Abstract
The prostaglandin PGE2 mediates estradiol-induced masculinization of sexual behavior in the rat during a perinatal sensitive period. PGE2 induces formation of dendritic spines on POA neurons and this synaptic pattern change is associated with the ability to express male sexual behavior as an adult. Whether PGE2 is released from astrocytes or neurons in the developing POA is unknown. To further understanding of how PGE2 induces dendritic spine formation at the cellular level, we have explored the PGE2 receptor subtype mediating this response. There are four receptors for PGE2, EP1, EP2, EP3 and EP4, each having unique but interacting signal transduction profiles. Treatment of newborn female rats with the EP receptor agonists Iloprost, Butaprost and Sulprostone indicated that stimulation of both the EP2 and EP3 receptors significantly increased spinophilin, a protein which levels positively correlate to the presence of dendritic spines and masculinization of the POA. Use of antisense oligonucleotides against the mRNA for each receptor reveals that either EP2 or EP3 receptor knockdown reduces spinophilin in PGE2 or estradiol-treated females, whereas reducing EP1 or EP4 receptor levels by the same means has a smaller but also significant effect. A developmental profile of EP receptor expression indicates EP1 in particular is elevated for the first few days of life, corresponding to the critical period for masculinization, whereas mRNA levels for the other three receptors remain relatively constant.
Keywords: cox-2, masculinization, male sexual behavior, sex differences
INTRODUCTION
The preoptic area (POA) is a subdivision of the rostral hypothalamus and serves an integral role in adult behavioral patterns associated with reproduction such as sexual behavior and parenting. Neurotoxic lesions of the POA disrupt normal copulation in males (Meisel and Sachs, 1997; Christensen et al., 1977) and direct androgen stimulation of the POA elicits male-typical sexual behaviors after castration (Christensen and Clemens, 1974). The capacity to express male sexual behavior in adult inbred laboratory rats is dependent upon developmental exposure to estradiol (Booth, 1977), which is aromatized from androgen precursors in neurons. The origin of the androgens is the fetal and neonatal testis, resulting in sexually dimorphic hormonal milieus during a critical perinatal sensitive window that organizes the neural substrate(s) mediating adult behavior. Developmental exposure to estradiol results in numerous sexually dimorphic aspects of the POA including cellular morphology of both neurons and astrocytes, and synaptic patterning. (Dohler et al., 1984; McCarthy et al., 2003; McCarthy et al., 2002; Amateau et al., 2004; Amateau and McCarthy, 2002a; Amateau and McCarthy, 2002b). Parameters seen normally in males can be mimicked in females by exogenous administration of high levels of estradiol or testosterone during a perinatal sensitive period. We recently reported that a principle action of estradiol in the developing POA is up regulation of the enzyme, cyclooxygenase-2 (COX-2), which converts arachidonic acid into PGG2, ultimately resulting in increased PGE2 via the enzyme PGE synthase acting on the substrate PGH2 (Blatteis et al., 2005). Elevated PGE2 in the developing POA results in increased dendritic spines via an AMPA receptor dependent mechanism and this morphological change to the neuronal network correlates with the expression of male sexual behavior in adulthood (Amateau and McCarthy, 2004). Treatment of newborn females with PGE2 directly into the POA results in the capacity to express the full complement of male sexual behavior (exclusive of intromission and ejaculation) as adults and is a first ever demonstration of masculinization in the absence of exogenous hormone administration (Amateau and McCarthy, 2004). Thus, PGE2 is a major mediator of estradiol-induced masculinization of male sexual behavior and increased dendritic spine density on POA neurons.
The role of prostaglandins in neuronal functioning appears highly varied and poorly understood, particularly in regards to brain development. In adults, PGE2 levels vary in response to nociceptive events such as inflammation (Barr et al., 2004) and cerebral ischemia (McCullough et al., 2004). PGE2 signaling is propagated by interaction with the EP class of receptors identified as EP1-4. Contrary to our previous findings it is clear that all four prostanoid receptors are present in the developing POA, but which are responsible for estradiol mediated masculinization of the region remains unknown. A reliable and robust marker for PGE2 mediated masculinization of the developing POA is the upregulation of spinophilin, a signaling protein preferentially localized to the necks of dendritic spines and strongly and positively correlated with the presence of spines themselves (Amateau and McCarthy, 2004).
In the present study we use selective agonists to stimulate EP receptor activity, as well as reduce receptor protein levels with antisense oligonucleotide (oligo) sequences to gain insight into which EP receptor subtypes mediate PGE2 effects on spinophilin levels. The results indicate that endogenous expression levels of all receptors are necessary for the full induction of POA spinophilin by estradiol and/or PGE2 but also indicate the combination of selective EP2 or EP3 activation produces a result identical to that of the hormones.
EXPERIMENTAL PROCEDURES
Animals
Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were mated in our animal facility. Pregnancy was confirmed by presence of sperm in a vaginal smear and pregnant dams were isolated and allowed to deliver normally. Animals were given free access to food and water and kept on a reverse 12h light/dark cycle. On the day of birth [postnatal day 0 (PN0)], pups from multiple litters were assigned to experimental groups All procedures were approved by the IACUC of the University of Maryland, Baltimore.
Intracerebroventricular (ICV) injections
Injections were performed by hand after pups were anesthetized by cold and placed under bright light where the cranial landmark Bregma is visible underneath the skin, and can be used to locate the ventricles. Injections were performed bilaterally with a 23 gauge 1 μl Hamilton syringe stereotaxically lowered to a depth of 4 mm. A volume of 1 μl was infused over a period of 60 seconds.
