Abstract
The glaucomas are a group of optic neuropathies comprising the leading cause of irreversible blindness worldwide. Elevated intraocular pressure due to a reduction in normal aqueous outflow is a major causal risk factor. We found that endothelial leukocyte adhesion molecule-1 (ELAM-1), the earliest marker for the atherosclerotic plaque in the vasculature, was consistently present on trabecular meshwork (TM) cells in the outflow pathways of eyes with glaucomas of diverse etiology. We determined expression of ELAM-1 to be controlled by activation of an interleukin-1 (IL-1) autocrine feedback loop through transcription factor NF-κB, and activity of this signaling pathway was shown to protect TM cells against oxidative stress. These findings characterize a protective stress response specific to the eye’s aqueous outflow pathways and provide the first known diagnostic indicator of glaucomatous TM cells. They further indicate that common mechanisms contribute to the pathophysiology of the glaucomas and vascular diseases.
The glaucomas, characterized by cupping of the optic nerve head and irreversible loss of retinal ganglion cells, affect approximately 70 million people worldwide1. Elevated intraocular pressure (IOP) due to reduction in aqueous outflow facility is a major causal risk factor. The main aqueous outflow pathway of the eye consists of a series of endothelial-cell–lined channels in the angle of the anterior chamber comprising the trabecular meshwork (TM), Schlemm’s canal, the collector channels and the episcleral venous system. In closed-angle forms of glaucoma, elevated IOP is due to anatomic obstruction of this pathway2,3. Factors causing reduced outflow facility in the open angle glaucomas may include accumulation of extraneous material or cells within the TM, alterations in junctional structures between cells of the TM or Schlemm’s canal, accelerated TM cell death and collapse of trabecular beams2–7.
Chronic sublethal injury due to cellular stress is a common theme in the pathogenesis of diverse diseases including atherosclerosis, glomerulonephritis and pulmonary fibrosis8,9. Previous studies indicate that the glaucomas are part of this disease group, with sublethal damage to the outflow pathways being the result of accumulated oxidative stress arising from the environment, vascular dysregulation, aging and/or the disease process itself3,10–12. The pathophysiology of diseases involving sublethal cell injury is determined largely by the cells surviving the damaging insult. These cells mount a protective response involving expression of new genes and other molecular changes8,9, dependent on the nature of the damaging stimulus and on tissue type13,14. A few studies have compared the molecular phenotypes of glaucomatous with normal TM (refs. 4,15); however, a diagnostic marker of diseased TM has not yet been identified.
ELAM-1 is a disease marker for glaucomatous TM
Cell-adhesion molecules (CAMs) are key functional components of biological structures for fluid containment and transport16. Moreover, upregulation of selectin- and immunoglobulin-type CAMs is a common response to cellular stress agents implicated in vascular disease17,18. To characterize CAMs in the aqueous outflow pathways of normal eyes, and to determine whether CAM expression might be altered in glaucoma, we performed an immunohistochemical screen using a battery of vascular endothelial CAM probes. Six of the eight vascular CAM probes consistently stained the TM and Schlemm’s canal of both normal and glaucomatous eyes. A representative experiment with the N-CAM-16 (neural cell adhesion molecule-16) marker is shown in Fig. 1. Staining for ICAM-1 (intercellular adhesion molecule-1), VCAM-1 (vascular cell adhesion molecule-1) and integrin α3 was also easily detected, and trace staining for integrin α2 and integrin α5 was apparent. A seventh vascular marker, PECAM-1 (platelet/endothelial cell adhesion molecule-1), stained only Schlemm’s canal. The level of immunostaining for each of the vascular CAMs was similar in normal and glaucomatous eyes. Normal and glaucomatous TM-cell lines retained these markers, as shown by N-CAM-16 staining (Fig. 1).
Fig. 1.
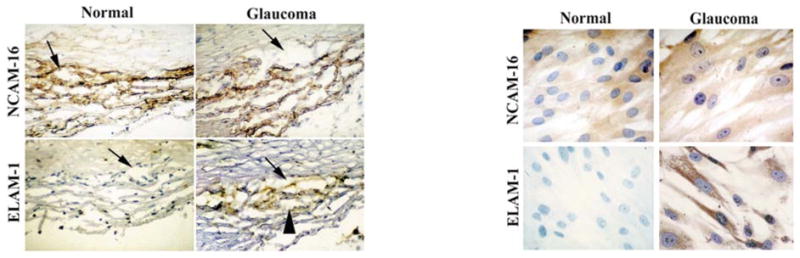
Immunohistochemical screen for CAMs altered in outflow pathways of glaucomatous eyes. Cross-sections through the angle of the anterior chamber of glaucomatous or normal eyes (left panels), or subcultured TM-cell lines from glaucomatous or normal eyes (right panels) were stained with N-CAM-16 or ELAM-1 antibody probes. Note the strong staining of Schlemm’s canal (arrow) and the surrounding TM (arrowhead) in glaucomatous eyes and TM cells from glaucomatous eyes. Tissue sections were negative for the inflammation marker LFA-1 and the fibrosis marker HPCA-2, and both tissue sections and cultured cells were positive for the TM cell marker HLA class I antigen (data not shown).
