Abstract
Studies have shown that fish oils, containing n-3 fatty acids, have protective effects against ischemia-induced, fatal cardiac arrhythmias in animals and perhaps in humans. In this study we used the whole-cell voltage-clamp technique to assess the effects of dietary, free long-chain fatty acids on the Na+ current (INa,α) in human embryonic kidney (HEK293t) cells transfected with the α-subunit of the human cardiac Na+ channel (hH1α). Extracellular application of 0.01 to 30 μM eicosapentaenoic acid (EPA, C20:5n-3) significantly reduced INa,α with an IC50 of 0.51 ± 0.06 μM. The EPA-induced suppression of INa,α was concentration- and voltage-dependent. EPA at 5 μM significantly shifted the steady-state inactivation relationship by −27.8 ± 1.2 mV (n = 6, P < 0.0001) at the V1/2 point. In addition, EPA blocked INa,α with a higher “binding affinity” to hH1α channels in the inactivated state than in the resting state. The transition from the resting state to the inactivated state was markedly accelerated in the presence of 5 μM EPA. The time for 50% recovery from the inactivation state was significantly slower in the presence of 5 μM EPA, from 2.1 ± 0.8 ms for control to 34.8 ± 2.1 ms (n = 5, P < 0.001). The effects of EPA on INa,α were reversible. Furthermore, docosahexaenoic acid (C22:6n-3), α-linolenic acid (C18:3n-3), conjugated linoleic acid (C18:2n-7), and oleic acid (C18:1n-9) at 5 μM and all-trans-retinoic acid at 10 μM had similar effects on INa,α as EPA. Even 5 μM of stearic acid (C18:0) or palmitic acid (C16:0) also significantly inhibited INa,α. In contrast, 5 μM EPA ethyl ester did not alter INa,α (8 ± 4%, n = 8, P > 0.05). The present data demonstrate that free fatty acids suppress INa,α with high “binding affinity” to hH1α channels in the inactivated state and prolong the duration of recovery from inactivation.
Keywords: transfection, eicosapentaenoic acid
Dietary long chain polyunsaturated fatty acids (PUFAs) are able to prevent ischemia-induced fatal arrhythmias in animals (1–4) and probably in humans (5–7). Their antiarrhythmic actions have been demonstrated in cultured cardiac myocyte preparations in the absence of neuronal or humoral influences (8) and have been shown to result from a stabilizing effect of the PUFAs on the electrophysiology of each individual myocyte (9). The electrophysiologic effects of the PUFAs in turn are caused by their modulation of several ion currents through the plasma membrane of the cardiomyocytes (10–12). One of the ion currents affected by the PUFAs is the voltage-dependent, fast sodium current, INa (10), which is responsible for initiating the action potential in cells of excitable tissues. Thus the effects of the PUFAs on INa must constitute an important factor in the ability of the PUFAs to prevent or terminate fatal cardiac arrhythmias.
We have used whole-cell voltage-clamp studies on cultured neonatal rat cardiomyocytes to examine the effects of the PUFAs on INa and have found that in their free fatty acid form they rapidly and strongly suppress INa and prolong the duration of the inactivated state of the sodium channels (10, 11). Our previous studies of the PUFAs on INa all have been on the cardiac myocytes of neonatal and adult rat hearts. To extend understanding of the electrophysiologic effects of PUFAs and to learn what effects PUFAs may have on INa in the human heart, we studied their effects on the major α-subunit (hH1α) of the sodium channel protein complex from the human heart when transiently transfected into stable cultured human embryonic kidney cells, HEK293t, which themselves lack a voltage-dependent INa.
MATERIALS AND METHODS
Cell Culture and Transient Transfection.
The methods for the culture of human embryonic kidney (HEK293t) cells and their transient transfection with the α-subunit of the human cardiac Na+ channel (hH1α) were similar to that described by Cannon and Strittmatter (13). Briefly, cells were grown to 50% confluence in DMEM (GIBCO) containing 10% fetal bovine serum (HyClone), 1% penicillin and streptomycin solution (Sigma), 3 mM taurine, and 25 mM Hepes (GIBCO). Cells were split twice per week. HEK293t cells were transfected with cloned hH1α Na+ channels (the α-subunit) by a calcium phosphate precipitation method in a TI-25 flask. A reporter plasmid CD8-pih3 m (1 μg, cell surface antigen) and hH1α Na+ channel cDNA (10 μg) in the pcDNA1/amp vector (Invitrogen) were prepared in 250 mM CaCl2, added to a test tube containing 0.36 ml of Hepes-buffered saline (2×) solution (274 mM NaCl/40 mM Hepes/12 mM dextrose/10 mM KCl/1.4 mM Na2HPO4, pH 7.05), and incubated at 22°C for 20 min. The DNA solution then was dripped over a cell culture (30–50% confluence) containing 7 ml of DMEM. The transfected cells were trypsinized and replated 15 hr later to an appropriate density in 35-mm tissue culture dishes (which also served as recording chambers) containing 2 ml of fresh DMEM. Transfected cells were incubated at 37°C in air with 5% CO2 added and 98% relative humidity and used within 3 days. Transfection-positive cells that were identified by binding immunobeads (CD8-Dynabeads M-450, Dynal, Oslo, Norway) coated with a mAb (ITI-5C2) specific for CD8 antigen were selected for patch-clamp experiments. Successful transfection was confirmed by the presence of voltage-dependent fast Na+ currents in these cells (14).