Experiment One: EP Receptor Agonist Manipulations
Female pups were treated within 6 hr of birth, and again ~24 hr later. Pups were assigned to one of six treatment groups and given either (1) vehicle alone (n=5), (2) 2.5μg PGE2 (Sigma) (n=5), (3) 22.7μg Iloprost, an EP1 agonist (n=6), (4) 25μg Butaprost, an EP2 agonist (n=6), (5) 7.14μg Sulprostone, an EP1/EP3 agonist (n=6), (6) 7.14 μg Sulprostone and 25μg Butaprost (n=6). The 2.5μg dose of PGE2 is consistent with previous studies in our lab and shown to be sufficient to induce masculinization (Amateau and McCarthy, 2002b). Doses of the synthetic agonists necessary to mimic the control dose of PGE2 were calculated by a ratio of disassociation constants (KdPGE2/KdAgonist) and then multiplied by 2.5. All EP receptor agonists were obtained from Cayman Chemical (Ann Arbor, MI). All drugs were diluted in 100μl 0.9% saline vehicle and administered by ICV injection. Brains were collected on PN2, ~24 hr after the second treatment.
Microdissections
Under aseptic conditions, animals were sacrificed on PN2. Brains were removed and placed in a Zivic Miller brain mold and sectioned at 1mm. The optic chiasm which appears at the rostral portion of the diencephalon, served as the marker to guide rostral-to-caudal sectioning within the mold. The preoptic area was then dissected out using the anterior commissure as the perimeter for both dorsal and lateral incisions. The next most caudal 1mm section was isolated, the cortex peeled from the dorsal end of the slice, and the 1mm thick section of both hippocampi were dissected. The dissected tissue was immediately collected, flash frozen in isopentane, and stored at −80°C.
Western Immunoblots
The microdissected POA was homogenized in RIPA buffer and then centrifuged at −9°C at 3000 RPM for 30 min. Supernatant was removed and used for subsequent experiments. For standardization, protein concentration of samples was determined by Bradford protein assay. Aliquots of 15μg of each protein sample were electrophoresed through an 8–16% precast SDS-polyacrylamide gel (Novex, San Diego, CA) and then transferred to a polyvinyl difluoride membrane (Bio-Rad, Hercules, CA). Blocking was done in 5% non-fat milk in 0.1% Tween TBS for 1hr at room temperature for hybridizations with anti-spinophilin. Membranes were then incubated with anti-spinophilin/neurabin II rabbit polyclonal IgG at 1:3000 (Upstate Biotechnology, Lake Placid, NY). Secondary incubations for 30 min at room temperature were done in either goat anti-rabbit HRP conjugated IgG at 1:10,000 dilution (New England Biolabs, Beverley, MA). The Phototope chemiluminescence system (New England Biolabs) was used to detect protein. All blots were exposed on Hyperfilm-ECL (Amersham, Arlington Heights, IL) for 30 seconds
Experiment Two: Antisense Oligonucleotide Interference with EP Receptor mRNA Translation
On the day of birth, female pups were assigned to 6 experimental groups. SCRAM + saline (n=6), SCRAM + PGE2 (n=6), EP1AS + PGE2 (n=6), EP2AS + PGE2 (n=6), EP3AS + PGE2 (n=6), or EP4AS + PGE2 (n=5). All oligonucleotides treatments were administered at a concentration of 0.5μg/μl saline via ICV injection as outlined above, followed ~4hr later by PGE2 infusion (2.5 μg/μl saline via ICV). This procedure was repeated on PN1 for a total of 4 infusions (2 of oligo, 2 of PGE2). Animals were sacrificed and brains dissected on PN2. Oligonucleotide sequences were generated from GenBank accession numbers for prostanoid receptor sequences: EP1 (NM_013100); EP2 (NM_031088); EP3α (NM_012704); EP3 β (X80133); EP4 (D28860). A random sequence of oligonucleotides (SCRAM) served as a control for oligonucleotide infusion. The synthetic oligonucleotide sequences are as follows: SCRAM C*C*G* ATG AAC TGT CGC GAT G*G*A*, EP1AS G*G*C* TCA TAT CAG TGG CCA A*G*A* (571–591), EP2AS A*A*G* AAT TGT CCA TGG TGG A*G*G* (35–55), EP3AS A*C*A* CGC CGG TAG TGG C*G*G* (78–98 EP3α, 96–116 EP3β), EP4AS A*C*T* CCA ACC ACC ATC CAG G*T*C* (62–82). All oligonucleotides contained locked nucleic acid (LNA) bases that are denoted by asterisks (*). Sequences were queried into the BLAST database (www.ncbi.nlm.nih.gov/BLAST/) and showed significant homology only to their specific target sequences within the relevant genome. The sequence SCRAM had no significant homology to any sequence in the genome. Oligonucleotides were obtained from Proligo (Boulder, CO).
Treatment with estradiol
To investigate the effects of antisense with estradiol, the previous experiment using antisense with PGE2 treatment was repeated in an identical manner with the exception of using estradiol in place of PGE2. Female pups were injected subcutaneously with estradiol benzoate (100μg) in 0.1 cc sesame seed oil or oil alone on PN0 and again on PN1. This dose is routinely used in our laboratory for induction of masculinization of the brain (Amateau and McCarthy 2004, Mong and McCarthy 1999). Treatment with either SCRAM or an antisense oligonucleotide sequence was done 4 hr prior to treatment with estradiol.
Western blot analysis
Microdissection of the POA and Western blot analysis for spinophilin protein were performed as described above. Western blots for EP receptors used the same tissue isolated from PGE2 treated groups. These samples were subjected to the same protocol as described above until the blocking step is reached. Blocking for hybridizations with anti-EP2, anti-EP3, and anti-EP4 was done in 10% non-fat milk in 0.1% Tween TBS overnight at 4°C. Blocking of the membranes for anti-EP1 was performed in 10% normal rabbit serum in 0.1% Tween TBS at room temperature for 1 hr. anti-EP1 goat polyclonal IgG at 1:750, anti-EP2 rabbit polyclonal IgG at 1:5000, anti-EP3 rabbit polyclonal IgG at 1:3000, or anti-EP4 rabbit polyclonal IgG at 1:3000 in Tween TBS for 3 hr at room temperature. All prostanoid receptor antibodies were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. Secondary incubations for 30 min at room temperature were done with rabbit anti-goat HRP conjugated IgG at 1:10,000. Western blots were analyzed by identifying the protein band relative to a mass of 120 kDa. The integrative grayscale pixel area-density (iad) was captured with a CCD camera and quantified with NIH image software. Comparisons were made as a ratio of the iad of the sample over the iad of vehicle controls on the same gel.