Of these, only the probe for ELAM-1 differentiated the glaucomatous from the normal eye aqueous outflow pathway (Fig. 1), regardless of glaucoma subtype or severity (Table 1), and independent of prior glaucoma therapy (four eyes had no treatment prior to surgical intervention). ELAM-1 staining was absent in the outflow pathway of normal eyes, but was clearly present in glaucomatous eyes in the region of Schlemm’s canal and the surrounding TM. The differential expression of ELAM-1 was retained in subcultured TM cells. ELAM-1 was consistently present in all glaucomatous tissue specimens and TM-cell lines examined. In contrast, ELAM-1 was consistently absent from the TM of all normal tissue specimens and TM-cell lines. This screen defines a molecular marker specific for glaucomatous TM cells that is retained even when the cells are subcultured.
Table 1.
Clinical findings in surgical specimens
| Patient (age/sex) | Diagnosis | IOP (mmHg) | Cup/Disc ratio | Visual field | Analyses |
|---|---|---|---|---|---|
| 73/M | POAG | 29 | 0.4 × 0.4 | INS | I |
| 58/M | PXFG | 25 | 0.8 × 0.6 | INS, SAS | I |
| 49/F | POAG | 50 | 0.95 × 0.95 | SAS, INS | I |
| 88/F | PXFG | 30 | 0.9 × 0.9 | SNS, SPC | I |
| 80/F | PXFG | 23 | 0.9 × 0.9 | Full | I |
| 75/F | POAG | 16 | 0.8 × 0.8 | SAS | I |
| 78/F | Inflammatory Glaucoma | 14 | 0.9 × 0.9 | Nonspecific loss | I |
| 59/M | Inflammatory Glaucoma | 30 | 0.3 × 0.3 | IAS, SNS | I |
| 60/M | Inflammatory Glaucoma | 16 | 0.9 × 0.8 | SNS | I |
| 40/M | POAG | 40 | 0.9 × 0.9 | Central island | I |
| 73/M | POAG | 18 | 0.95 × 0.95 | SAS, IAS | I |
| 85/M | POAG | 15 | 0.9 × 0.9 | SAS, IPC | I+N+R |
| 60/F | POAG | 18 | 0.1 × 0.1 | INS, IPC | I+N+R |
| 88/F | POAG | 25 | 0.8 × 0.8 | IAS, SAS | I |
| 68/F | PXFG | 22 | 0.9 × 0.9 | SAS | I |
| 69/F | PXFG | 15 | 0.9 × 0.9 | IHD, OS | I |
| 16/M | JOAG | 27 | 0.95 × 0.95 | Central island | I+N |
| 47/M | POAG | 18 | 0.95 × 0.95 | Central island | I+N+E |
| 41/M | OAG | 19 | 0.8 × 0.8 | Full | I |
| 81/F | PXFG | 30 | 0.8 × 0.8 | Full | I |
| 58/F | ACG | 42 | 0.3 × 0.3 | NA | I |
| 66/F | ACG | 50 | 0.3 × 0.3 | NA | I |
| 63/F | ACG | 38 | 0.3 × 0.3 | NA | I |
The analyses performed are: immunohistochemistry (I); northern-blot (N); RT-PCR (R); EMSA (E). ACG: angle-closure glaucoma, IAS: inferior arcuate scotoma, IHD: inferior hemifield defect, INS: inferior nasal scotoma, IPC: inferior paracentral scotoma, JOAG: juvenile open-angle glaucoma, POAG: primary open-angle glaucoma, PXFG: pseudoexfoliation glaucoma, SAS: superior arcuate scotoma, SHD: superior hemifield defect, SNS: superior nasal scotoma, SPC: superior paracentral scotoma, NA: not available
ELAM-1 expression is not due to inflammation
ELAM-1 expression is stimulated in normal vascular endothelium by inflammatory cytokines such as IL-1 (ref. 19). Many of the glaucomatous eyes that we examined had forms of disease associated with an inflammatory component. In addition, laser therapy or the preservatives in glaucoma medications could induce inflammation20. On gross examination, however, there was no evidence of inflammation or trauma in the glaucomatous cadaver eyes. Trabeculectomy was performed only on eyes in which inflammation was subdued or absent, and none of these eyes had received laser therapy within three months of surgery. The prostaglandin analog drugs might induce inflammatory cytokines21; however, only one of the cadaver eyes and only five of the trabeculectomy eyes had been treated with such drugs. One cadaver eye came from a patient whose disease was diagnosed only two months prior to death, so there was little opportunity for drug therapy. One trabeculectomy patient with primary open-angle glaucoma and three with angle-closure glaucoma had received no treatment whatsoever for their disease. Routine hematoxylin and eosin staining of tissue sections revealed no evidence of inflammation. Moreover, immunostaining revealed that all normal and glaucomatous eyes were positive for the TM-cell marker used in this study (HLA class I antigen), but negative for both the leukocyte marker and the fibrosis marker (data not shown). These results indicate that the commonality among all of the glaucomatous eyes leading to ELAM-1 expression is the disease process itself.