Recording of Na+ Current.
During an experiment the cells plated in a culture dish were continuously superfused (1–2 ml/min) with a Tyrode’s solution containing 137 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 10 mM Hepes, 10 mM glucose, pH 7.4. Recording glass electrodes had a resistance of 2–4 MΩ when filled with the pipette solution and were connected via a Ag-AgCl wire to an Axopatch 1D amplifier (Axon Instruments, Foster City CA). A cell coated with CD8 beads was chosen for patch clamp study. After forming a conventional “gigaseal” the capacitance of an electrode was compensated. Additional suction was used to form the whole-cell configuration (15). Whole-cell membrane capacitance was measured as described previously (16). Correction of cell capacitance and series resistance then was performed before application of experimental voltage-clamp protocols. Recordings of INa,α were made from the same cell before, during, and after exposure to fatty acids. Bath solutions with or without fatty acids were rapidly exchanged by using a modified puffer-pipette system (10). Experiments were conducted at room temperature of 22–23°C.
The pipette solution for recording the inward Na+ current contained 100 mM CsCl, 40 mM CsOH, 1 mM MgCl2, 1 mM CaCl2, 11 mM EGTA, 10 mM Hepes, 5 mM MgATP, pH adjusted to 7.3 with CsOH. In all experiments the bath solution contained 60 mM NaCl, 40 mM N-methyl-[scap]d-glucamine, 10 mM CsCl, 1 mM MgCl2, 1.8 mM CaCl2, 10 mM Hepes, 10 mM glucose, pH adjusted to 7.4 with HCl.
Materials.
All fatty acids were obtained from Sigma. Fatty acids were dissolved weekly in ethanol at a concentration of 10 mM and stored under nitrogen at −20°C before use. The experimental concentration of fatty acids was obtained by dilution of the stocks and contained negligible ethanol, which alone had no effect on INa,α.
Statistics.
Data from two groups were analyzed by paired or unpaired Student’s t test. Variance analysis (one-way ANOVA) was used to compare the difference derived from three or more group experiments. The level for statistical significance was set at P < 0.05. All data are presented as mean ± SEM. Some data were fit by least-squares fitting (y = A0 + A1exp−t/τ1 + A2exp−t/τ2; origin 4.1, Microcal Software, Northampton, MD) with single or double exponential functions.
RESULTS
Voltage-Gated Na+ Currents in HEK293t Cells Transfected with hH1α Channels.
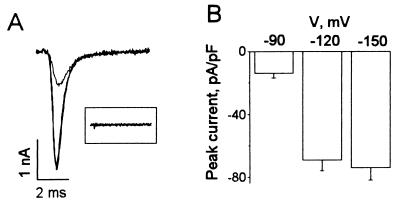
HEK293t cells successfully transfected with hH1α channels had voltage-activated Na+ currents with fast activation and fast inactivation kinetics (Fig. 1A). In contrast, cells without transfected hH1α channels did not have INa,α (Fig. 1A Inset, n = 16). The average capacitance of the cells studied was 43 ± 1 pF (n = 121). INa,α was activated at the threshold near −60 mV and reached a maximum at around −30 mV (see Fig. 3A, control, □). The averaged peak currents of INa,α were −14 ± 3, −69 ± 7, and −74 ± 8 pA/pF (n = 27) for single-step pulses to −30 mV from −90, −120, and −150 mV, respectively (Fig. 1B). The steady-state inactivation of INa,α in HEK293t cells transfected with hH1α channels had a mean value of −97.5 ± 7.3 mV (n = 6) at the V1/2 point (see Fig. 3B, control, □). This value is hyperpolarized (P < 0.001) compared with that of INa in neonatal rat cardiac myocytes that was −70.0 ± 4.6 mV (n = 7) at the V1/2 point (10).
Figure 1.
The voltage-dependent Na+ currents in HEK293t cells transfected with hH1α, the α-subunit of the human cardiac Na+ channel. (A) Superimposed Na+ current traces were evoked by 10-ms duration depolarizing stimuli from −150, −120, and −90 mV to −30 mV at 0.2 Hz in an HEK cell expressing hH1α Na+ channels. The decrease of the maximal Na+ current from −120 compared with −150 mV is very small. The inset shows that no INa,α was evoked by the same voltage steps in a HEK293t cell not expressing hH1α Na+ channels. Capacitance and leakage currents have been subtracted on line from the Na+ currents shown. (B) The averaged peak INa,α was elicited by voltage steps to −30 mV from −150, −120, and −90 mV, respectively, in HEK293t cells expressing hH1α (n = 27).