Experiment Three: Developmental Profile of EP Receptor mRNA
The POA was collected from four males and four females (n = 8) at the following time points: PN 0, 3, 20, 40. The treatment group for PN6 consisted of two males and four females (n = 6). Two females and four males (n =6) were used for PN10 measurements.
RNA Isolation
Isolation of RNA was carried out by utilizing Trizol reagent as described in Chomsky et al, excepting the modifications described below. Each POA was suspended in 1mL Trizol reagent at 4°C and immediately homogenized. The Trizol reagent/POA homogenate was passed through a 27.g gauge needle twice to decrease the viscosity. Heavy phase lock gel from Eppendorf was utilized for the separation of aqueous from organic phases in the RNA protocol and the separation of phases was carried out at 12000 g at 4 degrees. RNA was precipitated through the addition of 125 μL of ice cold isopropyl alcohol and 125 μL of a high salt solution containing .8 M sodium citrate and 1.2 M NaCl for every .5 μL of original Trizol added. The precipitation was allowed to occur for 30 min at 4°. 4) RNA was resuspended in 40 μL of pure H2O, followed by heating at 50° C for 15 min. RNA was assessed for purity and concentration at a 1:14 dilution by 260–280 nm absorbance spectroscopy. Acceptable purity ratios were between 1.76 and 2.05.
Reverse Transcription
Applied Biosystems (Foster City, CA) reverse transcription reagents were added to 1 μg of RNA suspended in 38.5 μL H2O in the following volumes: 10 μL 10 X TaqMan RT buffer; 22 μL 25 mM MgCl2; 20 μL dNTPs; 5μL random hexamers; 2 μL RNase inhibitor; 2.5 μL MultiScribe reverse transcriptase (total reaction volume 100 μL). The reaction was carried out as follows: 10 min primer incubation at 25° C; 60 min extension at 37° C; and 5 min rt inactivation at 95° C. The PN0 and PN40 samples plus 10 random samples from the intermediate time points were subject to controls for genomic DNA where a mock rt PCR reaction was carried out without reverse transcriptase. These samples plus the reverse transcribed samples were subject to a regular PCR reaction and products were run out on an agarose gel. Negative reverse transcriptase samples did not yield a PCR product controlling for genomic DNA contaminants.
Primer Design
Primers were designed using Primer Express from Applied Biosystems and used the GenBank Accession Numbers listed above. EP1 forward primer (AAGGCAGTGACAGGTGAAGTGG) started at position 453l; reverse (CGTCATGGAGAAGATAGGCAGC) started at position 722. Amplicon length was 270 bp. EP2 forward primer (CTTGCTCTTCTGTTCTCTGCCG) started at position 537; reverse (GCTTCTTTTGCTCCGAAGCTG) started at position 741. Amplicon length was 205 bp. EP3 forward primer (TTGTGTCGCGCAGCTATAGAC) started at position 231. Reverse primer (TCCGAACACTGTCATGGTCAG) started at position 429. Amplicon length was 209 bp. EP4 forward primer (TGCTCAGAGAGTCGGAGGACAAT) started at position 1276; reverse primer (GAAATTCGCAAAGTTCTCAGCG) started at position 1490. Amplicon length was 215 bp.
Real Time PCR
Real time PCR utilized SYBR green and the standard curve method for relative RNA quantification. Real-time PCR reactions were as follows: 5 μL DNA, 5 μL 10 nM primers, 10 μL 2X SYBR green Master Mix (New England Biolabs, Ipswich, MA). The reactions were as follows 1) 10 min 95° C start; 2) 10 sec 94° C melt step; 3) 15 sec primer incubation step; 4) 15 sec 72° C extension step; 5) 1sec 79 or 80° read fluorescence step 6) cycle from step 2 to step 5 40 times; 7) 10 min 72° product stabilization step; and 8) a melting curve. The primer incubation steps were performed at 58.7° C, 60.8° C, 60° C, 60° C for EP1-4 respectively. All results were normalized to reference RNA 18 S. Products were ran out on an agarose gel to establish correct band size, and a melting curve of the amplicon was performed. The melting curve was performed from 65° C to 95° C reading ever .2° C with 1 sec between reads.
Statistical Analysis
Statistics used for all experiments except the RT-PCR studies are as follows: For comparison of two groups, a Students t-test was used. Data sets that contained more than 2 groups were analyzed by a one-way ANOVA followed by a Student-Neuman-Keuls post hoc multiple comparison test for differences between treatment groups. A p<0.05 was used as the criteria for differences between treatment groups. Raw RT-PCR data was transformed using a logarithmic transformation to achieve homogeneity of variance and subjected to a two-way mixed ANOVA with time as a repeated measure. Post hoc analysis consisted of one way ANOVAs within each time point. The first demonstrated a simple effect. A second ANOVA compared EP2, EP3 and EP4 and found no difference between them and a third ANOVA compared EP1 to the others combined. This series of ANOVAs was applied to each time point until the time point was reached where no significance was observed.
