Abstract
The heptadecapeptide histogranin, synthesized by adrenal chromaffin cells, is implicated in the analgesia produced by transplanting chromaffin cells into the spinal cord, including block of hyperalgesia mediated by NMDA-subtype glutamate receptors. To examine the neurophysiological basis for this analgesia, we applied the stable analog [Ser1]-histogranin (SHG) by iontophoresis near extracellularly recorded wide-dynamic range (WDR) neurons in anesthetized rats. When SHG was applied during peripheral electrical stimulation of A and C fibers at 0.1 Hz, the C-fiber response was significantly inhibited but the A-fiber response was unaffected. SHG also opposed the NMDA-receptor-dependent post-tetanic facilitation (wind-up) of C-fiber responses produced by increasing the rate of peripheral afferent stimulation to 1 Hz for 20 seconds. To test whether block of NMDA-subtype receptors could be wholly or partially responsible for this suppression, SHG was applied during sequential pulsed iontophoresis of three agonists targeting distinct excitatory synaptic receptors: NMDA, kainate and substance P. All three excitatory effects were reversed by SHG; this reversal outlasted the 10-30 minute observation period when higher SHG doses were applied (>60 nA). Histogranin therefore probably produces prolonged spinal analgesia by opposing the basal and potentiating synaptic effects of C-fibers on dorsal horn neurons. Actions besides or in addition to NMDA-receptor antagonism (e.g., agonism at inhibitory postsynaptic receptors or block of voltage-gated cation channels on C-fibers) are implied by the diversity of excitatory transmitters opposed by SHG.
INTRODUCTION
Adrenal chromaffin cells synthesize a large number of releasable neuroactive substances, including several with known analgesic actions [22], and their transplantation into the rat's spinal subarachnoid space leads to long-term analgesia [13]. Both catecholamines and met-enkephalin are released into the subarachnoid space from chromaffin cell transplants and appear to play a role in the consequent analgesia [14, 15, 16, 21]. However, components of an NMDA-mediated cascade that initiates central pain sensitization are also suppressed by adrenal medullary transplants, including nitric oxide synthase [6], cyclic guanosine monophosphate [19] and c-fos protein [17, 20]. One substance released from chromaffin cells is known to have a fairly selective affinity for NMDA subtype receptors, namely the heptadecapeptide histogranin [9, 11]. Its stable analog, [Ser1]-histogranin (SHG), if administered intrathecally, blocks the hyperalgesia and allodynia produced in rats by intrathecal NMDA [7]. SHG can non-competitively inhibit the binding of NMDA receptor antagonists in rat membrane preparations, possibly by acting at the regulatory polyamine on the receptor [11, 18].
The mechanism of histogranin's spinal analgesic action has not previously been analyzed by electrophysiological recording. In the present experiments, we examined wide-dynamic range (WDR) mechanoreceptive neurons in the dorsal horn of halothane-anesthetized rats, which convey ascending signals that are important in the perception of pain [1]. Rapid repetitive stimulation of the C-fiber input to WDR cells produces a homosynaptic post-tetanic potentiation (wind-up) that requires activation of NMDA-subtype glutamate receptors [5, 23]. We had previously shown that wind-up is suppressed by adrenal medullary transplants [8]. Here we explore how iontophoresis of [Ser1]-histogranin near WDR neurons affects basal and wind-up responses to peripheral electrical stimulation, and whether histogranin's actions can be entirely accounted for by antagonism at NMDA receptors.
MATERIALS AND METHODS
Experimental procedures were carried out on female, Sprague-Dawley rats weighing 250-350 g (n=14). All research on animals was approved by a local Institutional Animal Care and Use Committee (University of Illinois College of Medicine at Rockford, #375-02). In 11 rats, anesthesia was induced with 3% halothane in oxygen inside a closed Plexiglass chamber. A rapid ventral neck incision allowed insertion of a tracheal tube, and halothane was then applied through this tube at a rate of 0.2-0.5 l/minute and a concentration of 1.1-1.3%. Sodium pentobarbital was employed as the anesthetic in 3 animals to verify that certain responses were not due to halothane; induction by 50 mg/kg (i.p.) pentobarbital was followed by continuous intravenous infusion of 10 mg/kg/hr. In all animals, the external jugular was cannulated, and a syringe pump delivered Ringers solution intravenously at a rate of 1-2 ml of per hour. A pulse oximetry probe was used to monitor blood oxygen saturation. A direct-current electric heating blanket, controlled by the feedback signal from a rectal thermistor kept the body temperature at 37°C. A laminectomy was performed over the T11 to L2 vertebrae, corresponding to T13 to S1 spinal segments. Spines rostral and caudally to the laminectomy were held by clamps to prevent motion. The dura was cut over the target spinal segments and the area covered by solidified agar (5%, dissolved in 0.9% saline) to further minimize tissue movement.