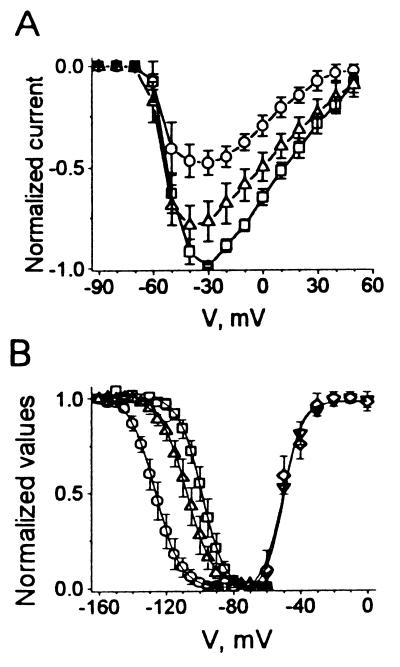
Figure 3.
The activation and deactivation of INa,α in hH1α expressed in HEK293t cells in the absence and presence of EPA (5 μM). (A) Averaged and normalized current-voltage relationships (n = 6) of INa,α were plotted in the absence (□), presence (○), and washout (▵) of 5 μM EPA. (B) The averaged relative activation of INa,α was determined by the ratio of conductances [G = I/(V − VRev), in which VRev was the reversal potential from each I–V curve] to maximal conductance (Gmax). The curves were fit by a Boltzmann function: y = 1/{1 + exp[V − V1/2)/k]}, which yields V1/2 of −50.1 ± 0.7 mV and a slope with k of 5.3 ± 0.7 mV in control (♦, n = 6). EPA (5 μM) did not significantly alter the activation with V1/2 of −50.8 ± 1.3 mV and a slope of k of 6.0 ± 1.1 mV (▿, n = 6, P > 0.05) − (Right). By contrast, EPA altered the steady-state inactivation of INa,α in HEK293t cells expressing hH1α (Left). Currents were evoked by two pulses at 0.1 Hz; a prepulse, 500-ms duration with 5-mV increments from −160 mV to −55 mV, and a test pulse of 10-ms duration from the various prepulses to −30 mV. The resting membrane potential was held at −80 mV. The normalized steady-state inactivation of peak INa,α shows the control (□) with fitting parameters (fit by a Boltzmann function: y = 1/{1 + exp[(V − V1/2)/k]}) of V1/2 = −97.5 ± 2.3 mV and k = 6.8 ± 1.7, EPA (5 μM, ○) with fitting parameters of V1/2 = −125.3 ± 2.5 mV and k = 7.6 ± 1.5, and washout (▵) with fitting parameters of V1/2 = −105.8 ± 3.4 mV and k = 7.9 ± 1.8. The shift caused by EPA at V1/2 is significantly different from control (P < 0.0001 n = 6).
Effects of PUFAs on INa,α of hH1α Expressed in HEK293t Cells.
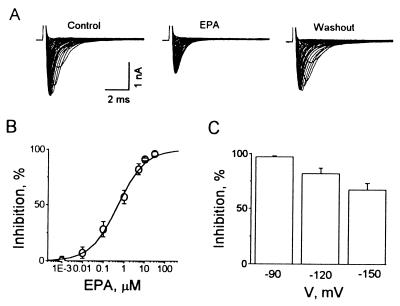
Addition of eicosapentaenoic acid (EPA) to the superfusate suppresses INa,α reversibly, as shown in Fig. 2A. Whole-cell voltage-clamp traces from HEK293t cells expressing hH1α elicited by 10-ms test pulses from −90 to 55 mV with 5 mV increments at 0.2 Hz for control, 5 μM EPA and washout are superimposed. The inhibitory effect of EPA on INa,α was initiated rapidly, within 20 s and reached a maximal level within 3 min, returning toward the pretreatment level after washout of the EPA with 0.2% fatty-acid free BSA added to the superfusate.
Figure 2.
Inhibitory effects of EPA on INa,α of hH1α channels expressed in HEK293t cells. (A) Whole-cell voltage-clamp traces from HEK293t cells expressing hH1α are superimposed. They were elicited by 10-ms test pulses from −90 mV to 55 mV with 5-mV increments at 0.2 Hz for control, 5 μM EPA and washout. The cell was held at −80 mV and hyperpolarized to −160 mV for 200 ms before a test pulse. (B) EPA suppressed INa,α is concentration-dependent with an IC50 of 0.51 ± 0.06 μM. INa,α was elicited by single voltage pulses from −120 to −30 mV. The sigmoidal curve is fit by y = {(A1 − A2)/[1 + (x/x0)p]}, origin 4.1, Microcal Software. Each value represents 6–12 individual preparations exposed to different concentrations of EPA. (C) Voltage-dependent suppression by EPA (5 μM) of INa,α in HEK293t cells expressing hH1α. The cells were held at −80 mV and hyperpolarized to −90, −120, and −150 mV for 200 ms before a test pulse to −30 mV.