RESULTS
Combined agonist activity at receptor subtypes EP2 and EP3 increases spinophilin levels
Treatment of PN0 female pups with PGE2 resulted in approximately a two-fold increase in spinophilin levels compared to those treated with saline vehicle (p<0.001; n = 5–6; Fig 1A), which is consistent with previous observations from this laboratory. To identify which prostanoid receptor(s) are involved in upregulating spinophilin levels, we used selective PGE2-like agonists. Agonist-treated groups were compared to the animals treated with saline as control or PGE2. Treatment with the EP1 agonist Iloprost (n=6) had no effect on spinophilin levels when compared to control animals. Treatment with the EP2 agonist Butaprost (n=6), or the EP1/EP3 agonist Sulprostone (n=6) individually resulted in a measurable, but not significant increase in levels of spinophilin. With coapplication of Butaprost and Sulprostone (n=6), the resulting spinophilin level was additive of the two treatments with each agonist alone and was significantly higher than both the saline- and PGE2-infused control groups (F=15.3; d.f.=5,29; p<0.05).
FIG 1. Treatment of animals with selective prostanoid receptor agonists induces spinophilin in the POA.

A) PGE2 treatment of female pups on the day of birth and PN1 induces a significant 88.4% increase in spinophilin in the POA by PN2 compared to females treated with saline (ANOVA; p < 0.5). There was no significant effect on spinophillin levels of treatment with the EP1 selective agonist Iloprost, the EP2 selective agonist, Butaprost, or the non-selective EP1/EP3 agonist, Sulprostone. However, co-application of Butaprost and Sulprostone induced a significant 222.2% increase in spinophilin over those treated with saline (n=5–6/group; p < 0.05). B) Representative Western blot for spinophilin protein. Each lane represents POA tissue from one animal. Each blot included saline control animals for standardization.
Treatment with antisense oligonucleotides against mRNA of specific EP receptor subtypes reduces the level of protein for each receptor
Antisense oligonucleotide sequences were designed against mRNA for each of the four receptors to specifically reduce expression of that receptor in vivo. To validate the efficacy and specificity of each sequence, animals were given injections of nucleotides, and dissected tissue was analyzed by Western blot with primary antibodies for each of the receptor proteins. Tissue analyzed for each receptor was compared to tissue treated with each of the three other antisense sequences (Figure 2A) after all samples were normalized to tissue treated with a scrambled control sequence (SCRAM). Antisense oligonucleotides against the mRNA for the EP1 receptor (EP1AS), reduced EP1 protein levels by 21.9% (n=4, F=10.23; p<0.01; Figure 2B) while other antisense treatments had no effect compared to control. Treatment with the sequence specific to the EP2 receptor mRNA (EP2AS) selectively reduced EP2 receptor protein level by 30.0% (n=5, F=4.63; p<0.04; Figure 2C). Treatment with antisense oligonucleotides against EP3 receptor mRNA (EP3AS) showed a 24.8% reduction exclusively of EP3 protein levels (n=5, F=11.73; p<0.01; Figure 2D). Administration of antisense oligonucleotides against EP4 receptor mRNA (EP4AS) selectively reduced EP4 protein expression by 19.6% (n=4, F=7.95; p<0.01; Figure 2E).
FIG 2. EP receptor protein levels are reduced after treatment with antisense oligonucleotide sequences specific for each prostanoid receptor.

Newborn pups were treated with 1μg of specific antisense (AS) oligonucleotide sequences or scrambled (SCRAM) oligonucleotide for two days and levels of receptor protein were measured on the third day. Measurements were normalized to control samples then compared against each other for significant differences A) Western Blot of EP receptors. Rows are different proteins being blotted for, columns represent specific sequence of antisense treatment. B) Treatment with the EP1 antisense oligonucleotide sequence reduced EP1 receptor protein by 21.9% 1 (n=4; ANOVA, p<0.01). C) Treatment with EP2 antisense oligo reduced protein levels for that receptor by 30.0% (n=5; ANOVA, p<0.04). D) Treatment with the EP3 sequence resulted in a 24.8% decrease in the EP3 receptor protein (n=5; ANOVA, p<0.01). E) Treatment with the EP4 sequence resulted in a 19.6% decrease in the EP4 receptor expression (n=4; ANOVA, p<0.01).
Treatment with antisense oligonucleotides against EP receptor mRNA lowers spinophilin expression induced by PGE2 in the POA
To examine the role of the different receptor subtypes in the upregulation of spinophilin in response to PGE2, animals were treated with receptor-specific antisense oligonucleotides or SCRAM and then treated with PGE2. When EP2 or EP3 expression was downregulated there was a significant decrease in spinophilin compared to the two groups which received either SCRAM + saline or SCRAM + PGE2 (Figure 3). Pretreatment with antisense oligonucleotides against EP2 (n=6) or EP3 mRNA (n = 6) completely blocked the induction of spinophilin normally observed in response to PGE2. (F=61.7; d.f.= 4,20; p<0.001) Further, antisense to either of the receptors blocked spinophilin expression to levels that were nearly undetectable. Identical treatment with antisense oligonucleotide against the EP1 or the EP4 receptor (n=6 and 5 respectively) blocked only the induction observed with PGE2, resulting in spinophilin levels comparable to SCRAM + saline treated animals (n=3). Thus reducing the level of EP1 or EP4 only blocked the PGE2 induced effect. It did not reduce spinophilin to levels significantly lower than control animals as was seen when knocking down EP2 or EP3.
FIG 3. Antisense oligonucleotides against EP receptors block PGE2 induction of spinophilin.