Recordings were made with multi-barrel micropipettes (5 or 7 barrels). These were fabricated from glass tubing with glass fibers inside (obtained from World Precision Instruments). The central tube was used for recording action potentials, and was filled with 3 M NaCl. Another barrel contained 2 M NaCl in which 2.5% fast-green dye had been dissolved, to act as a return current path for marking locations histologically. [Ser1]-histogranin (SHG) was obtained as its trifluoroacetate salt from Bachem (Torrance, CA) at >94% purity, and all other substances were obtained from Sigma/RBI (Natick, MA). SHG was dissolved at a concentration of 1.25 mM in 165 mM NaCl and its pH was adjusted to 5.5 with NaOH. A vehicle control was prepared by starting with 165 mM NaCl and adjusting its pH with NaOH to 5.5. NMDA, kainate and glycine were separately dissolved at 50 mM in Tris buffer at pH 8 and applied by iontophoresis as anions. Spermidine (N-[3-Aminopropyl] 1,4-butanediamine) and substance P were dissolved in Tris buffer at pH 4.5, 10 mM, and applied as cations. Iontophoresis employed isolated constant-current sources (±100 V compliance, World Precision Instruments model 260). Since only 2 cells were studied with glycine, they are not included in the Results.
The L4 dorsal horn was sampled from 0.2 to 0.8 mm lateral to the midline, and 0.0 to 0.8 mm below the dorsal surface. Filtered (0.1-10 kHz) extracellular recordings, along with stimulus and iontophoresis markers, were stored on videotape (processed by a Vetter 3000A PCM adaptor). Spike times were extracted off-line by voltage-time discrimination using a dedicated time/amplitude window discriminator and event detector (Dagan WD2). Overlapped oscilloscope traces were monitored to ensure exclusion of additional units when >1 spike shape was present. Wide-dynamic-range (WDR) nociceptive neurons were distinguished from low-threshold mechanoreceptors by means of flexible von Frey probes that delivered forces which were innocuous (2 mN/mm2) or noxious (20 mN/mm2). The WDR neurons were defined by an increase in firing when the stimulus became noxious. In some experiments, their receptive fields were activated by subcutaneous needle electrodes, placed 1 cm apart in the receptive field, delivering 1-ms pulses at a basal rate of 0.1 Hz. The numbers of spikes was counted in time windows 0-20 ms, 40-300 ms and 300-1000 ms, respectively representing the A-fiber, C-fiber and “post-discharge” responses [5]; the post-discharge occurred only during post-tetanic facilitation (wind-up). The stimulus amplitude (10-40 V) was adjusted to give a mean response of 3-5 action potentials in the C-fiber window. To produce wind-up, the stimulus rate was raised to 1 Hz, with at least a 2-minute interval between tests. Wind-up was quantified as a fractional increase in the average response in the last five stimuli over the first five stimuli in the 20 s (1 Hz) train.
At the end of a given vertical trajectory, pressure (5 p.s.i) was applied for 5 minutes to eject the fast green dye solution. Rats were sacrificed by an intravenous infusion of pentobarbital (100 mg/kg/min for 2 minutes), and, when breathing and all other reflexes had ceased, received intracardial perfusion of phosphate-buffered saline (pH 7.6, 4°C) followed with10% formaldehyde (pH 7.0). The spinal cord was removed, maintained overnight in formaldehyde, then embedded in agar and sliced in the coronal plane on a Vibratome at 50 μm thickness. Sections were stained with cresyl violet and scanned under a light microscope for dye marks. Recording positions with respect to laminar location in gray matter were verified from these marks in conjunction with micromanipulator readings.
Statistical assessment employed commercial software (SPSS 14.0). Changes in A-fiber and C-fiber responses (number of impulses) at different times relative to SHG delivery were assessed by multi-way analysis of variance (ANOVA), at a significance level of P<0.05 (2-sided). Significant effects on individual neurons were found by post-hoc contrasts, with Bonferroni adjustments made to significance levels. For wind-up, the change in spike numbers was analyzed similarly, with post-discharge responses also included as a dependent variable along with A-fiber and C-fiber responses. Analysis of the effect of SHG on responses to excitatory agonists was also subjected to ANOVA, taking as the dependent variable the spike count recorded during the last half of the agonist's application period plus the 5 subsequent seconds. The minimum percentage of neurons in the underlying target population responding to a particular intervention was estimated (at P<0.05) by applying the equation for the binomial distribution to the proportion of sampled neurons showing significant responses (responses were always in the same direction).
