Abstract
Melanopsin, a novel photopigment, has recently been localized to a population of retinal ganglion cells that display inherent photosensitivity. During continuous light and following light offset, primates are known to exhibit sustained pupilloconstriction responses that resemble closely the photoresponses of intrinsically-photoreceptive ganglion cells. We report that, in the behaving macaque, following pharmacological blockade of conventional photoreceptor signals, significant pupillary responses persist during continuous light and following light offset. These pupil responses display the unique spectral tuning, slow kinetics, and irradiance coding of the sustained, melanopsin-derived ganglion cell photoresponses. We extended our observations to humans by using the sustained pupil response following light offset to document the contribution of these novel ganglion cells to human pupillary responses. Our results indicate that the intrinsic photoresponses of intrinsically-photoreceptive retinal ganglion cells play a role in the pupillary light reflex and are primarily responsible for the sustained pupilloconstriction that occurs following light offset.
Keywords: Human, macaque, pupil, papillary, melanopsin
1. Introduction
The pupillary light reflex controls the light intensity reaching the retina by a simple and well characterized pathway that links a sensory input: irradiance level, to a motor output: pupil diameter (Loewenfeld, 1993; Gamlin et al., 1995; Pong & Fuchs, 2000; Clarke et al., 2003). Pupil area decreases with increasing irradiance over a ~9 log unit range. A key feature of the light reflex is its tonic nature in bright light: constriction is held steady under continuous illumination (Bouma, 1962; Loewenfeld, 1993; Gamlin et al., 1995; Pong & Fuchs, 2000; Clarke et al., 2003). This may be contrasted with ganglion cell recordings that show rapid desensitization – or light adaptation – of the cone pathways (Shapley & Enroth-Cugell, 1984). Another feature of primate pupil responses is that they often exhibit a brief dilation at light cessation followed by a sustained reconstriction (Alpern & Campbell, 1962; Newsome, 1971; Alpern & Ohba, 1972; Hansen & Fulton, 1986). There has not been a satisfactory explanation of this sustained post-stimulus pupil response, although it was suggested that it arose from bleached rhodopsin in rods (Alpern & Campbell, 1962; Alpern & Ohba, 1972; Hansen & Fulton, 1986).
In rodents, intrinsically photosensitive retinal ganglion cells have been described that influence non-image-forming functions, including the pupillary light reflex (Berson et al., 2002; Hattar et al., 2002, 2003; Gooley et al., 2003; Lucas et al., 2003; Panda et al., 2003). Several studies have recently provided evidence that melanopsin is the photopigment responsible for the photoresponse of intrinsically photoreceptive ganglion cells (Qiu et al., 2005; Panda et al., 2005; Melyan et al., 2005; Fu et al., 2005).
Mice lacking rod and cone photoreceptors still display pupil constriction in response to stimuli of high retinal irradiance (Lucas et al., 2001), mice lacking melanopsin show an abnormal pupillary light reflex in which the pupil fails to fully constrict at high retinal irradiance (Lucas et al., 2003), and mice lacking both melanopsin and functional rods and cones show no pupillary responses (Hattar et al., 2003; Panda et al., 2003). Recently we showed that a comparable population of retinal ganglion cells exists in primates, and these cells project to the pupillary control center in the pretectum (Dacey et al., 2005). When the melanopsin-associated response of these ganglion cells is isolated in vitro by pharmacological blockade of rod and cone transmission to the inner retina, they display a slow, maintained depolarization in response to long-duration light pulses and repolarize only slowly upon light OFF (Dacey et al., 2005). These data are consistent with the melanopsin-based response contributing significantly to the maintained pupil constriction in continuous light as well as to the sustained, post-stimulus pupil constriction seen after light OFF. The goal of this study was to test this hypothesis in the behaving macaque by evaluating pupillary behavior after pharmacological blockade of retinal rod-cone pathways. Our results indicate that indeed intrinsically-photoreceptive retinal ganglion cells contribute significantly to the sustained component of the pupillary light reflex and primarily to the sustained pupilloconstriction that occurs following light offset. We also provide evidence that this class of retinal ganglion cells plays the same functional roles in human pupillary responses.
2. Methods
2.1 In vitro preparation
The in vitro whole mount retina preparation and recording methods have been described previously (Dacey et al., 2003; Dacey et al., 2005). Eyes were removed from deeply anesthetized animals and the retina dissected free of the vitreous and sclera in oxygenated Ames’ Medium (Sigma Chemical Co., St. Louis, MO). The retina-RPE-choroid was placed flat, vitreal surface up, in a superfusion chamber mounted on the stage of a light microscope. Rhodamine-labeled cells were visualized as described above; autofluoresent granules were visualized with a blue filter block (excitation 490 nm; barrier 515 nm). Ganglion cells were targeted for intracellular recording using high impedence (~300 – 450 M) glass micropipettes filled with 2–3% Neurobiotin (Vector Labs, Burlingame, CA) and 1–2% pyranine (Molecular Probes, Eugene, OR) in 1.0 M potassium acetate. Voltage responses were amplified (Axon instruments; Axoclamp) and digitized at 10 kHz. Visual stimuli were delivered to the retina as previously described (Packer et al., 2001).
2.2 Subjects
Two juvenile, male Rhesus monkeys (Macaca mulatta) were used to investigate pupillary responses. All experimental procedures were approved by the UAB Institutional Animal Care and Use Committee, and complied with the USPHS Policy on Humane Care and Use of Laboratory Animals. All described surgical procedures were performed under sterile conditions using isoflurane anesthesia. Post-surgical animals received analgesics (Buprenex, 0.01 mg/kg) to minimize pain.