ELAM-1 expression and IL-1-NF-κB signaling
Autocrine cytokine feedback loops can be activated in tissue repair, disease and cellular aging, and serve as a mechanism for amplifying and sustaining expression of specific genes22. Exogenous IL-1 stimulated expression of ELAM-1 mRNA (Fig. 2a) and protein (Fig. 2c) in normal TM cells. The mRNA for IL-1α was undetectable in normal TM cells, and the mRNAs for IL-1β and IL-6 were found only at low levels (Fig. 2b). Exogenous IL-1 stimulated expression of each of these mRNAs (Fig. 2b). In contrast, IL-1α, IL-1β and IL-6 mRNAs were present at easily detectable levels in untreated TM cells derived from glaucomatous specimens (Fig. 2b), and correlated positively with ELAM-1 expression (Fig. 2a). Treatment with IL-1 receptor antagonist (IL-1ra), a naturally occurring analog of IL-1 that binds to IL-1 receptors but does not transduce a signal23, significantly downregulated ELAM-1 expression (Fig. 2c). These findings indicate that sustained ELAM-1 expression in glaucomatous TM cells is controlled largely by autocrine IL-1, although expression may be amplified by other autocrine cytokines such as IL-6.
Fig. 2.
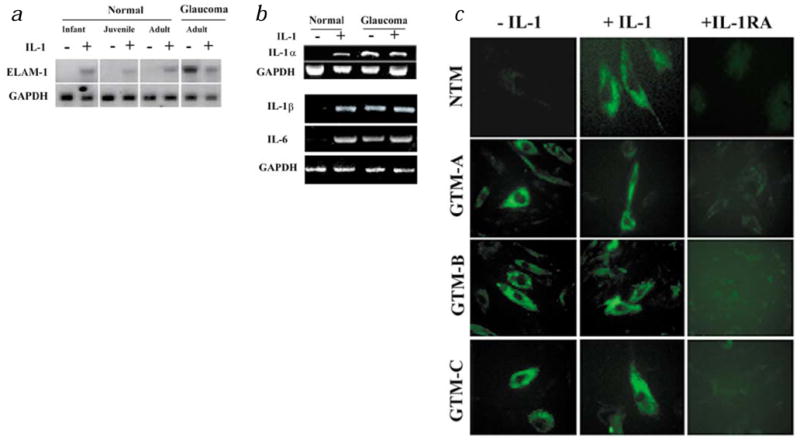
Regulation of ELAM-1 expression by exogenous and endogenous IL-1. Normal and glaucomatous TM cells were plated into replicate cultures and were untreated (−) or treated with IL-1 for 2 h (+). In some experiments, cells were also treated with receptor antagonist (IL-1ra) for 24 h. a, Northern blotting with ELAM-1 cDNA (ref. 42). The GAPDH probe35 served to determine the equality of RNA loading among lanes. b, RT-PCR analysis for IL-1α, IL-1β and IL-6 mRNA. The cDNA for GAPDH was amplified in parallel reactions to assess cDNA-loading equivalence among samples42. c, Indirect immunofluorescent staining with antibody to ELAM-1. NTM: normal TM cells. GTM-A: glaucomatous TM cells. GTM-B: glaucomatous TM cells, specimen-B. GTM-C: glaucomatous TM cells, specimen-C.