The EPA-induced suppression of INa,α was concentration-dependent with suppression evident at 10 nM and an IC50 of 510 ± 60 nM when INa,α was elicited by single-step pulses from −120 to −30 mV (Fig. 2B). Fig. 2C shows that 5 μM EPA inhibited INa,α by 97 ± 1%, 82 ± 5%, and 67 ± 6% (n = 12) for the currents evoked by a single-step voltage command with pulses to −30 mV from −90, −120, and −150 mV, respectively. Therefore, the EPA-induced suppression of INa,α was voltage-dependent.
Effects of EPA on Activation of INa,α.
The EPA-induced suppression of INa,α did not alter the current-voltage relationship (n = 6, Fig. 3A). The Na+ current was activated at −60 mV and attained maximal amplitude at −30 mV in the absence and presence of 5 μM EPA.
The activation curves calculated from relative conductances in the absence and presence of 5 μM EPA overlapped (n = 6, Fig. 3B, Right). The 50% channel activation was at −50.1 ± 0.7 mV and −50.8 ± 1.3 mV for the control and EPA, respectively.
Effects of EPA on the Steady-State Inactivation.
Fig. 3B (Left) illustrates that 5 μM EPA modified the steady-state inactivation. The membrane potential of the cells was held at −80 mV. Currents were elicited with a double-pulse protocol that consisted of a 10-ms test pulse to −30 mV after a 500-ms prepulse varying from −160 mV to −55 mV in 5-mV increments at 0.1 Hz. Bath perfusion of 5 μM EPA solution significantly suppressed INa,α (Fig. 3B, ○, EPA). The inhibition was reversed by washout of EPA (Fig. 3B, ▵, Washout). INa,α was completely inhibited in the presence of EPA when the prepulses were set from −80 to −100 mV (Fig. 3B). The normalized steady-state inactivation curve of INa,α was significantly shifted toward the hyperpolarizing direction, from −97.5 ± 2.3 mV for the control to −125.3 ± 2.5 mV (n = 6, P < 0.0001) in the presence of 5 μM EPA (Fig. 3B). After washout of EPA with 0.2% fatty acid-free BSA, the steady-state inactivation curve was shifted back to −105.8 ± 3.4 mV at the V1/2 point. Although the values at the V1/2 point between the control and washout are not significant (P > 0.05, ANOVA), the difference between EPA and washout was statistically significant (P < 0.01, ANOVA). The mean shift for INa,α caused by 5 μM EPA was −27.8 ± 1.2 mV, which is different (P < 0.05) from the −19 ± 3 mV shift (n = 7) caused by 10 μM EPA in neonatal rat cardiac myocytes (10).
“Binding Affinity” of EPA to hH1α Channels in the Resting and Inactivated States.
Channels in the resting and inactivated states are in dynamic equilibrium during depolarization and repolarization. Only closed resting channels generally are thought able to open during depolarizations. Therefore, current amplitude would be proportional to the number of channels in the resting state before a depolarization pulse. The EPA-induced suppression of INa,α was voltage-dependent (Fig. 2C) and the steady-state inactivation of INa,α was shifted −27.8 mV in the presence of EPA (Fig. 3B). The voltage-dependent block and the negative shift in channel availability may be interpreted as caused by preferential EPA “binding” to inactivated channels compared with closed resting channels (17). Therefore, we used different protocols to test “binding affinities” of EPA to hH1α channels in the resting state and in the inactivated state.
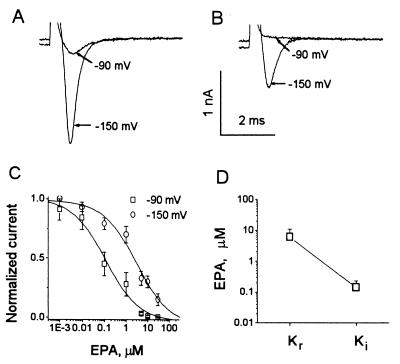
Fig. 4A indicates that in the absence of EPA most hH1α channels are in the resting state at a holding potential of −150 mV as seen by Na+ currents evoked by depolarizing pulses to −30 mV. By contrast, at −90 mV most channels are inactivated and the presence of 5 μM EPA inactivates and suppresses virtually all hH1α channels (Fig. 4B) at the holding potential of −90 mV. To examine the EPA “binding affinity” to resting channels, we assessed the EPA-induced concentration-dependent suppression of INa,α evoked by depolarization pulses from a holding potential of −150 mV to −30 mV (Fig. 4C). At −150 mV holding potential, nearly all channels are in the resting state. The concentration-dependent curve gave an estimated Kr, the equilibrium constant for drug “binding” of EPA to resting channels (17) of 6.03 ± 4.78 μM (Fig. 4D).