Two male humans with normal corrected vision also participated in this study. All experimental procedures were approved by the UAB Institutional Review Board, and were undertaken with the understanding and written consent of each subject.
2.3 Recording procedures
For eye-movement recording in macaques, scleral search coils were implanted under the conjunctiva of each eye (Judge et al., 1980), and the horizontal and vertical gains of each eye were calibrated at the beginning of each recording session. Human eye movements were monitored by video camera. Pupil diameters were measured in both eyes under infra-red illumination using video cameras and ISCAN RK406 pupillometer systems. The positions of the right eye, left eye, and pupil diameters were sampled at 500 Hz. All samples were stored on computer disk for later analysis.
2.4 Behavioral task, methods common to macaque and human subjects
The pupil of the right eye was dilated with 1.0% tropicamide/2.5% phenylephrine. Therefore, pupillary responses elicited by stimuli presented to the right eye were measured by evaluating the consensual pupil response of the left eye. At the beginning of a trial, the subject fixated with the left eye on a target (2° Maltese cross; 5 cd/m2) presented on a computer monitor at a distance of 58 cm. Pupil diameter in the left eye was monitored continuously. After 5 seconds of fixation, a stimulus subtending 36° was presented in Maxwellian view to the right eye for 10 seconds. The stimulus (8.5–15.7 log photons/cm2/sec) was presented at selected narrow-band wavelengths (8–10 nm full width at half maximum, Thermo-Oriel) between 430 nm and 613 nm. The stimulus was then extinguished, and the subject maintained fixation for another 16 – 30 seconds. In all cases, stimulus generation was under computer control, including a stepper-controlled variable neutral-density filter, a 10-position filter wheel, and a mechanical shutter. At each wavelength, an IL1700 Radiometer/Photometer System was used to calibrate retinal irradiance for the Maxwellian view (Nygaard and Frumkes, 1982).
2.6 Intravitreal injections and ERG monitoring
To study the influence of the intrinsically-photoreceptive retinal ganglion cells on the pupillary light reflex, we eliminated the reflex component normally mediated by rods and cones by blocking ON and OFF retinal channels with intravitreal injections of L-2-amino-4-phosphobutyrate (L-AP4) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (Dolan & Schiller, 1994; Sieving et al., 1994). In one animal, D-2-amino-5-phosphonovaleric acid (D-AP5) was also included in the cocktail to block any potential involvement of NMDA receptors. Prior to the intravitreal injection, the animal was anesthetized with a mixture of 4–5% isoflurane and 95–96% oxygen. Topical 0.5% Proparacaine HCl was instilled in the right eye. Then, using a 28-gauge needle inserted through the pars plana, we injected 100 μl of a cocktail of 20–50 mM L-AP4, 2 mM CNQX, and 5 mM D-AP5 diluted in sterile saline. Assuming complete mixing in a vitreal volume of 2.0 ml, the retinal concentrations of these drugs would be 1 – 2.5 mM L-AP4, 100 μM CNQX, and 250 μM D-AP5. To monitor the effectiveness of the intravitreal injection, we recorded the electroretinogram (ERG) (4ms duration; 2.25 Cd/m2/sec, 6500K) with an Espion system (Diagnosys) equipped with a ColorBurst hand-held mini-Ganzfeld simulator. The right eye was dilated with 1.0% tropicamide/2.5% phenylephrine for ERG recording. Once the ERG confirmed that the injection had been effective, the animal was allowed to recover from the anesthesia for 1–2 hours, and testing began. Since the right eye was dilated for the pharmacological blockade sessions, it was maintained in a fully dilated state for all recording sessions. Previous studies have shown that such intravitreal pharmacological blockades remain effective for at least five hours, and weeks are required for full recovery (e.g. Sieving et al., 1994; Kondo & Sieving, 2001). Furthermore, we could confirm the continued effectiveness of the blockade from the measured pupillary responses.
2.7 Data analysis
Individual trials showing relevant pupil traces were displayed and analyzed off-line by computer. For measurements of the light-evoked pupilloconstriction, the average pupilloconstriction was measured over the 5-second period between 5 and 10 seconds after light onset. For measurements of the sustained pupillary constriction after termination of the light stimulus, the average pupilloconstriction was measured over the 10-second period between 6 and 16 seconds after light offset in macaques. In humans, for measurements of the sustained pupillary constriction after termination of the light stimulus, the average pupilloconstriction was measured over the 15-second period between 15 and 30 seconds after light offset. The relation between irradiance and pupilloconstriction was fit with the Hill equation:
where I is irradiance, Pmax is maximal pupilloconstriction, B is a constant, and C is the irradiance at which half-maximal pupilloconstriction is produced. For measurements of light-evoked pupillary responses during pharmacological blockade and for sustained pupilloconstriction following light termination, Pmax and B were determined at 493 nm and kept constant. Only C was varied to obtain best fits at other wavelengths. For measurements of light-evoked pupillary responses under normal conditions, Pmax, B and C were varied to obtain best fits. We then generated plots of relative quantal sensitivity as a function of wavelength.