RESULTS
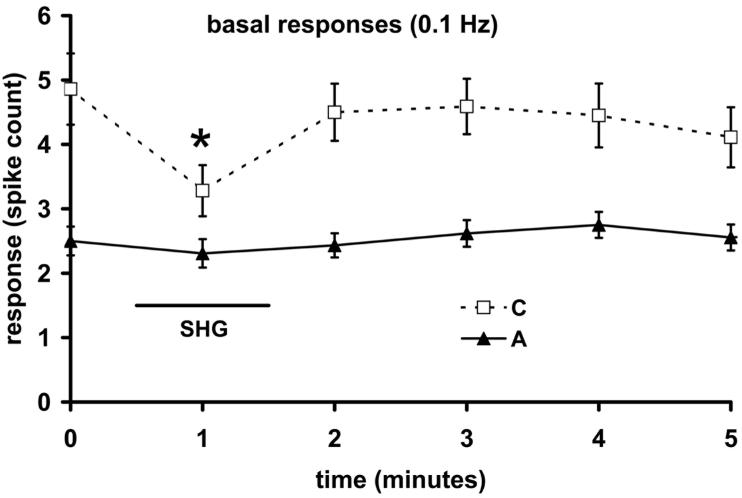
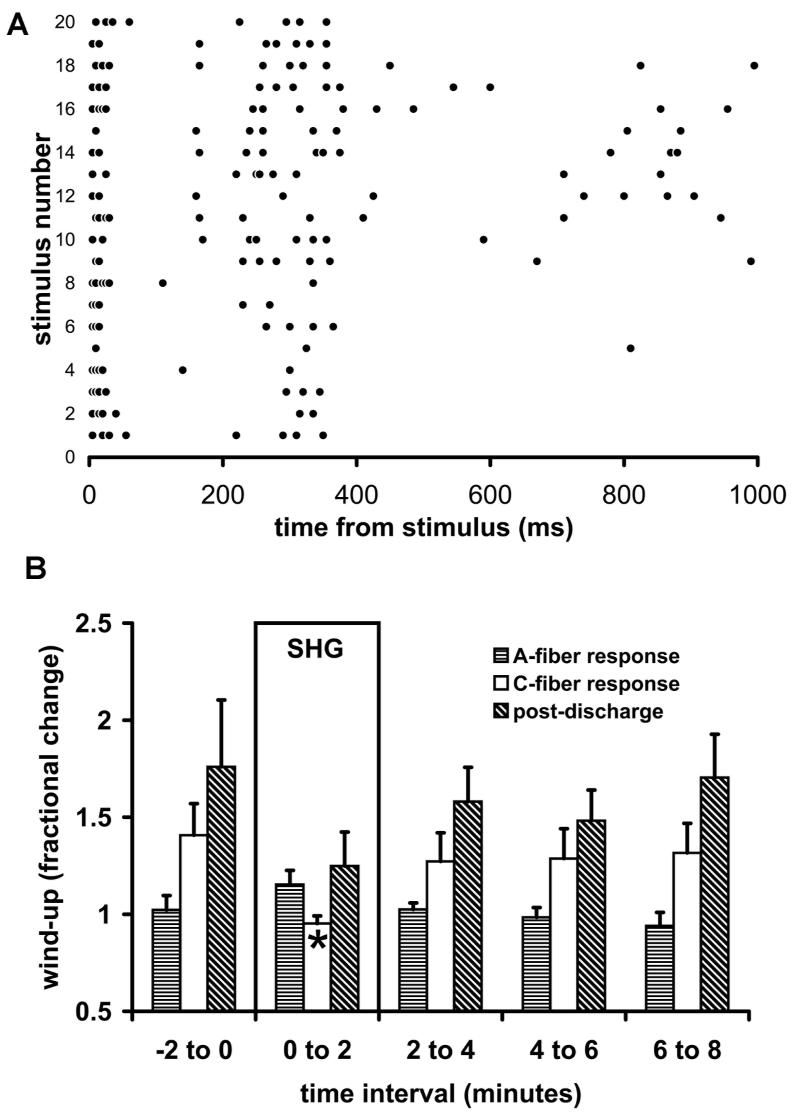
All cells studied (n=27) were of the WDR type and were found at depths of 418-753 μm, which was within or near lamina V according to histological analysis. The effect of SHG on responses to cutaneous electrical stimulation was studied under halothane anesthesia, by applying SHG iontophoretically for 1 minute. During basal stimulation (0.1 Hz), application of SHG (60-100 nA) caused the response to C-fiber input to fall significantly (P<0.001, n= 8 cells) as shown in Figure 1. No significant effect was seen in the mean A-fiber response, and the iontophoresis of a vehicle control was without significant effect. Given that all 8 neurons showed this effect, >67% are expected in the underlying population of WDR neurons (exact binomial distribution, 5% confidence level). The effect on the C-fiber response took 1-3 minutes from the end of SHG application to recover. Wind-up was tested by raising the stimulation rate to 1 Hz for 20 seconds during delivery of SHG (60-100 nA), as illustrated in Figure 2A. In the absence of applied SHG, wind-up was lacking in the A-fiber response, whose mean fractional increase was 1.02 (±0.07 s.e.m., n=8 neurons). However, it was present for all neurons' C-fiber responses (1.41 ±0.16) and post-discharges (1.76 ± 0.34). The relatively low fractional increase in wind-up can be ascribed to the C-fiber stimulus having been set quite near the C-fiber threshold (see Methods). During and immediately following iontophoresis of SHG, wind-up of the C-fiber response was significantly reduced, on average to (0.95 ±0.04; P<0.001). The post-discharge wind-up also appeared to be dampened by SHG (1.25 ±0.17), but this did not reach significance, and the A-fiber responses were the least changed (1.15 ±0.07). The normal level of wind-up exhibited completely recovery by the 2-4 minute time interval after the end of delivery of SHG (Figure 2B).
Figure 1.