Figure 4.
“Binding affinity” of EPA to hH1α channels in the resting and inactivated states. Current traces were evoked by voltage steps to −30 mV from the holding potentials of −90 mV or −150 mV in the absence (A) and presence (B) of 5 μM EPA. (C) The concentration-dependent suppression of resting (○) and inactivated (□) hH1 channels by EPA is shown. The data points are fit by the same equation as in Fig. 3B. Each value represents 6–12 cells (mean ± SEM). (D) Mean Kr and Ki of EPA in HEK293t cells expressing hH1α channels is shown. The concentration-dependent curves in C give a Kr from the −150 mV holding potential of 6.03 ± 4.78 μM when all of the hH1α channels are in the resting, activatable state. Ki is the equilibrium constant, 0.14 ± 0.09 μM of EPA, from the −90 mV holding potential when nearly all of the channels are in the inactive, closed state. Normalized current was calculated as INa,α(EPA)/INa,α(control) from the same cell.
Because inactivated channels do not open during depolarizations, the effects of drug “binding” to the inactivated state must depend on a reduced portion of channels being in the resting state. Therefore, we set the membrane holding potential at −90 mV and assessed the midpoint of the concentration-dependent relationship of EPA, Ki (Fig. 4C). Here, Ki is the equilibrium constant for “binding” of EPA at holding potentials at which most channels are inactivated (17). When the membrane potential was set at −90 mV in the presence of 5 μM EPA, INa,α was reduced more than 90% (Figs. 3B and 4B). Ki measured at this holding potential was 0.14 ± 0.09 μM of EPA (Fig. 4D). Thus, hH1α channels in the inactivated state displayed a 43-fold higher “affinity” to EPA than channels in the resting state.
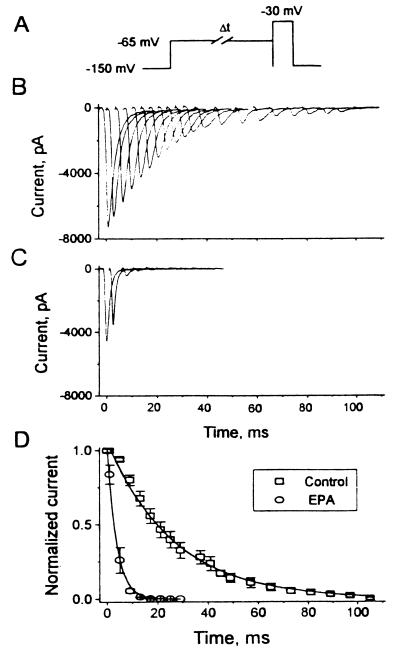
Effects of EPA on the Development of Resting Inactivation.
The traditional view has been that membrane fast sodium channels go through a cycle of responses to electrical stimulation. From a resting, activatable state they respond to a depolarizing stimulus by opening and producing an action potential. After this open state they enter an inactive state during which they are not excitable. With repolarization of their resting membrane potential they return again to the resting, activatable state and the cycle is repeated. However, in cardiac myocytes Na+ channels may directly transit from the resting state to the inactivated state without opening (18, 19). To assess the effects of EPA on the direct transition from the resting to the inactivated states from a holding potential of −150 mV, a conditioning prepulse to −65 mV was held for progressively longer intervals and followed by a test pulse to −30 mV (Fig. 5A). Because no Na+ currents were evoked by pulses to −65 mV (Fig. 3B), the development of inactivation was directly from the resting state. Fig. 5B shows that INa,α amplitude decreased progressively as the duration of conditioning prepulses increased, indicating that more and more channels entered from the resting into the inactive state. In the presence of 5 μM EPA INa,α amplitudes were even more dramatically decreased by increasing the duration of conditioning prepulses (Fig. 5C). The time course of this process, which was well fit by a single exponential, is shown in Fig. 5D. Compared with the control cells (τ = 26.2 ± 0.78 ms, n = 6), the time constant for the development of inactivation was significantly decreased in cells treated with EPA (τ = 3.67 ± 0.22 ms, P < 0.01, Fig. 5D).
Figure 5.
Development of resting inactivation of INa,α in HEK293t cells expressing hH1α directly from the resting, activatable state. (A) The pulse protocol shows a depolarizing pulse from a holding potential of −150 mV to −65 mV. With progressively longer values of Δt at −65 mV an increasing proportion of hH1α transited directly to their inactivated state. This is followed by a 10-ms test pulse to −30 mV. Current traces of INa,α in the absence (B) and presence (C) of 5 μM EPA (n = 6). The membrane potential was held at −150 mV and cycling frequency was 0.2 Hz. (D) The effects of prolonging the time at −65 mV (during which increasing numbers of hH1α channels slipped directly from their resting state into their inactivated state) on the normalized currents in the presence and absence of EPA are shown. In the presence of EPA the time constant for the development of inactivation was significantly decreased [control 26.2 ± 0.78 ms, n = 6 and EPA (5 μM) 3.67 ± 0.22 ms, n = 6, P < 0.01)].