In humans, we estimated the absorbance of the lens of each subject using D&H Color Rule settings (Coren, 1987) and an algorithm to convert to lens density functions (Pokorny et al., 1987), and corrected quantal sensitivity appropriately. These resultant spectral sensitivity functions were analyzed by optimizing fits of the data to a single pigment absorbance template for a retinal1-based pigment (Kraft et al., 1998). To determine λmax, we fit a sixth order polynomial to a plot of log sensitivity versus wavelength (Baylor et al., 1984; Baylor et al., 1987). Each term of that polynomial has the form an [log(λmax/λ · 561)]n.
3. Results
We first examined the correspondence between the response characteristics of the intrinsically photoreceptive ganglion cells recorded in vitro with those of pupillary responses recorded in vivo under equivalent conditions. Figure 1A shows the response of the macaque pupil to a 10-second pulse of light (493 nm) under photopic conditions. At light ON, the pupil rapidly constricted and the constriction was maintained for the duration of the stimulus. At light OFF, the pupil dilated transiently, but then reconstricted and displayed a sustained, post-stimulus constriction in darkness. Figure 1B shows the response of an intrinsically-photoreceptive retinal ganglion cell recorded from the in vitro macaque retina under similar conditions to those in Figure 1A. Overall, the retinal ganglion cell activity closely matched the observed pupillary responses in time course. At light ON, the ganglion cell depolarized rapidly (latency to first spike ~35 msec) and exhibited sustained firing for the stimulus duration. (Dacey et al., 2005). At light OFF, the cell hyperpolarized transiently to briefly cancel the sustained intrinsic response; the cell then repolarized to give rise to the sustained late discharge in darkness before slowly returning to the resting potential in darkness (Dacey et al., 2005). The complex dynamics of the ganglion cell response derives from an interaction between cone input, which provides the short-latency responses at light onset and offset, and the inherent photoresponse, which maintains depolarization and firing for the duration of the stimulus followed by an extremely slow decay at light OFF (Dacey et al., 2005).
Figure 1.
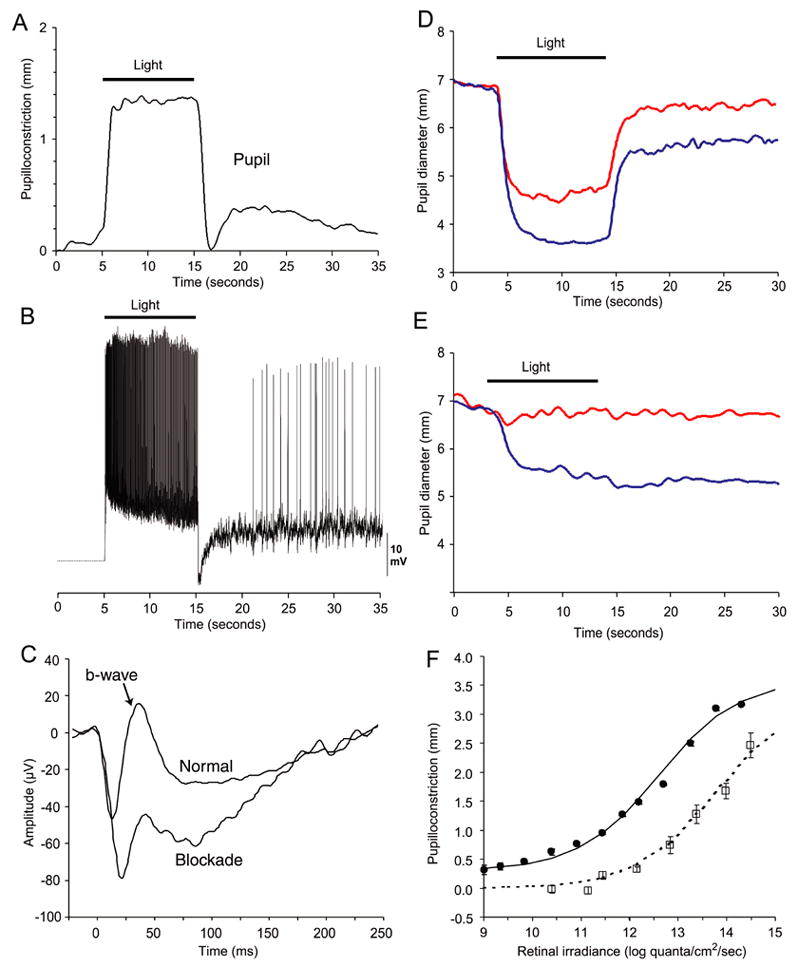
Pupillary and ganglion cell responses in macaques. (A) Averaged pupillary responses (n=5) showing sustained pupilloconstriction to a 10-second pulse of light (493 nm, 13.3 log quanta/cm2/second). At light OFF, there is a transient pupil dilation followed by a sustained pupilloconstriction. The transient pupil dilation is not always evident, since it depends on prior stimulus conditions and the magntitude of the sustained, post-stimulus pupil constriction. (B) In vitro, intracellular recording from an intrinsically-photoreceptive retinal ganglion cell. For further details see Dacey et al. (2005). A 10-second pulse of light (470 nm, 13.5 log quanta/cm2/second) was presented. Note the similarity between the retinal ganglion cell response and the pupillary response. (C) In vivo, flash electroretinogram under normal conditions (upper trace) and following intravitreal injection of L-AP4/CNQX/D-AP5 (lower trace). The b wave is virtually eliminated following this pharmacological blockade. (D) Normal condition. Averaged responses (n=3) of the pupil to 493 nm light of 14.0 log quanta/cm2/second irradiance (blue trace), and 613 nm light of 14.1 log quanta/cm2/second irradiance (red trace). Following light extinction, there is a pronounced sustained pupilloconstriction that, in this case, masks the transient pupil dilation often seen at light OFF. (E) Pharmacological blockade condition. Averaged responses (n=3) of the pupil to 493 nm light of 14.0 log quanta/cm2/second irradiance (blue trace), and 613 nm light of 14.1 log quanta/cm2/second irradiance (red trace). Note the robust and sustained pupillary responses elicited by light of 493 nm but not of 613 nm. (F) Retinal irradiance-pupillary response plots for 532 nm irradiance for the normal condition (●) and during pharmacological blockade (□) (SEM error bars).