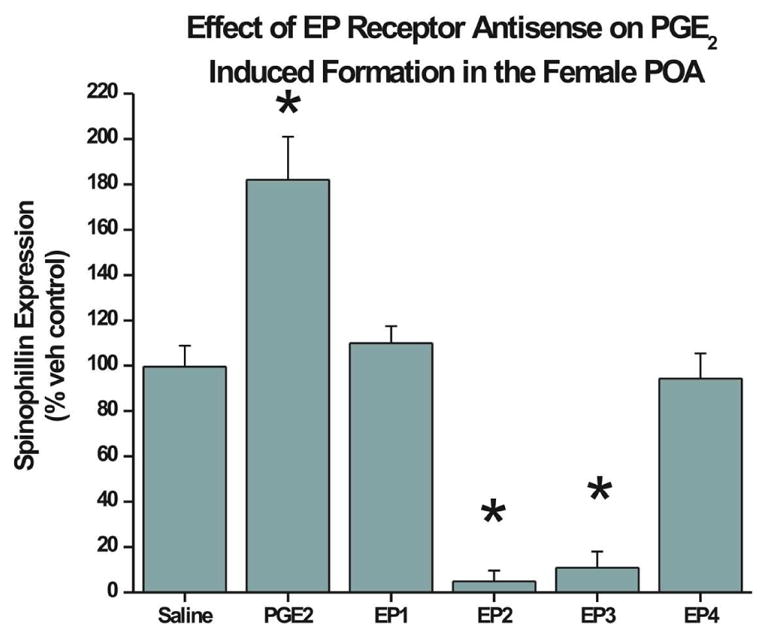
Treatment of newborn female pups with PGE2 + scrambled oligo (SCRAM) on PN0 and PN1 induces a significant increase in spinophilin by PN2 (n=4; ANOVA; p<0.05). Pretreatment with antisense oligo against either EP2 or EP3 receptor for 4 hrs prior to PGE2, reduced spinophilin levels to almost undetectable and significantly below SCRAM + saline treated controls (n=6 for both groups; ANOVA; p<0.01). Pretreatment with antisense oligo against the EP1 (n=6) or EP4 (n=5) receptors before PGE2 reduced spinophilin levels comparable to those seen in SCRAM + saline treated females.
Treatment with antisense oligonucleotides against EP receptor mRNA prevents the estradiol-induced increase in spinophilin in the POA
Estradiol increases spinophilin protein in the developing POA by up regulating the COX-2 enzyme and the production of PGE2 (Amateau and McCarthy, 2002b). Therefore, we next sought to determine if reducing EP receptors levels with antisense oligonucleotide treatment would block estradiol-induced increases in spinophilin with the same profile as that seen for PGE2 induction of spinophilin. Using the identical antisense oligonucleotide treatment protocol as above and treating with estradiol instead of PGE2, we observed the same pattern of effects as previously. Estradiol treatment significantly increased spinophilin levels over control (n = 4 and 5 respectively; p < 0.01) and antisense oligos against EP1 (n = 4) and EP4 (n = 6) reduced spinophilin levels in estradiol treated females to control levels while antisense oligos against EP2 (n=5) and EP3 (n = 5) reduced spinophilin levels to almost undetectable (Figure 4, F=11.8; d.f.=3,14; p<0.01).
FIG 4. Antisense oligonucleotides against EP receptors block the effects of estradiol on spinophilin.

Treatment with estradiol + SCRAM induced a significant increase in spinophilin (n=4) compared to animals treated with vehicle plus scrambled oligo. Pretreatment with antisense oligos against either EP2 or EP3 receptor decreased spinophilin levels to almost undetectable (n=5 for both groups), while treatment with sequences against EP1 (n=4) or EP4 (n=6) produced levels of spinophilin comparable to treated with oil vehicle (control for estradiol) and SCRAM oligo (ANOVA; p < 0.01)
Treatment with antisense oligonucleotides against prostanoid receptor mRNA does not reduce spinophilin expression induced by estradiol in the hippocampus
To further evaluate the specific effects of our oligonucleotide sequences, spinophilin protein was measured in the rostral hippocampus. Estradiol increases protein levels of spinophilin in immature hippocampal neurons through a PGE2 independent mechanism (Amateau and McCarthy, 2002b). We reasoned that if antisense oligos to EP receptors were inducing a sickness effect (i.e. nonspecific) that impaired spine formation or spinophilin synthesis, this would be observed throughout the brain, including the estradiol-induced increase in hippocampal spinophilin. Consistent with our previous observation, estradiol significantly increased spinophilin in the immature hippocampus (n=3–6, F=26.52; d.f.=4,14; p<0.01) and this increase was unaffected by treatment with antisense oligos against any of the EP receptors (Figure 5).
FIG 5. Antisense oligonucleotides against EP receptors do not inhibit estradiol induction of spinophilin in the hippocampus.

Animals were treated with estradiol with and without specific antisense oligo sequences. Hippocampal tissue from the same animals as in Figure 2 were assayed for spinophilin expression. While estradiol treatment significantly increases spinophilin protein compared to the vehicle treatment group, the increase is not attenuated by the prior application of antisense oligonucleotides (all groups n=4, ANOVA, p<0.01).
Time Course of Relative Prostanoid Receptor Expression in the Developing POA
Using real-time polymerase chain reaction (RT-PCR), we quantified mRNA levels of each of the four receptors in the POA from PN0 through PN40 (Figure 6). The mRNA for each of the four receptors was detectable at every developmental time point. There were no sex differences detected in mRNA level for any of the receptors at any time point measured (data not shown). The critical period for estradiol-induced masculinization of the POA begins prenatally and extends to ~PN6. A two-way mixed ANOVA with time as a repeated measure shows a significant effect across time (F = 8.51, d.f. = 5,15; p<.001). Post hoc analysis reveals that mRNA levels for EP2, EP3 and EP4 receptors remained relatively constant across development and do not vary from each other (F = 0.056; p>0.1), whereas the level of EP1 mRNA was significantly higher than all of the other receptors on PN0, PN3 and PN6 (F = 38.6; p<0.001) before declining to the same relative level as all others at PN10.
FIG 6. Relative Expression of Prostanoid Receptors throughout development.