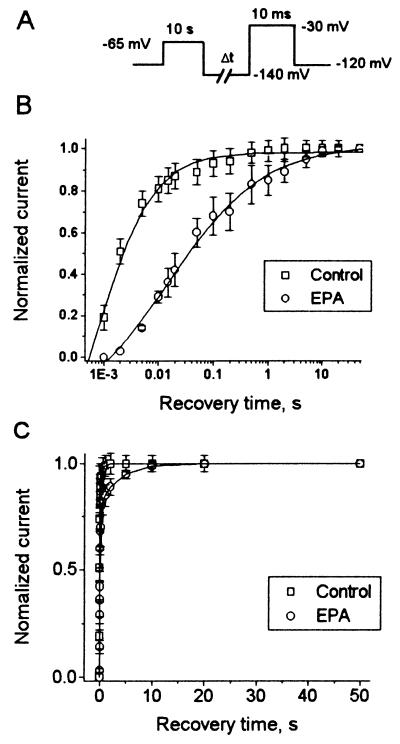
Effects of EPA on the Recovery from Inactivation.
The kinetics of INa,α recovery from inactivation were examined in the absence and presence of EPA. The pulse protocol consisted of a 10-s depolarizing pulse to −65 mV followed by a recovery interval with variable duration at −140 mV and then a subsequent test pulse to −30 mV (Fig. 6A). The time for 50% recovery from inactivation of INa,α was significantly delayed in the presence of 5 μM EPA, from 2.1 ± 0.8 ms for the control to 34.8 ± 2.1 ms (Fig. 6B, n = 5, P < 0.001).
Figure 6.
Kinetics of recovery of INa,α from inactivation. (A) Pulse protocol composed of a 10-s depolarizing pulse from −120 mV to −65 mV followed by a hyperpolarizing pulse to −140 mV of progressively longer durations and then a test pulse to −30 mV of 10-ms duration. The membrane holding potential was −120 mV and cycling frequency was 0.1 Hz. (B) The time course of recovery of peak INa,α in the absence (□, Control, n = 8) and presence (○, EPA, n = 5) of 5 μM EPA is shown. The test currents have been normalized to their maximal steady-state values of INa,α. Recovery of INa,α was markedly delayed; Δt1/2 in the presence of 5 μM EPA was 34.8 ± 2.1 ms compared with 2.1 ± 0.1 before EPA was present (n = 5, P < 0.001). Note a logarithmic scale of the abscissa. Data are fit by the same equation as in Fig. 3B. (C) The recovery times of INa,α were well fitted by a double exponential function.
The time course of recovery from inactivation of INa,α in the absence of EPA was well fit by a double exponential function, (y = A0 + A1exp−t/τ1 + A2exp−t/τ2, Fig. 6C, □). The amplitudes (A) and time constants (τ) for the recovery from inactivation of INa,α in the absence of EPA were 78 ± 6% (A1), 21 ± 2% (A2), 2.1 ± 0.3 ms (τ1), and 232 ± 78 ms (τ2). Recovery from inactivation of INa,α in the presence of EPA was also well fit by a double exponential function (Fig. 6C, ○). The amplitudes and time constants for the recovery from inactivation of INa,α were 67 ± 8% (A1), 28 ± 5% (A2), 12.6 ± 1.3 ms (τ1), and 440 ± 69 ms (τ2). Though τ2 in the presence of 5 μM EPA was 208 ± 73 ms greater than τ2 in the absence of EPA, the difference did not reach significance (P > 0.05), because of the variability of the measurements. In contrast, τ1 in the presence of 5 μM EPA was significantly increased (n = 5, P < 0.001). These results suggest that EPA acts on the fast inactivation component of hH1α and possibly on its slow component as well.
Effects of Other Fatty Acids on INaα.
Table 1 summarizes the effects of other fatty acids, docosahexaenoic acid (DHA, C22:6), α-linolenic acid (α-LNA, 18:3), conjugated linoleic acid (CLA, C18:2), oleic acid (OA, C18:1), stearic acid (SA, C18:0), palmitic acid (PA, C16:0), all-trans-retinoic acid (RA), and the ethyl ester of eicosapentaenoic acid (EPA EE), on INa,α in HEK293t cells expressing hH1α channels. DHA, α-LNA, CLA, and OA at 5 μM produced significant inhibition of INa,α, respectively. RA, but at 10 μM, caused an inhibition of INa,α similar to that of 5 μM EPA. Interestingly, SA and PA, two saturated fatty acids, at 5 μM also significantly suppressed INa,α by 20 ± 3% (n = 8, P < 0.01) and 34 ± 8% (n = 5, P < 0.05), respectively. In contrast, extracellular application of 5 μM EPA EE only caused 8 ± 4% (n = 8, P > 0.05) inhibition of INa,α.