The hypothesis that combined cone and melanopsin-derived light responses account respectively for transient and sustained pupil behavior can be evaluated by measurement of the spectral sensitivity of these responses. The slow and sustained intrinsic photoresponse of the retinal ganglion cells is characterized by an absorbance template corresponding to a single retinal1-based pigment with a peak at 482 nm, whereas the fast and relatively transient cone-mediated response is complex and broadband, with significant sensitivity to longer wavelengths derived from L and M cone inputs (Dacey et al., 2005). Under normal photopic conditions, substantial and prompt pupilloconstriction was elicited by retinal illumination at both 493 nm (activating both cones and the intrinsic photoresponse) and 613 nm (activating predominantly cones) (Fig. 1D). However, during pharmacological blockade of rod and cone inputs (confirmed by the substantial reduction in the b-wave of the electroretinogram; see Fig. 1C), the pupillary response was delayed by approximately one second and was more sluggish than normal (Fig. 1E); at the same time, substantial pupilloconstriction was elicited only by retinal illumination at 493 nm, consistent with the idea that the intrinsic photoresponse of ganglion cells controls this pupillary response. Similarly, sustained pupilloconstriction after light cessation was present only after retinal illumination at 493 nm (Fig. 1 D,E).
To more completely characterize the spectral sensitivity of these macaque pupillary responses, the irradiance-response relations for pupil constriction were measured at ten wavelengths between 430 nm and 613 nm, and fitted with the Hill equation. As an example, the filled circles in Fig. 1F show the normal relation during the light stimulus at 532 nm. During pharmacological blockade (open squares), the response was absent at irradiance levels below ~11 log quanta/cm2/sec, presumably due to the elimination of rod-driven input. At higher irradiance levels, substantial pupilloconstriction occurred, despite the elimination of cone-driven responses.
Figure 2A shows Hill equation fits to the irradiance-response relations of the sustained response during illumination for all ten wavelengths under normal conditions. Pupil response-irradiance functions obtained for stimuli between 452 nm and 552 nm were comparable, with a light-evoked pupil constriction of ~3.2 mm at a retinal irradiance of 14.0 log quanta/cm2/sec. However, at longer wavelengths, pupil constriction was reduced. The collective spectral sensitivity data could not be well fit by a single pigment absorbance template for a retinal1-based pigment (Fig. 2C, triangles), indicating the presence of more than one receptoral component. Figure 2B shows Hill equation fits to the irradiance-response relations during pharmacological blockade to isolate the intrinsic photoresponse. The pupillary responses for a given retinal irradiance were largest for wavelengths between 453 and 510 nm, with light-evoked pupil constriction of ~2.1 mm at a retinal irradiance of 14.0 log quanta/cm2/sec. At longer wavelengths, light-evoked pupillary responses were significantly reduced, and were essentially absent at 613 nm (Fig 2B). The collective spectral sensitivity data were well fit by the melanopsin spectrum (λmax 482 nm) (Fig. 2C, circles).
Figure 2.
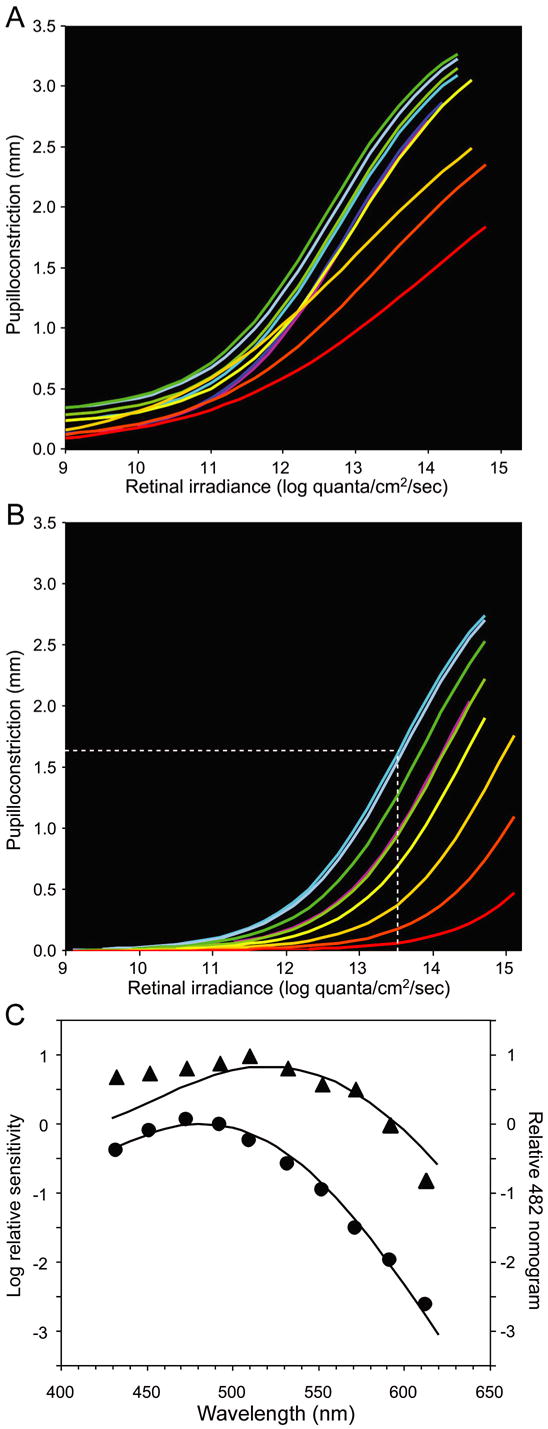
Pupillary responses of macaques during a light stimulus under normal conditions and during pharmacological blockade. (A) Retinal irradiance-pupillary response plots under normal conditions. (B) Retinal irradiance-pupillary response plots after pharmacological blockade. The white dotted line indicates the retinal irradiance at 470 nm required to produce half-maximal pupilloconstriction. In panels A and B, line color represents an approximation of stimulus wavelength. (C) Spectral sensitivity data derived from panels A and B. The data in normal condition (▲) is poorly fit (R2 = 0.77) by a best-fit, Vitamin A1 pigment nomogram (peak sensitivity at 522 nm). The data obtained during pharmacological blockade (●) is well fit (R2 = 0.99) by a Vitamin A1 pigment nomogram with peak sensitivity at 482 nm.