Modulation by SHG (60-100 nA) of responses to peripheral electrical stimulation of WDR neurons. The mean effect of SHG (1 minute) on responses to 0.1 Hz stimulation is shown (n=8 neurons). The asterisk shows a significant change in the group mean from the point prior to SHG administration (P<0.05).
Figure 2.
Effect of SHG on wind-up. A: An example of a wind-up response in the absence of SHG to a 20-scond trial of 1-Hz peripheral stimulation in one cell. Dots represent single action potentials and each line of dots is shifted upward for each succeeding stimulus. The clustering of A-fiber and C-fiber responses in their time-windows, and the growth of the post-discharge is illustrated. B: Mean change in A-fiber responses, C-fiber responses and post-discharge following application of SHG (60-100 nA) for 2 minutes (n=8 neurons).
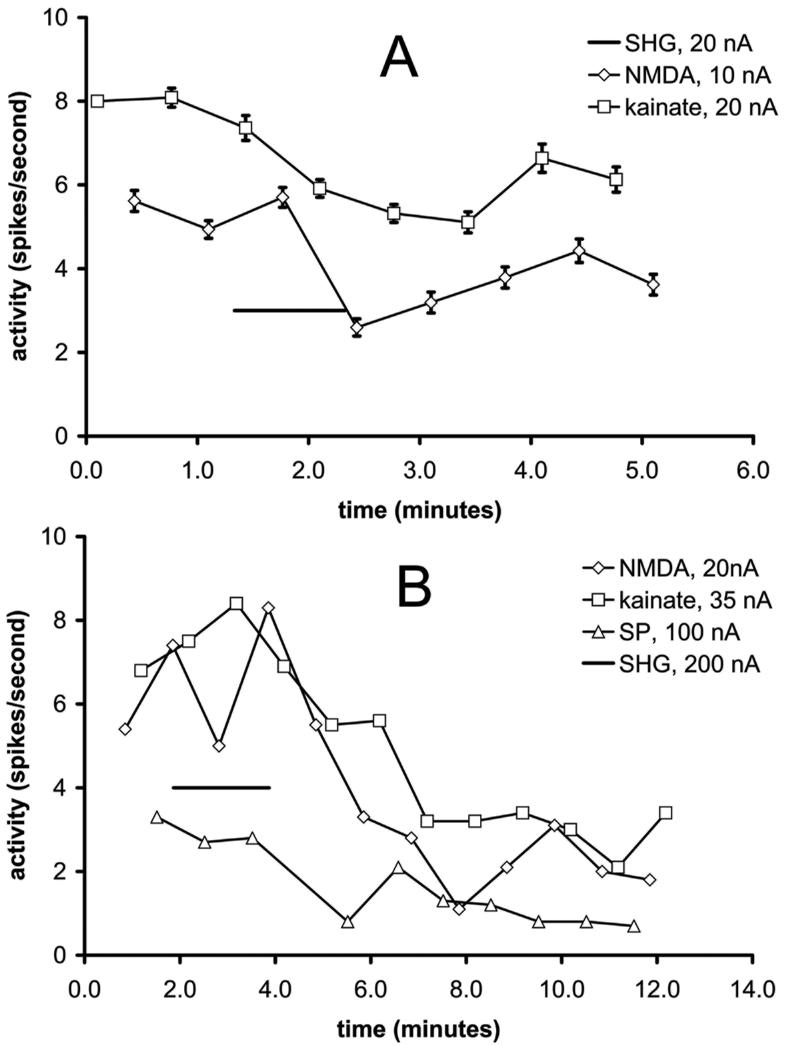
Reversal of iontophoretic glutamatergic excitation by SHG was studied by alternately applying NMDA and kainate to activate their separate, eponymous subtypes of glutamate receptor, again under halothane anesthesia. The alternating periods of application lasted 7 seconds, with 7-second intervals between applications (n=12 cells). With some cells (n=6), substance P was added to the repeating sequence. Iontophoresis currents were chosen to achieve similar levels of excitation, about 5-10 action potentials per second (spikes/s). This current level varied considerably among cells (5-80 nA), probably because of varying distances between the cell body and the microelectrode. When SHG (20-200 nA) was applied for one or two minutes during such a sequence, the effects of all 3 substances were significantly suppressed in all cells (e.g., Figures 3A, 3B). The inhibitory effect of SHG did not outlast the application period by more than 3 minutes with lower iontophoretic doses of SHG (e.g., Figure 3A). In contrast, with high iontophoretic currents (100-200 nA), the inhibitory effect increased over several minutes and prior levels of excitation were never restored (e.g., Figure 3B). The exact binomial distribution estimates >75% responding when the sample proportion is n=12/12 (kainate and NMDA) and >60% when it is n=6/6 (substance P).