Expression of ELAM-1 (ref. 17), IL-1α, IL-1β and IL-6 (ref. 21) is dependent on activity of the NF-κB family of dimeric DNA-binding complexes24. Electrophoretic mobility shift assay (EMSA) revealed that exogenous IL-1 stimulated NF-κB DNA-binding activity in normal TM cells (Fig. 3a). This binding activity was present constitutively in glaucomatous TM cells, and was further stimulated by exogenous IL-1. Antibodies to NF-κB family member p50 (NF-κB1) completely super-shifted the inducible complexes from normal cells and the constitutive complex from glaucomatous cells, whereas antibodies to p65 (Rel A) super-shifted a distinct subcomponent of these complexes (Fig. 3a). This is consistent with the identities of the complex of faster mobility as p50–p50 homodimers and the complex of slower mobility as p50–p65 heterodimers. Interestingly, p50 antibodies did not alter the electrophoretic mobility of the inducible complexes from glaucomatous cells, although these complexes were shifted completely with p65 antibodies (Fig. 3a). This indicates that disease alters the expression of NF-κB family members. IL-1ra treatment eliminated the constitutive NF-κB DNA-binding complex from glaucomatous cells (Fig. 3b). Treatment with the NF-κB antagonist, SN50 peptide, reduced ELAM-1 expression in glaucomatous TM cells, whereas treatment with control peptide again had no effect (Fig. 3c). These findings indicate that IL-1 sustains ELAM-1 expression in the TM cells of glaucomatous eyes through a signaling pathway that culminates in activation of NF-κB.
Fig. 3.

NF-κB activity in normal and glaucomatous TM cells. a, TM cells isolated from normal and glaucomatous eyes were left untreated (−) or treated with IL-1 for 2 h (+). EMSA was performed. Arrowhead indicates the migration of NF-κB protein–DNA complex. Specificity is indicated by supershift analysis with antibodies to the p50 or p65 subunits of NF-κB and competition analysis with ‘cold’ NF-κB probe (1:50). b, EMSA from TM cells left untreated (−), treated with IL-1 for 2 h, or treated overnight with IL-1ra. Specificity was demonstrated using a control probe corresponding to the E-box-like element in the human IL-1α gene promoter35. Binding to this probe was unaffected by treatment with either IL-1 or IL-1ra (data not shown). c, Indirect immunofluorescent staining with antibody to ELAM-1.
Activation of NF-κB in response to stimulators involves release from an inhibitor, I-κB, exposing a protein domain which enables translocation to the nucleus24,25. An antibody against the nuclear localization epitope which recognizes only the active form of p65 showed substantial binding to TM cells surrounding Schlemm’s canal in glaucomatous tissue specimens, but binding was not detectable in the outflow pathways of normal eyes (Fig. 4). The localization pattern of activated p65 antigen overlapped substantially with that of ELAM-1 antigen. These data support the hypothesis that NF-κB activation stimulates ELAM-1 expression in situ.
Fig. 4.

Active NF-κB in glaucomatous TM cells in situ. Double-label immunofluorescent localization was performed using antibody that binds ELAM-1 and antibody against the p65 nuclear localization signal, which binds only the activated form of this subunit free of I-κB. Sections were further stained with Hoechst dye to visualize nuclei. ‘Triple’ indicates the overlap of all images. Magnification: ×40.
IL-1-NF-κB signaling protects cells from oxidative stress
IL-1 and NF-κB are reported to be protective of cells subjected to stress26,27. We performed an experiment to determine whether endogenous IL-1 protects glaucomatous TM cells (Fig. 5). Normal TM cells exhibited a dose-dependent apoptotic response when treated with the oxidant tBH. In contrast, glaucomatous TM cells were resistant to the oxidant, with apoptosis apparent only at the highest dose in the range. When normal TM cells were treated with IL-1, the threshold oxidant dose for eliciting apoptosis was also increased. Conversely, when glaucomatous TM cells (which produce IL-1 endogenously) were treated with the IL-1 antagonist, IL-1ra, they exhibited less resistance to the oxidant. The NF-κB antagonist SN50 also decreased resistance, whereas control peptide had no effect. These results support the hypothesis that activation of NF-κB through IL-1 is protective of glaucomatous TM cells.
Fig. 5.

Resistance of normal and glaucomatous trabecular meshwork cells to apoptosis in response to an oxidant, and protective role of IL-1 and NF-κB. Normal TM cells or glaucomatous cells were treated with indicated concentrations of the oxidant tBH for 6 hours and stained with fluorescein-TUNEL assay. Groups of cells were pre-treated with IL-1 (10μg/ml), IL-1ra (500 μg/ml) or SN-50 peptide (NF-κB antagonist, 50 μg/ml) then assayed for the apoptotic response. Cells were also stained with propidium iodide to stain the nucleus. Arrows indicate apoptotic cells which stain yellow because of the colocalization of TUNEL staining and propidium iodide.