Table 1.
Suppressant effects of fatty acids on Na+ currents in HEK293t cells transfected with the α-subunit of the human cardiac Na+ channel
| Fatty acids | n | μM | % inhibition | P |
|---|---|---|---|---|
| EPA | 12 | 5 | 82 ± 5 | <0.01 |
| DHA | 6 | 5 | 52 ± 10 | <0.01 |
| α-LNA | 6 | 5 | 64 ± 9 | <0.01 |
| CLA | 6 | 5 | 49 ± 9 | <0.01 |
| OA | 9 | 5 | 53 ± 6 | <0.01 |
| SA | 8 | 5 | 20 ± 3 | <0.01 |
| PA | 5 | 5 | 34 ± 8 | <0.05 |
| RA | 5 | 10 | 55 ± 9 | <0.01 |
| EPAEE | 8 | 5 | 8 + 4 | >0.05 |
Values are means ± SEM. Currents were elicited by single-step voltage pulses from −120 mV to −30 mV at 0.2 Hz. n, number of cells; P, statistical difference versus the corresponding control. Inhibition was calculated from the same cell by the equation of {[(1 − INa,α(fatty acid)/INa,α(control)) × 100]/100}. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; α-LNA, α-linolenic acid; CLA, conjugated linoleic acid; OA, oleic acid; SA, stearic acid; PA, palmitic acid; RA, all-trans-retinoic acid; and EPAEE, eicosapentaenoic ethyl ester.
DISCUSSION
There is now considerable direct evidence that N-3 polyunsaturated fatty acids can prevent ischemia-induced fatal ventricular fibrillation in animals (1–4) and probably in humans (5–7). We have demonstrated their antiarrhythmic effects in neonatal rat cardiomyocytes that beat rhythmically and spontaneously in culture (8). Free, nonesterified PUFAs (<10 μM) affect the electrophysiology of each cardiomyocyte to make it resistant to arrhythmias (9). They slightly hyperpolarize the resting membrane potential and make more positive the threshold for the gating of the fast Na+ channels so that a 40–50% stronger electrical stimulus is required to elicit an action potential. They also prolong the relative refractory period 2- to 3-fold despite slight shortening of the action potential duration. These stabilizing effects result from their ability to modulate several transmembrane ion currents (10, 11) including INa.
To extend our earlier observations (10) we report the effects of the PUFAs on the α-subunit of the human myocardial Na+ channel, hH1α, transiently transfected into a stable human embryonic cell line, HEK293t, which lacks a voltage-dependent, fast Na+ current. Micro injection or transfection of nucleic acid encoding only the α-subunit is sufficient for functional expression of voltage-gated Na+ channels (20–24), as we also observed. Extracellular application of EPA significantly suppressed INaα of hH1α, in a concentration and voltage-dependent manner. EPA (5 μM) shifted the steady-state inactivation relationship by −27 ± 1.2 mV (P < 0.0001) at the V1/2 point. EPA blocked INaα with a 43-fold higher “binding affinity” to hH1α in the inactivated state than in the resting state. The transition from the resting to the inactivated state was markedly accelerated in the presence of EPA and the time for recovery from the inactivated state was significantly slowed. Fifty percent recovery from inactivation in the presence of EPA (5 μM) was prolonged from 2.1 ± 0.8 ms to 34.8 ± 2.1 ms (P < 0.001). The results indicate that EPA prolongs the fast recovery component from inactivation of hH1α, as reported by Bendahhou et al. (25), and possibly the slow component of recovery, as well. All these effects of PUFAs were prompt and reversible. These results with the α-subunit of the Na+ channel of human cardiac myocytes alone transiently transfected into a stable human renal embryonic cell, which lacks a fast, voltage-dependent Na+ channel, extend our previous report on the inhibition of INa in cultured neonatal rat cardiomyocytes (10) by these PUFAs.
The steady-state inactivation of INa,α of HEK293t cells transfected with hH1α alone was shifted by −27.5 mV at the V1/2 point compared with the INa,α in neonatal rat cardiac myocytes and 5 μM EPA shifted the V1/2 point a further −27.8 mV. This is why at a holding potential of −90 mV less than 10% of the channels are in the resting activatable state and why we had to hyperpolarize the cells to a seemingly unphysiological −160 mV to assure that most channels were in that state.