Figure 3.
Examples of the modulatory influence of SHG on repeated cycles of excitation by NMDA and kainate (A), or by NMDA, kainate and substance P (B), under halothane anesthesia. Figure 3A depicts average responses to 5 trials of SHG, and figure 3B shows the result of a single trial. Responses are plotted in terms of the total number of action potentials (spikes) measured during and 5 seconds after the 7-s application of the excitatory substances, which were given in consecutive rotating order.
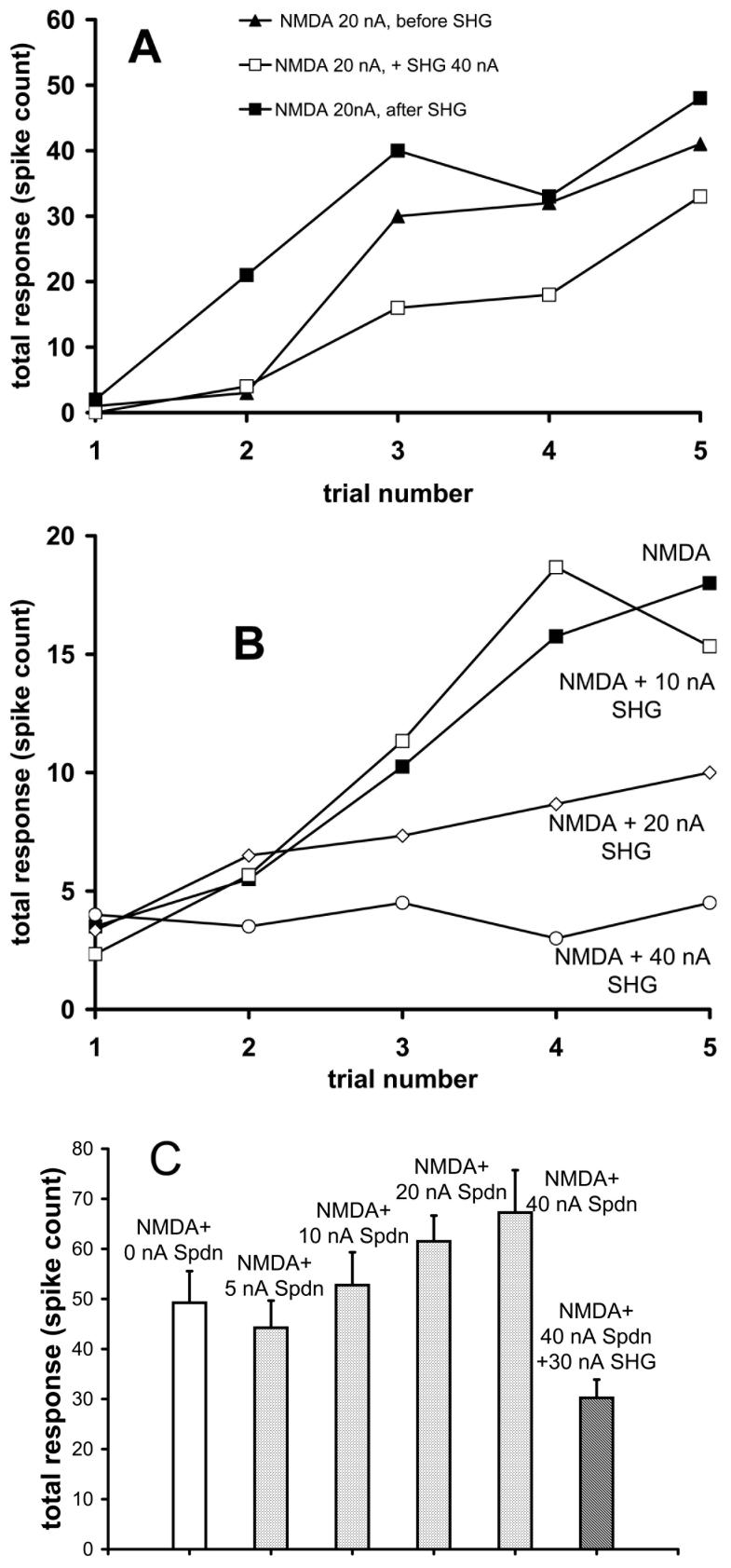
The effect of SHG on neuronal responses to NMDA was tested also under pentobarbital anesthesia. A train of 5 pulses of NMDA was applied, each pulse lasting 5 s, with 5 s separations between them and an interval of 50 s between trains. SHG was applied for 1 minute during one pulse train. SHG (5-40 nA) reduced the excitatory action of the NMDA (Figure 4A, 4B) in all cells tested (n=9; binomial estimated proportion >70%). The inhibitory effect of SHG increased with the dose (Figure 4B). To examine the spermidine regulatory site on NMDA receptors as a target for SHG, different doses of spermidine (5-80 nA) were applied continuously during pulsed application of NMDA (Figure 4C). An enhancement of NMDA-evoked excitation was produced dose-dependently by spermidine. This enhancement was opposed by SHG (n=4 neurons, binomial estimated proportion >47%), but the response was then reduced below that produced by NMDA alone.
Figure 4.
A, B. Examples of SHG opposing NMDA-induced excitation (20 nA) in WDR neurons under pentobarbital anesthesia. Responses are plotted in terms of the total number of action potentials (spikes) measured during the 5 seconds of NMDA application and 5-seoncds after. The trial number represents the order of the consecutive pulse applications, separated by 5-second intervals. The build-up of excitation