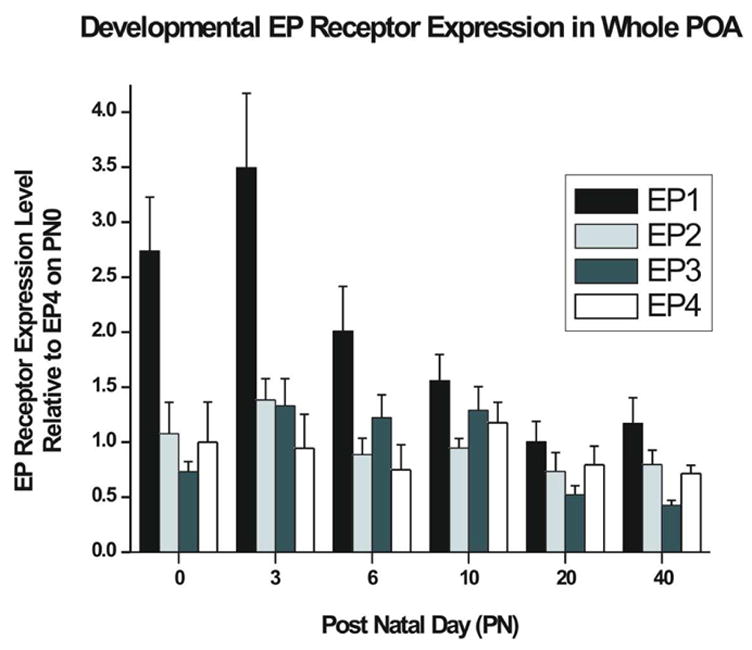
Relative quantification of EP Receptor gene expression by RT-PCR show that EP1 mRNA is at significantly higher levels than the other EP receptors from PN0 through PN6 (For PN0, 3, 20, and 40, n=8 [4 male and 4 female] PN6, n=6 [2 male and 4 female] and PN10 n=6 [4 male and 2 female], ANOVA; p<0.05). This corresponds to the perinatal time period where the POA is sensitive to estradiol induced masculinization (data from males and females are combined for presentation).
DISCUSSION
Previous studies in our laboratory demonstrate that PGE2 is a major mediator of masculinization of the POA neurocircuitry controlling sex behavior; however, the specific prostanoid receptor(s) activated by PGE2 to induce masculinization were unknown. Data presented here suggest the prostanoid receptor subtypes EP2 and EP3 are the predominant mediators of spinophilin expression that underlie masculine patterning in the developing preoptic area, and that the EP1 and EP4 receptors are also involved in some necessary, but peripheral capacity.
We detected mRNA for all four of the EP receptors in the developing and adult POA. Only EP1 showed any developmental change, being at substantially higher levels than all three of the other receptors for the first postnatal week of life and then declining to adult levels. Previously we had not detected EP4 receptor mRNA in the newborn POA (Amateau and McCarthy, 2004), most likely due to the weaker sensitivity of standard PCR compared to the use of real time PCR here.
Spinophilin is an excellent proxy marker for the formation of dendritic spines on developing POA neurons. The current results again confirm that estradiol increases spinophilin in the neonatal POA and that this effect is mimicked by PGE2. Treatment of newborn females with the selective EP2 agonist, Butaprost, combined with the EP1/EP3 agonist, Sulprostone, induced a significant increase in spinophilin protein in the neonatal POA. Neither agonist alone significantly altered spinophilin levels, nor did the EP1 agonist, Iloprost. Given the lack of effect with Iloprost alone, and a large difference (~75 fold) in the EC50 of Sulprostone for the EP1 and EP3 receptors, we conclude the effect of Sulprostone is the result of EP3 activation, although an involvement of EP1 cannot be ruled out. We did not explore the effects of an EP4 agonist in this study but conclude that activating EP2 and EP3 is sufficient to induce POA spinophilin levels similar to that observed by either PGE2 or estradiol.
Reduction in EP receptor protein with the use of antisense oligonucleotides indicated both EP2 and EP3 were essential for the ability of either estradiol or PGE2 to increase spinophilin in the POA. In fact, levels of spinophilin were reduced to almost undetectable levels by treatment with antisense oligos against either EP2 or EP3 receptor, suggesting these receptors’ activation may be essential for spinophilin production in the developing POA. However, antisense oligos against EP1 or EP4 receptor mRNA also reduced spinophilin levels in both PGE2- and estradiol-treated females, but only to those seen in their respective control vehicle-treated animals. This suggests EP1 and EP4 receptors are also involved in production of spinophilin and that they may be necessary but not sufficient to increase spinophilin independent of the other receptor types.
Legitimate concerns in the use of antisense oligonucleotide treatment to study the role of particular proteins include non-specific effects on other untargeted proteins (McCarthy 1998). Each of the antisense oligo constructs used here reduced only their targeted EP receptors, and more importantly, treatment with the oligos did not impair estradiol-mediated increases in spinophilin in the developing hippocampus from the same animals where effects were observed in the POA. Thus the effects of the EP receptor antisense oligos on spinophilin in developing POA appear to be specific, strengthening the conclusion that multiple receptors are involved.
Prostaglandin-mediated biological responses often require the simultaneous activation of more than one EP receptor subtype. For example, both EP2 and EP4 are required for PGE2 differentiation of chondrocytes (Miyamoto et al., 2003), combined EP1 and EP3 receptor activation is essential for induction of aromatase by PGE2 in the stromal cells of breast tumors (Richards and Brueggemeier, 2003) and both EP1 and EP4 are involved in carcinogenesis of colon tissue (Kitamura et al., 2003). The signal transduction pathways activated by EP receptors are complex and variable (see below). Moreover, signaling responses of one EP receptor subtype can be due to EP subtype’s activation modulating the signal pathway of another subtype, and in some cases that modulated pathway acting back on the first (Hatae, 2003; Regan, 2003). This complements our observation of combined activation of only EP2 and EP3 being sufficient, but also that activation is insufficient without the presence of normal levels of both EP1 and EP4. For example, EP1 and EP4 signaling impinge downstream on the already active signaling cascade of EP2 and EP3 in such a necessary way as to produce high levels of spinophilin.