Discussion
The vascular endothelium and the TM have a common role in the formation of biological structures for containment and conduction of bodily fluids. As such, they share many properties, including the expression of the CAM markers defined in this study. Nonetheless, the vascular endothelium and the TM can be clearly distinguished by presence or absence of the vascular endothelial-cell marker, factor VIII (ref. 28), and they are derived from different embryological precursors29. ELAM-1 upregulation in the vascular endothelium has been associated with a broad range of acute and chronic disease states in all organs of the body, including the eye30–32. A few reports also describe ELAM-1 expression in the inflamed corneal endothelium30, a tissue that is continuous with the TM and of similar embryonic origin. However, this is the first report of which we are aware that identifies ELAM-1 expression in the TM and Schlemm’s canal. We note that the juxtacanalicular location of ELAM-1 expression that we observed corresponds to the site of maximal resistance to aqueous outflow, thought to be the location of the glaucomatous lesion in primary open angle glaucomas3–5.
Considering the structural and functional similarities between the vasculature and the eye’s aqueous outflow pathway, it is not surprising that diseases of these tissues share many points of convergence. For example, oxidized low-density lipoprotein8 is a major initiating factor in atherosclerosis, and oxidative stress is also implicated in glaucoma pathogenesis10. Fluid pressure gradients in the form of shear stress are implicated in stimulating ELAM-1 expression in vascular disease14,17,18, and elevated blood pressure and IOP are key risk factors in the pathophysiology of vascular disease14,17 and glaucoma5, respectively. Inflammation and inflammatory cytokines17 are central to the stimulation of ELAM-1 expression in the diseased vasculature, and the inflammatory cytokine IL-1α is upregulated in vascular endothelial cells isolated from the atherosclerotic lesion33. Only a few glaucomas are associated with inflammation; however, we found in this study that IL-1 is consistently upregulated by glaucomatous TM cells and acts as a key autocrine regulatory factor for ELAM-1 expression, much as in vascular disease. This difference may be due to environment—the cellular and extracellular composition of arterial matrices are more complex than those of TM, and the acellular aqueous humor which percolates through the aqueous outflow pathways is different from the cellular blood that flows through arterial endothelial vessels. Inflammatory mediators may stimulate systemic effects when expressed by vascular endothelia, but effects may be locally contained when expressed by TM.
Activation of the transcription factor NF-κB is a common end point for diverse stress signals, including oxidative stress34. The gene for ELAM-1 has NF-κB response elements in its transcriptional promoter14,17. We show here that endogenous IL-1 (possibly augmented by IL-6) controls ELAM-1 expression in glaucomatous TM cells through NF-κB. NF-κB is also known to mediate expression of the inflammatory cytokines IL-1α, IL-1β and IL-621,24,35. This suggests a model in which sublethal damage to TM cells initiates NF-κB activation, which in turn activates the genes for both ELAM-1 and the inflammatory cytokines. At first, ELAM-1 and IL-1/IL-6 gene expression would occur only at very low levels depending on the strength and duration of the initial damaging stimulus. However, this signal would be progressively amplified by the cytokines through further activation of NF-κB. Ultimately, NF-κB activation would become self-sustaining, and ELAM-1 and IL-1/IL-6 expression would reach levels easily detectable by the methods used in this study.
The genetic response to sublethal cellular stress confers protection to the surviving tissue in a variety of disease states8,9. Autocrine IL-1 expression has been associated with replicative senescence in cultured fibroblasts36, and treatment with exogenous IL-1 extends the replicative lifespan of cultured endothelial cells26. In addition, a previous study has demonstrated that activity of NF-κB prevents cells from undergoing apoptosis in response to stressful stimuli by controlling expression of genes encoding anti-apoptotic proteins27. Our results now place glaucoma in this group, as we show that IL-1 produced endogenously by glaucomatous TM cells inhibits the apoptotic response to oxidative stress through NF-κB. IL-1 may have additional beneficial effects in glaucoma, as it has been reported to increase outflow facility37, perhaps through its ability to stimulate expression of matrix metalloproteinases (MMPs)38.
Although the tissue response to stress may be protective in the short run, if continued on a chronic basis it has the potential to become the disease entity itself, a lesson already learned for vascular disease8. In open-angle forms of glaucoma, IOP would increase as TM damage accumulated, secondarily amplifying NF-κB activation12,18. In closed-angle forms of glaucoma, elevated IOP resulting from anatomic obstruction of the angle would be the primary oxidative stress initiating NF-κB activation, but this would be subsequently amplified through autocrine cytokine loops. The IL-1–NF-κB pathway generates oxygen free radicals as signaling intermediates12,34, which could cause cell damage10. Moreover, downstream targets of this pathway such as MMPs could ultimately cause irreversible damage to the trabecular beams. Thus, activation of the IL-1/NF-κB pathway may provide a unifying disease mechanism for pathophysiology in glaucomas of diverse etiology.