is caused by the rapidly repeated applications. In A, recovery from suppression induced by 100 s of SHG application is illustrated; in B, a dose-response experiment with SHG is displayed. C: Effect of spermidine (Spdn, 0-40 nA) applied continuously at different doses on responses to 20 nA NMDA, including at the largest Spdn dose during continuously applied SHG (30 nA). Responses were averaged from the final 4 of 5 applications, parallel to those shown in Figures 4A and 4B. The response in the presence of SHG can be noted to be less than with NMDA alone in the absence of Spdn.
DISCUSSION
The above results provide the first physiological evidence for an inhibitory effect of the naturally derived peptide SHG on the response of WDR neurons in the spinal cord. From the perspective of clinical analgesia, it is of interest that the block was selective for C-fiber input. This selectivity extended to the blocking of wind-up, an effect that is that is both produced by C-fiber input and confined to C-fiber responses. The neurocytological sites of SHG's action is thus likely to lie at some point in the dorsal horn before A-fiber and C-fiber inputs converge on the WDR cells, but its locus remains unclear. The initial hypothesis for the receptor target was based on SHG acting exclusively as an antagonist of NMDA subtype glutamate receptors. This now seems unlikely, since SHG also blocked excitation by kainate (targeting kainate-subtype glutamate receptors) and substance P (targeting tachykinin receptors). On the other hand, the strong blocking of NMDA-mediated excitation and of wind-up suggests that the NMDA receptor could be a one of the targets of histogranin and SHG, which would be consistent with previous chemical binding and pharmacological findings [7, 9, 11]. However, the tests with spermidine make it unlikely that the polyamine facilitatory site is where SHG binds on the NMDA receptor, unless competition with an unknown endogenous modulator or an inverse agonistic effect of SHG was occurring.
