Niznik HB, Weiner DM, Stormann TM, Brann MR, Kennedy JL, Gelernter JE, Rozmahel R, Yang YL, Israel Y. Human dopamine D1 receptor encoded by an intronless gene on chromosome 5. Nature. 1990;347:80–83. doi: 10.1038/347080a0. [DOI] [PubMed] [Google Scholar]
- Torvinen M, Kozell LB, Neve KA, Agnati LF, Fuxe K. Biochemical identification of the dopamine D2 receptor domains interacting with the adenosine A2A receptor. J Mol Neurosci. 2004;24:173–180. doi: 10.1385/JMN:24:2:173. [DOI] [PubMed] [Google Scholar]
- Torvinen M, Marcellino D, Canals M, Agnati LF, Lluis C, Franco R, Fuxe K. Adenosine A2A receptor and dopamine D3 receptor interactions: evidence of functional A2A/D3 heteromeric complexes. Mol Pharmacol. 2005;67:400–407. doi: 10.1124/mol.104.003376. [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Selective dopaminergic mechanism of dopamine and SKF38393 stimulation of inositol phosphate formation in rat brain. Eur J Pharmacol. 1992;226:297–302. doi: 10.1016/0922-4106(92)90046-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30:56–61. doi: 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wyrick SD, Mailman RB. Tritium-labeled (+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4, 5-tetrahydro-1H-3-benzazepine (SCH23390) J Label Compd Radiopharm. 1985;22:189–195. [Google Scholar]