Corresponding experiments on the sustained pupillary constriction after light OFF showed that this response was driven exclusively by the intrinsic photoresponse. More specifically, a large pupilloconstriction was produced by prior exposure to 10-second light pulses between 432 nm and 510 nm (Fig. 3A, blue and green traces), whereas little response was observed after 10-second light pulses at 613 nm, a wavelength that does not readily evoke the intrinsic response (Fig. 3A, red trace). At all wavelengths, the magnitude of the sustained pupilloconstriction after light pulses of a given retinal irradiance in the absence of pharmacological blockers was comparable to that of the sustained light-evoked pupillary response evoked by light of the same irradiance in the presence of blockers (cf. Figs 2B and 3A). Further, a comparable retinal irradiance was required in both cases to produce half-maximal pupilloconstriction at 493 nm. With or without pharmacological blockade, the irradiance-response relation for sustained, post-stimulus pupilloconstriction was the same at all wavelengths (Fig. 3A, B), and had the same spectral sensitivity, being well fit by the melanopsin spectrum (λmax 482 nm) (Fig. 3C).
Figure 3.
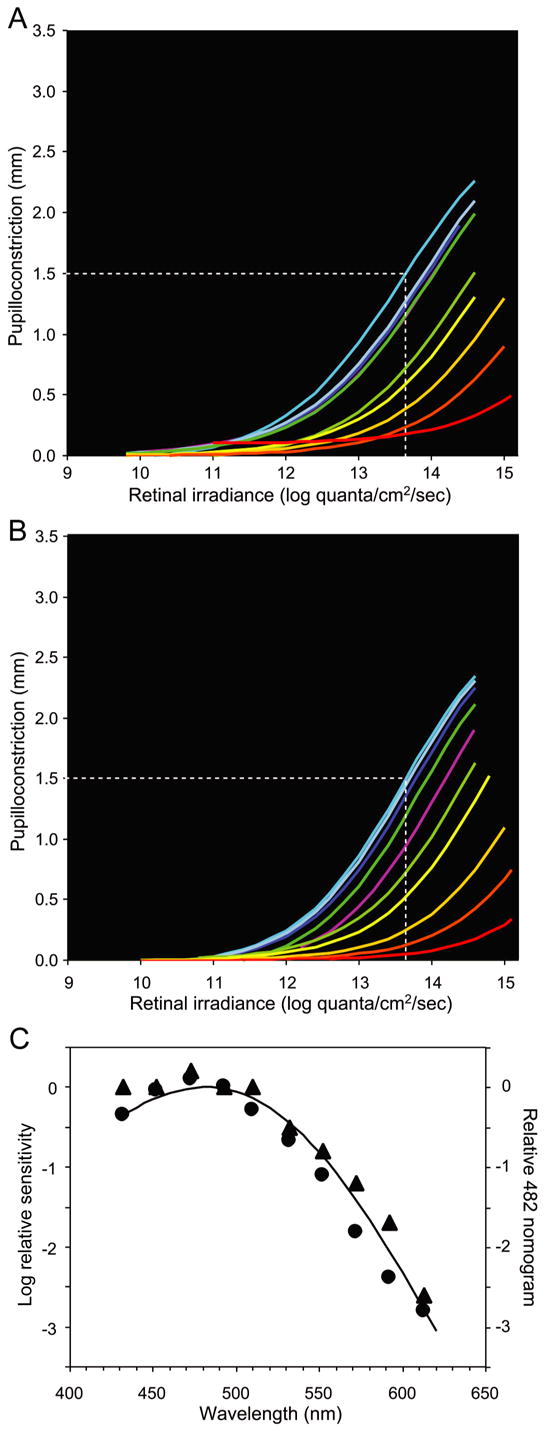
Post-stimulus, sustained pupillary responses of macaques under normal conditions and during pharmacological blockade. (A) Retinal irradiance-pupillary response plots under normal conditions. (B) Retinal irradiance-pupillary response plots after pharmacological blockade. In both A and B, the white dotted line indicates the retinal irradiance at 470 nm required to produce half-maximal pupilloconstriction, line color represents an approximation of stimulus wavelength, and Pmax = 3.0 mm was used for fitting the Hill equations. (C) Spectral sensitivity data derived from panels A and B. The solid curve, a Vitamin A1 pigment nomogram with peak sensitivity at 482 nm, closely matches the data obtained both under normal conditions (▲, R2 = 0.98) (Best fit λmax 483 nm) and during pharmacological blockade (●, R2 = 0.97) (Best fit: λmax 476 nm).