Histogranin is apparently absent in the rat's normal spinal cord, although a relatively high level of histogranin mRNA has been found [10]. Thus the biological basis for the spinal cord's affinity for this chromaffin cell constituent peptide remains enigmatic. Histogranin was first isolated by its affinity to an antibody against bombesin (the vertebrate, non-mammalian analogue of neuromedins), but has no sequence in common with other peptides found in the dorsal horn. Because the QRLG sequence of bombesin is found alternated within histogranin (QGRTLYG), and the full-length histogranin peptide shows maximum antibody affinity in analog binding studies [11], secondary conformation may be a significant determinant of binding, so that neuromedin receptors cannot be entirely rules out as sites of SHG-mediated antinociception in the spinal dorsal horn. Indeed, bombesin, neuromedin B and neuromedin C block activity in nociceptive neurons, although, unlike histogranin, their intrathecal administration facilitates the tail-flick reflex to a noxious thermal stimulus [2, 3]. SHG could also bind to inhibitory monoamine receptors, given its affinity for dopamine D2 receptors [12], and this offers one mechanism for the blockade of the excitatory effects of kainate, NMDA and substance P, given that binding to these diverse receptors seems less likely. Another mechanism worth further study is block at non-synaptic receptors, such as nociceptive-specific voltage-gated sodium-channels [4].
SHG has a prolonged action when given intrathecally, such that it prevents the 1-2 hour period of hyperalgesia and allodynia produced by applying intrathecally NMDA 15 minutes later [7]. The present experiments demonstrated a prolonged action of SHG only when iontophoretically applied excitatory substances were opposed by the highest doses of SHG, whereas blocking of peripherally evoked responses at any SHG dose always disappeared rapidly. This difference can be explained by the geometrical distributions of peripherally and iontophoretically activated synapses on the recorded cell and the spatiotemporal pattern of diffusion from a point-source. Excitation by iontophoresis of agonists occurs predominantly near the micropipette, where the high levels of iontophoretically delivered SHG take longer to fall below threshold. Peripheral activation occurs throughout the dendritic tree and is dominated by the more numerous distal synapses (in a much larger volume) where SHG concentration will be much lower on average and will fall below threshold sooner. This prolonged blocking could perhaps be due to the SHG reaching a threshold for producing either intracellular enzymatic changes or toxicity.
In sum, the main finding above was that SHG selectively blocked the basal and potentiating synaptic effects of C-fibers on dorsal horn WDR neurons, while sparing activity produced by fast A-fiber afferents. This provides a physiological basis for the previously reported analgesic effects of SHG, shown with a variety of behavioral pain models. Remarkably in these behavioral experiments, the antinociception was unaccompanied by locomotor dysfunction, a side-effect that has limited exploitation of current NMDA receptor antagonists as clinical analgesics. This has encouraged the idea that histogranin mimetics may be useful in pharmacotherapy against hitherto problematic forms of chronic pain, a promise supported by the presently demonstrated specificity of SHG's inhibitory action for C-fiber input.
Acknowledgement
Supported by NIH grant DA10546 and the Campus Research Board of the University of Illinois Chicago
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Coghill RC, Mayer DJ, Price DD. Wide dynamic range but not nociceptive-specific neurons encode multidimensional features of prolonged repetitive heat pain. J Neurophysiol. 1993;69:703–16. doi: 10.1152/jn.1993.69.3.703. [DOI] [PubMed] [Google Scholar]
- 2.Cridland RA, Henry JL. Bombesin, neuromedin C and neuromedin B given intrathecally facilitate the tail flick reflex in the rat. Brain Res. 1992;584:163–8. doi: 10.1016/0006-8993(92)90890-l. [DOI] [PubMed] [Google Scholar]
- 3.De Koninck Y, Henry JL. Bombesin, neuromedin B and neuromedin C selectively depress superficial dorsal horn neurones in the cat spinal cord. Brain Res. 1989;498:105–17. doi: 10.1016/0006-8993(89)90404-6. [DOI] [PubMed] [Google Scholar]