To our knowledge, ELAM-1 represents the first molecular marker of the glaucoma disease phenotype, that is, it is expressed in an all-or-none fashion, and could be used diagnostically. However, activation of the NF-κB signaling pathway in TM cells may be the more comprehensive determinant of disease, because it controls not just one gene (ELAM-1), but a whole battery24. We detected upregulation of three additional members of this battery (IL-1α, IL-1β and IL-6) in glaucomatous TM, although we reserve judgement on their designation as true disease markers since we did not rigorously document their absence from normal TM. We predict that other disease markers besides ELAM-1 will be found once additional members of the gene battery stimulated by NF-κB in the TM are identified. The possible role of ELAM-1 in protection or pathophysiology of the glaucomas remains to be learned.
The continued capacity to distinguish normal and diseased TM cells on the basis of ELAM-1 expression even after subculture indicates that TM cells retain a memory of their disease history when removed from the glaucomatous conditions in the eye. This means that TM cells can be amplified in culture to provide material for molecular characterization of the disease lesion by application of differential cloning and gene profiling strategies. All current therapeutic modalities for glaucoma are aimed at reducing IOP by decreasing aqueous humor production or by increasing outflow facility through alternative pathways5. Greater understanding of the mechanisms leading to TM-cell damage in the glaucomatous eye will facilitate development of new management strategies that target the primary disease lesion.
Methods
Tissue specimens, embedding and cell culture
Sixteen normal (age range: 4 d to 88 y) and 12 glaucomatous (age range: 67 to 86 y) cadaver eyes were obtained from the National Disease Research Interchange and the New England Eye Bank. Eyes were enucleated within 2–4 h after death and processed for experiments within 20–36 h. Anterior segments were isolated, lenses were removed and the specimens were halved; one portion was then embedded for frozen sectioning and the TM was isolated from the other portion and explanted to culture. Surgical specimens from 23 glaucoma patients (age range 16–88 y) were used for either frozen section or culture (Table 1). Cultures from single individuals were maintained as separate lines and used before fourth passage. In some experiments, cells were treated with IL-1α at 10 ng/ml and/or IL-1ra at 100–1000 ng/ml (R&D Systems, Minneapolis, Minnesota). In other experiments, cells were treated with the NF-κB antagonist, SN50 or control peptide (Biomol, Plymouth Meeting, Pennsylvania) at 50 μg/ml.
Immunolocalizations
Nine different CAM monoclonals were used as primary antibodies for indirect immunolocalization on frozen tissue sections (4 μM) or cells cultured on glass slides. These were the selectin family member ELAM-1 (E-selectin; CD62E); the immunoglobulin superfamily members N-CAM-16 (CD56), ICAM-1 (CD54), PECAM-1 (CD31) and VCAM-1 (CD106); and the integrin family members integrin α2 (VLA-2; CD49b), integrin α3 (VLA-3; CD49c) and integrin α5 (VLA-5; CD49d). Control antibody probes included the inflammation marker LFA-1α (lymphocyte function-associated antigen-1α; CD11a)39, the fibrosis marker HPCA-2 (human progenitor cell antigen-2; CD34)40, and the TM cell marker HLA class I (major histocompatibility complex reagent-1; HLA-ABC)41. Each probe was purchased from Becton Dickinson (San Jose, California) with the exception of PECAM-1 antibody, which was purchased from Dako (Carpinteria, California). Antibody used to detect activity of the NF-κB p65 subunit in tissue sections was purchased from Boehringer. Eight to 20 tissue sections and 4 coverslips of cultured cells were probed with each primary antibody. The avidin–biotin–peroxidase complex technique was used to visualize antibody binding in initial experiments, but later experiments used a fluorescent secondary antibody. Appropriate controls for antibody specificity were included. Stained slides were examined by light microscopy and positivity was scored in a double-masked analysis.
RNA analysis
Northern blotting and reverse transcription (RT)-PCR were performed as described35,42. Sequences for construction of the IL-1α oligonucleotide primer pair were obtained from Genbank (primer: 5′-CGGCTGCTGCATTACATAATCTGG; reverse: 5′-TGAAAGTCAGTGATAGAGGGTGGC-3′). The IL-1β primer pairs were obtained from Clontech (forward: 5′-ATGGCAGAAGTACCTAAGCTCGC-3′; reverse: 5′-ACACAAATTGCATGGTGAAGTCAGTT-3′). The IL-6 primer pair was purchased from R&D Systems (proprietary sequences).