A melanopsin-associated photosensitive pathway appears to exist in humans (Dacey et al., 2005; Rollag et al., 2003; Hannibal et al., 2004), but definitive evidence linking it to a functional role is still lacking. Because the sustained, post-stimulus pupil response appears to arise primarily from the melanopsin pathway in macaque, we sought to investigate the same phenomenon in normal human subjects. We found that pupilloconstriction in human subjects persisted after exposure to a 10-second light at 493 nm (Fig. 4A, blue trace), but not at 613 nm for the same irradiance (Fig. 4A, red trace). The irradiance-response relation at 493 nm (Fig 4B) showed a half-maximal retinal irradiance (13.6 log quanta/cm2/sec) very similar to that in macaque (cf. Fig 3A). Next, for eight wavelengths between 452 nm – 592 nm, we calculated the retinal irradiance required to produce a given criterion pupil response if mediated by a Vitamin A1 pigment nomogram with a peak sensitivity at 482 nm (pilot data suggested a spectral sensitivity peaking between 480 and 485 nm). Repeated measures were obtained for the sustained pupil response at each wavelength and retinal irradiance. We found that the actual data obtained in this experiment departed only slightly from the predicted values (Fig. 4C). Indeed, these data, following correction for minor departures from the predicted values as described in the legend to figure 4, were well fit (R2 = 0.99) by a Vitamin-A1 pigment nomogram with a peak sensitivity at 482 nm (Fig. 4D), closely matching our results in macaques.
Figure 4.
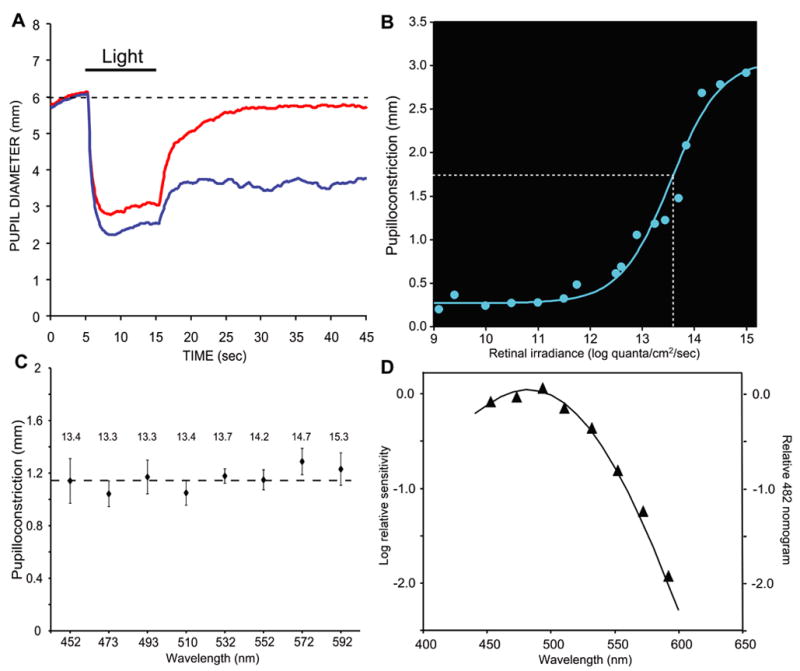
Post-stimulus, sustained pupillary responses in humans. (A) Averaged responses (n=3) of the pupil to 493 nm light of 14.1 log quanta/cm2/second irradiance (blue trace), and 613 nm light of 14.1 log quanta/cm2/second irradiance (red trace). (B) Retinal irradiance-pupillary response plot for 493 nm irradiance. The white dotted line indicates the retinal irradiance required to produce half-maximal pupilloconstriction. (C) Criterion pupil response. Magnitude of the sustained post-stimulus pupillary response after light OFF at eight different wavelengths (SEM error bars). The retinal irradiance used at each wavelength is indicated above the data point. Each stimulus irradiance was calculated to produce the same sustained pupil constriction if these responses were mediated solely by a Vitamin A1 pigment nomogram with a peak sensitivity at 482 nm. (D) Spectral sensitivity data derived from the results shown in C. For each wavelength, the difference between the measured and criterion pupilloconstriction in C was converted into an irradiance difference value based on the irradiance-pupilloconstriction response curve in B. This difference value was then combined with the stimulus irradiance value to determine the retinal irradiance required to produce the criterion pupil response at that wavelength. The solid curve, a Vitamin A1 pigment nomogram with peak sensitivity at 482 nm, closely matches the data (▲, R2 = 0.99).
4. Discussion
Our results clearly demonstrate in macaques that the melanopsin signal contributes significantly to sustained light-evoked pupillary responses; they also show in primates, including humans, that the melanopsin signal is the primary source of the sustained, post-stimulus pupilloconstriction observed following light offset.