Prostanoid signaling is an immensely complex picture with many differences between individual tissues and systems. PGE2 interacts with all four receptor subtypes, EP1–4, all of which are G-protein coupled receptors. (Coleman et al., 1994; Narumiya et al., 1999; Breyer and Breyer, 2001; Namba et al., 1993; Hatae et al., 2002). The pathways activated by the receptors vary widely and have mainly been categorized in epithelial tissue of renal, gastrointestinal, or reproductive systems. The signaling mechanisms characterized in other tissues have been largely consistent with the minimal studies involving prostanoid signaling in the CNS (Barr et al., 2004; McCullough et al., 2004). However a multitude of differences in signaling outcomes have been reported. The EP1 receptor is a G coupled protein whose signaling pathway leads to the activation of Phospholipase C and subsequent lipid signaling pathways. Phospholipase C cleaves PIP2 into membrane localized DAG and the cytosolic IP3 which interacts with IP3 receptors, and leads to a rise in cytosolic Ca2+ through release from ER stores and subsequent calcium signaling events (Chell et al., 2006). The EP2 receptor couples to a Gs protein and activates adenylyl cyclase to increase concentrations of cAMP (Regan et al., 1994) and activate PKA. While a multitude of PKA phosphorylation targets exist, the release of inhibition of glycogen synthase kinase (GSK-3α) and the phosphatidylinositol 3-kinase (PI3 kinase) have been recognized as PKA targets in EP2 signaling cascades. These phosphorylation events result in the inactivation of GSK-3β, and the release of inhibition on β-catenin mediated Tcf transcriptional activity (Cadigan and Nusse, 1997).
EP3 receptor signaling is much more complex because differential splicing generates multiple isoforms of the receptor. Thus, EP3 has been categorized as coupling with Gi (Sugimoto et al., 1993; Irie et al., 1993), Gs (Namba et al., 1993), or G13 (Hatae et al., 2002), proteins depending on the isoform. As few as 3 isoforms of the EP3 receptor exist in mice (Sugimoto et al., 1993), and up to 7 isoforms have been reported in humans (Adam et al., 1994). Several signaling pathways can result from EP3 activation, including modulation of adenylyl cyclase activity via Gs or Gi activity. EP3 receptor isoforms can also couple to G13 proteins and modulate the functions of the small GTPases of the Rho family. Specifically, they alter Rac, CDC42, and Rho (Hatae et al., 2002). Rac and CDC42 are required for neurite outgrowth, while Rho is necessary for neurite retraction. Downstream, components activated by G13 proteins such as Rho (Hatae et al., 2002) appear to be regulated differently by different isoforms. Bovine EP3α, when coupled to G13 results in constitutive activation of Rho. The β form coupled to the same G protein only activates Rho in an agonist dependent manner (Hatae et al., 2002). The EP3 receptor, in an agonist-dependent and Pertussis toxin-insensitive manner, also causes an intracellular mobilization of Ca2+ (Bezzi et al., 1998). This is known not to occur via the Gβγ activation of PLCβ since adenylyl cyclase activity was not attenuated by Gβγ inhibitors. Conversely, it could be attenuated with the addition of calcium chelators or calmodulin inhibitors. This suggests that following the increase in intracellular calcium, a Ca2+/Calmodulin dependent pathway activates adenylyl cyclase (Hatae et al., 2002). Our observation of a synergy in effects of an EP2 and EP3 agonist on spinophilin levels suggests that EP3 is positively coupled to adenylyl cyclase in the neonatal POA.
EP4 signaling is conducted through the G-protein coupled EP4 receptor. This G protein is mainly responsible for the activation of the PI3 kinase, though lesser activation of cAMP and PKA does occur as well. As with EP2 signaling, the PI3 kinase can activate the Akt kinase which inhibits GSK-3α leading to β-catenin modulated Tcf transcripsional activation. It also however, is responsible for the activation of ERK pathways that culminate in induction of PGE2 synthase and induction of COX-2. This leads to the synthesis of more PGE2, creating a positive feedback loop for prostaglandin signaling (Regan, 2003). The current observation that only EP2 and EP3 combined agonists were sufficient to induce spinophilin above baseline, but that EP1 and EP4 are required for the full effects of PGE2 to be observed, may be a function of the positive feedback effects that this prostaglandin can have via its own receptors. Moreover, we have recently observed that as little as one injection of PGE2 to a neonatal female rat will masculinize adult sexual behavior (Wright and McCarthy, unpublished observation). The ability of PGE2 to positively reinforce its own signaling via the EP2 and EP4 receptors may explain this improbable observation.
In summary, based on the data presented here, we conclude that all four of the EP receptors are present in the developing POA during the critical period for male-typical sexual differentiation of the brain and that each of them contributes to the process of estradiol-induced PGE2-mediated masculinization. Activation of EP2 and EP3 by receptor specific agonists is sufficient to increase spinophilin to the same level as PGE2, but both EP1 and EP4 are necessary for PGE2 to exert its effects. Future studies of the behavioral effects of EP receptor activation in the neonatal brain, as well as EP receptor signaling in POA neurons and investigation into the cross-talk involved between the four receptors, will further elucidate the role of each receptor.