Electrophoretic mobility shift assay (EMSA)
Nuclear lysates were prepared from cultured TM cells and EMSA was performed using a protein equivalent amount of each lysate as described35. The radiolabeled NF-κB probe was 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (Promega, Madison, Wisconsin). Supershift analysis was performed with p50 (NF-κB1) and p65 (RelA) antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, California).
Apoptosis assay
TdT-mediated dUTP nick-end labeling (TUNEL) was performed using the in situ cell death detection kit with fluorescein (Boehringer). For this assay, TM cells plated in 8-well chamber slides at 1 × 105 cells/well were treated with 125–500 μM tert-butyl hydroperoxide (tBH, Sigma) for 6 h.
Acknowledgments
We thank members of the Schuman and Fini labs for their technical assistance. Some surgical specimens were provided by the Institute of Ophthalmology & Eye Hospital, Shandong Academy of Medical Science, and the Department of Ophthalmology, Wuhan Municipal First Hospital, P.R. China. The ELAM-1 cDNA was a gift from B. Seed and the GAPDH cDNA was a gift from R. Allen. This work was supported by the Glaucoma Foundation, New York (J.S.S.), the American Health Assistance Foundation (J.S.S.), NEI grant EY09828 (M.E.F.), the Massachusetts Lions Eye Research Fund and Research to Prevent Blindness.
References
- 1.Quigley HA. Number of people with glaucoma world-wide. Brit J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 3.Epstein DL, Allingham RR, Schuman JS, editors. Chandler and Grant’s Glaucoma. 4. Williams and Wilkins; Baltimore, Maryland: 1996. pp. 18–24. [Google Scholar]
- 4.Underwood JL, et al. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intracellular junctions. Am J Physiol. 1999;277:C330–342. doi: 10.1152/ajpcell.1999.277.2.C330. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi RC. Mechanism of the aqueous outflow across the trabecular wall of Schlemm’s canal. Exp Eye Res. 1971;11:116–121. doi: 10.1016/s0014-4835(71)80073-8. [DOI] [PubMed] [Google Scholar]
- 6.Schuman JS, Wang N, Eisenberg DL. Leukemic glaucoma: the effects on outflow facility of chronic lymphocytic leukemic lymphocytes. Exp Eye Res. 1995;61:609–617. doi: 10.1016/s0014-4835(05)80054-5. [DOI] [PubMed] [Google Scholar]
- 7.Lütjen-Drecoll E, Shimizu T, Rohrbach M, Rohen JW. Quantitative analysis of “plaque” material in the inner and outer wall of Schlemm’s canal in normal and glaucomatous eyes. Exp Eye Res. 1986;42:443–455. doi: 10.1016/0014-4835(86)90004-7. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Cell Biology of Atherosclerosis. Annu Review Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 9.Dunn CJ. Cytokines as mediators of chronic inflammatory disease. In: Kimball ES, editor. Cytokines and Inflammation. CRC Press; London: 1991. pp. 1–34. [Google Scholar]
- 10.Green K. Free radicals and aging of anterior segment tissues of the eye: a hypothesis. Ophthalmic Res. 1995;27:143–149. doi: 10.1159/000267860. [DOI] [PubMed] [Google Scholar]
- 11.Flammer J, Haefliger IO, Orgul S, Resnick T. Vascular deregulation: a principal risk factor for glaucomatous damage? J Glaucoma. 1999;8:212–219. [PubMed] [Google Scholar]
- 12.Yeh LH, et al. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am J Physiol. 1999;276:C838–847. doi: 10.1152/ajpcell.1999.276.4.C838. [DOI] [PubMed] [Google Scholar]
- 13.Mercurio F, Manning AM. NF-κB as a primary regulator of the stress response. Oncogene. 1999;18:6163–6171. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- 14.Itoh H, Nakao K. Vascular stress response and endothelial vasoactive factors for vascular remodeling. Diabetes Res Clin Pract. 1999;45:83–88. doi: 10.1016/s0168-8227(99)00035-2. [DOI] [PubMed] [Google Scholar]
- 15.Polansky JR, et al. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica. 1997;211:126–139. doi: 10.1159/000310780. [DOI] [PubMed] [Google Scholar]
- 16.Petruzelli L, Takami M, Mimi T, Humes HD. Structure and function of cell adhesion molecules. Am J Med. 1999;106:467–476. doi: 10.1016/s0002-9343(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 17.Price DT, Loscalzo J. Cellular adhesion molecules and atherosclerosis. Am J Med. 1999;107:85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 18.Gimbrone MA, Nagel T, Topper JN. Perspective series: Cell adhesion in vascular biology. Biomechanical activation: an emerging paradigm in endothelial cell adhesion biology. J Clin Invest. 1997;99:1809–1813. doi: 10.1172/JCI119346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulson JC. In: Adhesion: Its role in Inflammatory Disease. Harlan JM, Liu DY, editors. W.H. Freeman; New York: 1992. pp. 19–42. [Google Scholar]