- 4.Hains BC, Saab CY, Klein JP, Craner MJ, Waxman SG. Altered sodium channel expression in second-order spinal sensory neurons contributes to pain after peripheral nerve injury. J Neurosci. 2004;24:4832–9. doi: 10.1523/JNEUROSCI.0300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-Daspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–26. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- 6.Hama AT, Sagen J. Induction of spinal NADPH-diaphorase by nerve injury is attenuated by adrenal medullary transplants. Brain Res. 1994;640:345–51. doi: 10.1016/0006-8993(94)91892-9. [DOI] [PubMed] [Google Scholar]
- 7.Hama AT, Siegan JB, Herzberg U, Sagen J. NMDA-induced spinal hypersensitivity is reduced by naturally derived peptide analog [Ser1]histogranin. Pharmacol Biochem Behav. 1999;62:67–74. doi: 10.1016/s0091-3057(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 8.Hentall ID, Noga BR, Sagen J. Spinal allografts of adrenal medulla block nociceptive facilitation in the dorsal horn. J Neurophysiol. 2001;85:1788–92. doi: 10.1152/jn.2001.85.4.1788. [DOI] [PubMed] [Google Scholar]
- 9.Lemaire S, Rogers C, Dumont M, Shukla VK, Lapierre C, Prasad J, Lemaire I. Histogranin, a modified histone H4 fragment endowed with N-methyl-D-aspartate antagonist and immunostimulatory activities. Life Sci. 1995;56:1233–41. doi: 10.1016/0024-3205(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 10.Poirier R, Lemaire I, Lemaire S. Characterization, localization and possible anti-inflammatory function of rat histone H4 mRNA variants. Febs J. 2006;273:4360–73. doi: 10.1111/j.1742-4658.2006.05444.x. [DOI] [PubMed] [Google Scholar]
- 11.Rogers C, Lemaire S. Characterization of [125I][Ser1]histogranin binding sites in rat brain. J Pharmacol Exp Ther. 1993;267:350–6. [PubMed] [Google Scholar]
- 12.Ruan H, Lemaire S. Interactions of histogranin and related peptides with dopamine D2 receptor in rat brain membranes. Synapse. 2001;39:270–4. doi: 10.1002/1098-2396(20010301)39:3<270::AID-SYN1009>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Sagen J, Castellanos D, Gajavelli S. Transplants for chronic pain. In: Halberstadt C, Emerich D, editors. Cellular Transplants: From Lab to Clinic. Elsevier; Burlington, MA: 2006. [Google Scholar]
- 14.Sagen J, Kemmler JE. Increased levels of Met-enkephalin-like immunoreactivity in the spinal cord CSF of rats with adrenal medullary transplants. Brain Res. 1989;502:1–10. doi: 10.1016/0006-8993(89)90455-1. [DOI] [PubMed] [Google Scholar]
- 15.Sagen J, Kemmler JE, Wang H. Adrenal medullary transplants increase spinal cord cerebrospinal fluid catecholamine levels and reduce pain sensitivity. J Neurochem. 1991;56:623–7. doi: 10.1111/j.1471-4159.1991.tb08195.x. [DOI] [PubMed] [Google Scholar]
- 16.Sagen J, Pappas GD, Pollard HB. Analgesia induced by isolated bovine chromaffin cells implanted in rat spinal cord. Proc Natl Acad Sci U S A. 1986;83:7522–6. doi: 10.1073/pnas.83.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagen J, Wang H. Adrenal medullary grafts suppress c-fos induction in spinal neurons of arthritic rats. Neurosci Lett. 1995;192:181–4. doi: 10.1016/0304-3940(95)11640-i. [DOI] [PubMed] [Google Scholar]
- 18.Shukla VK, Lemaire S, Dumont M, Merali Z. N-methyl-D-aspartate receptor antagonist activity and phencyclidine-like behavioral effects of the pentadecapeptide, [Ser1]histogranin. Pharmacol Biochem Behav. 1995;50:49–54. doi: 10.1016/0091-3057(94)00247-g. [DOI] [PubMed] [Google Scholar]
- 19.Siegan JB, Hama AT, Sagen J. Alterations in rat spinal cord cGMP by peripheral nerve injury and adrenal medullary transplantation. Neurosci Lett. 1996;215:49–52. doi: 10.1016/s0304-3940(96)12962-1. [DOI] [PubMed] [Google Scholar]
- 20.Siegan JB, Herzberg U, Frydel BR, Sagen J. Adrenal medullary transplants reduce formalin-evoked c-fos expression in the rat spinal cord. Brain Res. 2002;944:174–83. doi: 10.1016/s0006-8993(02)02742-7. [DOI] [PubMed] [Google Scholar]
- 21.Siegan JB, Sagen J. Attenuation of formalin pain responses in the rat by adrenal medullary transplants in the spinal subarachnoid space. Pain. 1997;70:279–85. doi: 10.1016/s0304-3959(97)03335-6. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SP, Chang KJ, Viveros OH. Proportional secretion of opioid peptides and catecholamines from adrenal chromaffin cells in culture. J Neurosci. 1982;2:1150–6. doi: 10.1523/JNEUROSCI.02-08-01150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]