ABBREVIATIONS
- AMPA/KA
Alpha-amino-3-hydroxy-5-methylisoxazol-4-proprionic acid/kainic acid
- CNS
Central Nervous System
- COX-2
Cyclooxygenase-2
- DAG
Diacylglycerol
- GSK-3α
Glycogen Synthase Kinase
- IAD
Integrative Grayscale Pixel Area Density
- ICV
Intracerebroventricular
- PGE2
Prostaglandin E2
- PGG2
Prostaglandin G2
- PGH2
Prostaglandin H2
- PIP2
Phosphatidylinositol bisphosphate
- POA
Preoptic Area
- RIPA
Radio-immunoprecipitation Assay
- TBS
Tris Buffered Saline
- TCF
Ternary Complex Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam M, Boie Y, Rushmore T, Muller G, Bastien L, Mckee K, Metters K, Abramovitz M. Cloning and expression of three isoforms of the human EP3 prostanoid receptor. FEBS Lett. 1994;338:170–174. doi: 10.1016/0014-5793(94)80358-7. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: Sex differences, regional heterogeneity and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002a;14:904–910. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002b;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau S, McCarthy M. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neuroscience. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Natura G, Telleria-Diaz A, Teschner P, Vogel R, Vasquez E, Schaible HG, Ebersberger A. Changes in the effect of spinal prostaglandin E2 during inflammation: prostaglandin E (EP1–4) receptors in spinal nociceptive processing of input from the normal or inflamed knee joint. J Neurosci. 2004;24:642–651. doi: 10.1523/JNEUROSCI.0882-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Lodi-Rizzini B, Pozan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Li S, Li Z, Feleder C, Perlik V. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostaglandins Other Lipid Mediat. 2005;76:1–18. doi: 10.1016/j.prostaglandins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Boie Y, Stocco R, Sawyer N, Slipetz D, Ungrin M, Neuschafer-Rube F, Puschel G, Metters K, Abramovitz M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur J Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- Booth JE. Sexual behaviour of neonatally castrated rats injected during infancy with oestrogen and dihydrotestosterone. J Endocrinol. 1977;72:135–141. doi: 10.1677/joe.0.0720135. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Breyer RM. G protein-coupled prostanoid receptors and the kidney. Annu Rev Physiol. 2001;63:579–605. doi: 10.1146/annurev.physiol.63.1.579. [DOI] [PubMed] [Google Scholar]
- Cadigan K, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Chell S, Kadi A, Caroline-Williams A, Paraskeva C. Mediators of PGE2 synthesis and signaling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochem Biophys Acta. 2006;1776:104–199. doi: 10.1016/j.bbcan.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Christensen LW, Clemens LG. Intrahypothalamic implants of testosterone or estradiol and resumption of masculine sexual behavior in long-term castrated male rats. Endocrinology. 1974;95:984–990. doi: 10.1210/endo-95-4-984. [DOI] [PubMed] [Google Scholar]
- Christensen LW, Nance DM, Gorski RA. Effects of hypothalmalic and preoptic lesions on reproductive behavior in male rats. Brain Res Bull. 1977;2:137–141. doi: 10.1016/0361-9230(77)90010-7. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- Dohler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen antagonist. Neuroendocrinology. 1984;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- Hatae N. Cooperation of two subtypes of PGE2 receptor, Gi coupled EP3 and Gs coupled EP2 or EP4 subtype. Yakugaku Zasshi. 2003;123:837–843. doi: 10.1248/yakushi.123.837. [DOI] [PubMed] [Google Scholar]
- Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin Receptors: Advances in the Study of EP3 Receptor Signaling. J Biochem. 2002;131:781–784. doi: 10.1093/oxfordjournals.jbchem.a003165. [DOI] [PubMed] [Google Scholar]
- Irie A, Sugimoto Y, Namba T, Harazono A, Honda A, Watabe A, Negishi M, Narumiya S, Ichikawa A. Third isoform of the prostaglandin E receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur J Biochem. 1993;217:313–318. doi: 10.1111/j.1432-1033.1993.tb18248.x. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Itoh M, Noda T, Tani K, Kobayashi M, Maruyama T, Kobayashi K, Ohuchida S, Sugimura T, Wakabayashi K. Combined effects of prostaglandin E receptor subtype EP1 and subtype EP4 antagonists on intestinal tumorgenesis in adenomatous polyposis coli gene knockout mice. Cancer Sci. 2003;94:618–621. doi: 10.1111/j.1349-7006.2003.tb01492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Boston: Kluwer Academic Publishers; 1998. Modulating Gene Expression by Antisense Oligonucleotides to Understand Neural Functioning. [Google Scholar]
- McCarthy MM, Amateau SK, Mong JA. Steroid modulation of astrocytes in the neonatal brain: implications for adult reproductive function. Biol Reprod. 2002;67:691–698. doi: 10.1095/biolreprod.102.003251. [DOI] [PubMed] [Google Scholar]
- McCullough L, Wu l, Haughey N, Liang X, Hand T, Wang Q, Breyer R, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. Physiology of Reproduction. Vol. 2. New York: Raven Press; 1997. pp. 3–106. [Google Scholar]
- Miyamoto M, Ito H, Mukai S, Kobayashi T, Yamamoto H, Kobayashi M, Maruyama T, Akiyama H, Nakamura T. Simultaneous stimulation of EP2 and EP4 is essential to the effect of prostaglandin E2 in chondrocyte differentiation. Osteoarthritis Cartilage. 2003;11:644–652. doi: 10.1016/s1063-4584(03)00118-3. [DOI] [PubMed] [Google Scholar]
- Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, Ito S, Ichikawa A, Narumiya S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993;365:166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Regan J. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Regan J, Bailey T, Pepperl D, Pierce K, Bogardus A, Donello J, Fairbairn C, Kedzie K, Woodward D, Gil D. Cloning of a novel human prostaglandin receptor with characteristics of the pharmacologically defined EP2 subtype. Mol Pharmacol. 1994;46:213–220. [PubMed] [Google Scholar]
- Richards JA, Brueggemeier RW. Prostaglandin E2 regulates aromatase activity and expression in human adipose stromal cells via two distinct receptor subtypes. J Clin Endocrinol Metab. 2003;88:2810–2816. doi: 10.1210/jc.2002-021475. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Negishi M, Hayashi Y, Namba T, Honda A, Watabe A, Hirata M, Narumiya S, Ichikawa A. Two isoforms of the EP3 receptor with different carboxyl-terminal domains. J Biol Chem. 1993;268:2712–2718. [PubMed] [Google Scholar]