- 20.Kawa JE, Higginbotham EJ, Chang IL, Yue BYJ. Effects of antiglaucoma medications on bovine trabecular meshwork cells in vitro. Exp Eye Res. 1993;14:560–565. doi: 10.1006/exer.1993.1160. [DOI] [PubMed] [Google Scholar]
- 21.Dinarello CA. Biology of interleukin 1. FASEB J. 1988;2:108–115. [PubMed] [Google Scholar]
- 22.West-Mays JA, Strissel KJ, Sadow PM, Fini ME. Competence for collagenase gene expression by tissue fibroblasts requires activation of an interleukin 1α autocrine loop. Proc Natl Acad Sci USA. 1995;92:6768–6772. doi: 10.1073/pnas.92.15.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 24.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 25.Bauerle PA, Baltimore D. IκB: a specific inhibitor of the NF-κB transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 26.Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990;249:1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- 27.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-—induced cell death. Science. 1996;274:782–789. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 28.Krohn J. Expression of factor VIII-related antigen in human aqueous drainage channels. Acta Ophthalmol Scand. 1999;77:9–12. doi: 10.1034/j.1600-0420.1999.770102.x. [DOI] [PubMed] [Google Scholar]
- 29.Foets B, van den Oord J, Engelmann K, Missotten L. A comparative immunohistochemical study of human corneotrabecular tissue. Graefes Arch Clin Exp Ophthalmol. 1992;230:269–74. doi: 10.1007/BF00176303. [DOI] [PubMed] [Google Scholar]
- 30.Whitcup SM, Wakefield D, Li Q, Nussenblatt RB, Chan CC. Endothelial leukocyte adhesion molecule-1 in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci. 1992;33:2626–2630. [PubMed] [Google Scholar]
- 31.Bacon AS, et al. Adhesive molecules and relationship to leukocyte levels in allergic eye disease. Invest Ophthalmol Vis Sci. 1998;39:322–330. [PubMed] [Google Scholar]
- 32.Pappa A, Calder V, Fells P, Lightman S. Adhesion molecule expression in vivo on extraocular muscles (EOM) in thyroid-associated ophthalmopathy (TAO) Clin Exp Immunol. 1997;108:309–313. doi: 10.1046/j.1365-2249.1997.3621258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loppnow H, Libby P. Functional significance of human vascular smooth muscle cell-derived interleukin 1 in paracrine and autocrine regulation pathways. Exp Cell Res. 1992;198:283–90. doi: 10.1016/0014-4827(92)90381-h. [DOI] [PubMed] [Google Scholar]
- 34.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook JR, Mody MK, Fini ME. Failure to activate transcription factor NF-κB in corneal stromal cells (keratocytes) Invest Ophthalmol Vis Sci. 1999;40:3122–3131. [PubMed] [Google Scholar]
- 36.Kumar S, Millis AJT, Baglioni C. Expression of interleukin 1-inducible genes and reduction of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci USA. 1992;89:4683–4687. doi: 10.1073/pnas.89.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kee C, Seo K. The effect of interleukin-1alpha on outflow facility in rat eyes. J Glaucoma. 1997;6:246–249. [PubMed] [Google Scholar]
- 38.Alexander JP, Samples JR, Acott JS. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Curr Eye Res. 1998;17:276–85. doi: 10.1076/ceyr.17.3.276.5219. [DOI] [PubMed] [Google Scholar]
- 39.Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- 40.Schuman JS, Wang N, Eisenberg DL. Expression of cell adhesion molecules in the trabecular meshwork and aqueous outflow pathways. In: Nussenblatt RB, Whitcup SM, Caspi RR, Gery I, editors. Advances in Ocular Immunology. Elsevier, Amsterdam; The Netherlands: 1994. pp. 47–50. [Google Scholar]
- 41.Lynch MG, Peeler JS, Brown RH, Niederkorn JY. Expression of HLA Class I and II antigens on cells of the human trabecular meshwork. Ophthalmology. 1987;94:851–857. doi: 10.1016/s0161-6420(87)33539-0. [DOI] [PubMed] [Google Scholar]
- 42.Fini ME, Girard MT, Matsubara M, Bartlett JD. Unique regulation of the matrix metalloproteinase, gelatinase B. Invest Ophthalmol Vis Sci. 1995;36:622–636. [PubMed] [Google Scholar]