4.1 Melanopsin-containing RGCs contribute significantly to the primate pupillary light reflex
Melanopsin-containing RGCs provide the major retinal input to the pretectal olivary nucleus in primates (Dacey et al., 2003) and rodents (Morin et al., 2003; Hattar et al., 2006). Consistent with this projection, substantial light-evoked pupillary responses occur despite pharmacological inactivation of conventional photoreceptor signals. For example, during blockade, a stimulus with a retinal irradiance of approximately 14.5 log quanta/cm2/sec at 493 nm elicits a pupil constriction of 2.7 mm (Fig 2b), while half maximal pupil constriction is elicited by a retinal irradiance of approximately 13.5 log quanta/cm2/sec (Fig 2b). The action spectrum of these pupillary responses is well fit by the melanopsin spectrum, and the irradiance sensitivities and kinetics of these responses are also comparable to the intrinsic photoresponses of melanopsin-containing RGCs in vitro (Dacey et al., 2005). These results clearly indicate that melanopsin-containing RGCs contribute significantly to the primate pupillary light reflex. Further, these results suggest that in primates the role of the melanopsin signal is to combine with the cone signal over the photopic range, serving to maintain pupil constriction during continuous daylight illumination. Indeed, data obtained in humans prior to the discovery of intrinsically-photoreceptive ganglion cells supports the importance of the intrinsic photoresponse for maintaining pupil constriction during continuous daylight illumination. Bouma (1962) reported on the size of the static pupil as a function of wavelength and retinal irradiance. He obtained a spectral sensitivity curve with a λmax at 490 nm, which he interpreted as resulting from S-cone and rod inputs driving the sustained pupil response during maintained illumination. Since this interpretation could not be reconciled with the known properties of these photoreceptors, it did not gain standing. It is now clear that Bouma’s data approximate the action spectrum of intrinsically-photoreceptive retinal ganglion cells in primates.
4.2 Melanopsin-containing RGCs drive sustained, post-stimulus pupil constriction
Our data show that there is a sustained, post-stimulus pupil constriction following light offset in primates. The threshold for this response is high in both macaques and humans. For example, at 30 seconds post-stimulus, a retinal irradiance of 12 log quanta/cm2/sec (492 nm) is required to produce any sustained pupil constriction in darkness. However, as the retinal irradiance of the stimulus increases, substantial sustained pupil constriction is produced 30 seconds following light offset. For example, a stimulus with a retinal irradiance of 13.5 log quanta/cm2/sec produces a sustained pupil constriction of > 1.5 mm in macaques and humans (Figs 3a,b; Fig. 4b); while in humans, a stimulus with a retinal irradiance of 15 log quanta/cm2/sec produces a sustained pupil constriction of 2.9 mm (Fig. 4b). The action spectrum of these responses is very well fit by the melanopsin spectrum, and the kinetics and sensitivity of these responses closely match the intrinsic photoresponses of melanopsin-containing RGCs in vitro (Dacey et al., 2005). These results clearly demonstrate that sustained, post-stimulus pupil constriction is mediated predominantly by the melanopsin-driven, intrinsic photoresponse and not by sustained rod activity resulting from bleached rhodopsin as had previously been suggested (Alpern & Campbell, 1962; Alpern & Ohba, 1972; Hansen & Fulton, 1986).
4.3 Comparison to rodent studies
Rodents possess melanopsin-containing, intrinsically photosensitive retinal ganglion cells that influence non-image-forming functions, including the pupillary light reflex (Berson et al., 2002; Hattar et al., 2002, 2003; Gooley et al., 2003; Lucas et al., 2003; Panda et al., 2003). It has been reported that mice lacking rod and cone photoreceptors, but presumably with intact melanopsin-driven intrinsic photoresponses, display substantial pupil constriction in response to stimuli of high retinal irradiance (Lucas et al., 2001). In these animals, pupil constriction “is driven by a single opsin/vitamin A-based photopigment with peak sensitivity around 479 nm” (Lucas et al., 2001). These results closely match our results in primates, including humans, which demonstrate that the intrinsic photoresponse driving pupillary responses can be well fit by a single pigment nomogram with a λmax of 482 nm. Further, mice lacking melanopsin show an abnormal pupillary light reflex in which the pupil fails to fully constrict at high retinal irradiance (Lucas et al., 2003, while mice lacking both melanopsin and functional rods and cones show no pupillary responses (Hattar et al., 2003; Panda et al., 2003). These results in rodents are consistent with our suggestion that the melanopsin signal contributes significantly to the sustained pupilloconstriction component of the pupillary light reflex in primates. To our knowledge, no study in rodents has examined the sustained, post-stimulus pupil constriction that occurs following light offset.
4.4 Evidence for a functional, melanopsin-driven inner retinal pathway in humans
Previous studies in humans have shown that a novel photopigment is responsible for melatonin regulation (Thapan et al., 2001; Brainard et al., 2001) and cone adaptation (Hankins & Lucas, 2002), but could not identify the photopigment involved. More recently, melanopsin has been shown to be present in human retina and retinohypothalamic tract (Dacey et al., 2005; Rollag et al., 2003; Hannibal et al., 2004), but these studies did not directly address the physiological role played by this photopigment in humans. Our experiments extend these previous studies by demonstrating the influence of melanopsin-containing retinal ganglion cells on human pupillary responses, and hence provide further evidence for the existence of a functional, melanopsin-driven inner retinal pathway in humans.
Acknowledgments
The authors thank Dr. Timothy Kraft for help with ERG recording, Drs. Michael Loop and Thomas Norton for comments on the manuscript, and Sam Hayley and Jill Woods for technical assistance. This work was supported by NIH grant EY09380 and EyeSight Foundation of Alabama (PDG), EY06678 and EY09625 (DMD), Kayser Award, EY00901 (JP), and EY06837 and EY14596 (K-WY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpern M, Campbell FW. The behaviour of the pupil during dark-adaptation. J Physiol Lond. 1962;165:5–7p. [Google Scholar]
- Alpern M, Ohba N. The effect of bleaching and backgrounds on pupil size. Vision Res. 1972;12:943–951. doi: 10.1016/0042-6989(72)90016-8. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Nunn BJ, Schnapf JL. Spectral sensitivity of cones of the monkey Macaca fascicularis. J Physiol. 1987;390:145–160. doi: 10.1113/jphysiol.1987.sp016691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bouma H. Size of the static pupil as a function of wavelength and luminosity of the light incident on the human eye. Nature. 1962;193:690–691. doi: 10.1038/193690a0. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans:evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RJ, Zhang HY, Gamlin PDR. The primate pupillary light reflex: receptive field characteristics of pretectal luminance neurons. J Neurophysiol. 2003;89:3168–3178. doi: 10.1152/jn.01130.2002. [DOI] [PubMed] [Google Scholar]
- Coren S. A rapid method to assess crystalline lens pigment density in vivo. Acta Ophthalmol. 1987;65:575–578. doi: 10.1111/j.1755-3768.1987.tb07043.x. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao H, Peterson B, Robinson F, Smith V, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal color and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Dolan RP, Schiller PH. Effects of ON channel blockade with 2-amino-4-phosphonobutyrate (L-AP4) on brightness and contrast perception in monkeys. Vis Neurosci. 1994;11:23–32. doi: 10.1017/s095252380001107x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Liao HW, Do MT, Yau KW. Non-image-forming ocular photoreception in vertebrates. Curr Opin Neurobiol. 2005;15:415–422. doi: 10.1016/j.conb.2005.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PDR, Zhang H, Clarke RJ. Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Exp Brain Res. 1995;106:177–180. doi: 10.1007/BF00241367. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Ostergaard J, Georg B, Heegaard S, Larsen PJ, Fahrenkrug J. Melanopsin is expressed in PACAP-containing retinal ganglion cells of the human retinohypothalamic tract. Invest Ophthalmol Vis Sci. 2004;45:4202–4209. doi: 10.1167/iovs.04-0313. [DOI] [PubMed] [Google Scholar]
- Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12:191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- Hansen RM, Fulton AB. Pupillary changes during dark adaptation in human infants. InvestOphthalmol & Vis Sci. 1986;27:1726–1729. [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells:architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BS, Chu FC. Implantation of magnetic search coils for measurement of eye position: An improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kondo M, Sieving PA. Primate photopic sine-wave flicker ERG: vector modeling analysis of component origins using glutamate analogs. Invest Ophthalmol Vis Sci. 2001;42:305–312. [PubMed] [Google Scholar]
- Kraft TW, Neitz J, Neitz M. Spectra of human L cones. Vision Res. 1998;38:3663–3370. doi: 10.1016/s0042-6989(97)00371-4. [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE. Anatomy, physiology, and clinical applications. Vol. 1. Iowa State University Press/Ames, Wayne State University Press/Detroit; 1993. The Pupil. [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving papillary constriction in mice. Nature Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Newsome DA. Afterimage and pupillary activity following strong light exposure. Vision Res. 1971;11:275–288. doi: 10.1016/0042-6989(71)90191-x. [DOI] [PubMed] [Google Scholar]
- Nygaard RW, Frumkes TE. Calibration of the retinal illuminance provided by maxwellian views. Vision Res. 1982;22:433–434. doi: 10.1016/0042-6989(82)90189-4. [DOI] [PubMed] [Google Scholar]
- Packer O, Diller LC, Verweij J, Lee BB, Pokorny J, Williams DR, Dacey DM, Brainard DH. Characterization and use of a digital light projector for vision research. Vision Res. 2001;41:427–439. doi: 10.1016/s0042-6989(00)00271-6. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Optics. 1987;26:1437–1440. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- Pong M, Fuchs AF. Characteristics of the pupillary light reflex in the macaque monkey: metrics. J Neurophysiol. 2000;84:953–963. doi: 10.1152/jn.2000.84.2.953. [DOI] [PubMed] [Google Scholar]
- Pong M, Fuchs AF. Characteristics of the pupillary light reflex in the macaque monkey: discharge patterns of pretectal neurons. J Neurophysiol. 2000;84:964–974. doi: 10.1152/jn.2000.84.2.964. [DOI] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- Rollag MD, Berson DM, Provencio I. Melanopsin, ganglion-cell photoreceptors, and mammalian entrainment. J Biol Rhythms. 2003;18:227–234. doi: 10.1177/0748730403018003005. [DOI] [PubMed] [Google Scholar]
- Shapley R, Enroth-Cugell C. In: Visual Adaptation and Retinal Gain Controls, Progress in Retinal Research. 3. Osborne N, Chader G, editors. Pergamon; London: 1984. pp. 263–346. [Google Scholar]
- Sieving PA, Murayama K, Naarendorp F. Push-pull model of the primate photopic electroretinogram: A role for hyperpolarizing neurons in shaping the b-wave. Visual Neurosci. 1994;11:519–532. doi: 10.1017/s0952523800002431. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