By contrast with its marked effects on the inactivated state of the Na+ channel, the activation of the channel by depolarization was unaffected. The slopes of the normalized conductance-voltage curves were the same. In a classic study Stühmer et al. (27) showed that a reduction in the net positive charge in segment S4 of repeat 1 leads to a decrease in the steepness of the potential dependence of activation, a finding that provides experimental evidence for the direct involvement of the positive charges in the voltage sensor of segment S4. That the suppression of conductance of the hH1α by EPA is not accompanied by a change in the steepness of the slope of voltage-activation indicates that EPA does not reduce the positive charges on the voltage sensor peptide, as we had previously hypothesized (26). The marked effects of the PUFAs on the inactivated state of the hH1α is consistent, however, with an effect of the PUFAs on the short cytoplasmic segment of the hH1α channel linking transmembrane repeats III and IV (28, 29).
To us a surprising finding was that the INa of hH1α was suppressed not only by polyunsaturated fatty acids with two or more C=C unsaturated bonds but also by the dietary monounsaturated oleic acid and even, though to a lesser extent, the saturated palmitic and stearic acids. Significant inhibition by mono or saturated fatty acids of any electrophysiologic or functional effects previously had not been seen with external application of the fatty acids to cardiomyocytes in our studies (9–12). The characteristic specificity of the effects on INa of PUFAs alone was lost with only the α-subunit of the Na+ channel present. This suggests, that in the absence of other components of the intact voltage-dependent human cardiac Na+ channel, the configuration of the α-subunit may be more open or uncovered, allowing even fatty acids that lack the two or more double C=C bonds access to the site(s) at which polyunsaturated fatty acids affect the modulation of the channel conductance in the complete channel. Possibly the effects are caused by the fact that in this transfected hH1α preparation at physiologic resting potentials, circa −90 mV, a large percentage of the hH1α channels are in the inactivated state (30). Nevertheless, the failure of the EPA ethyl ester to affect INAα, indicates that a free, negatively charged carboxyl group is still essential for the electrophysiologic effects of the fatty acids.
We have enclosed the words “bind” and “binding affinity” in quotation marks to indicate that their designation by purely electrophysiologic evidence is a logical but not rigorous construct. When it was shown by photo affinity labeling of the calcium channel by the antagonists nifedipine (31), rigorous evidence was provided for binding of a ligand to the protein of the Ca2+ channel. In this case it was established that the binding of the ligands was a direct and primary action of the ligand on the Ca2+ channel protein. When the PUFAs displaced [3H]nitrendipine from its binding site on the Ca2+ channel, however, the displacement was found to be noncompetitive (32). The same occurs in the case of the displacement by PUFAs of [3H]batrachotoxinin A 20α-benzoate (3HBTX) from its binding site on the Na+ channel protein (26). Though in the case of the displacement of the 3HBTX, it may be correct that the PUFAs are binding to the Na+ channel protein in its inactive state and allosterically displacing the 3HBTX from its binding site on the open state of the channel, the fact that the displacement was noncompetitive (26) makes this an unproven claim. The PUFAs may be binding or acting primarily elsewhere—possibly even on the membrane phospholipids, the physical state of which they are known to modulate (33)—and allosterically displacing the 3HBTX from its binding site on the Na+ channel protein. It is in this sense we state that at present it has not been rigorously proven that the PUFAs in fact have a primary effect on ion channels by directly binding to the channel proteins. Further research is required to test that possibility. All that can be rigorously claimed from the stated electrophysiologic formalism is that the effect of the ligand rather than its “binding” is to cause the functional channel changes observed.
In conclusion, our results indicate that the marked prolongation of the inactivated state of the Na+ channels is the major inhibitory effect of the PUFAs on INa,α in this preparation. They shorten the time required to enter the inactivated state from the open state and delay the recovery from the inactivated state to the closed, resting state.
Acknowledgments
We thank Dr. R. G. Kallen for the hH1 clone and Dr. S. C. Cannon for the CD8 clone and the HEK293t cell line. This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK38165 (A.L.), National Heart, Lung, and Blood Institute Grant HL51307 (J.P.M.), and National Institute of General Medical Sciences Grant GM35401 (G.K.W.).
ABBREVIATIONS
- EPA
eicosapentaenoic acid
- INa,α
the Na+ current of HEK293t cells transfected with the α-subunit of the human cardiac Na+ channel, hH1α
- PUFAs
long chain polyunsaturated fatty acids
- HEK
human embryonic kidney
References
- 1.McLennan P L, Abeywardena M Y, Charnock J S. Can J Physiol Pharmacol. 1985;63:1411–1417. doi: 10.1139/y85-232. [DOI] [PubMed] [Google Scholar]
- 2.McLennan P L, Bridle T M, Abeywardena M Y, Charnock J S. Am Heart J. 1992;123:1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- 3.Hock C E, Beck L D, Bodine L C, Reibel D K. Am J Physiol. 1990;259:H1518–H1526. doi: 10.1152/ajpheart.1990.259.5.H1518. [DOI] [PubMed] [Google Scholar]
- 4.Billman G E, Hallaq H, Leaf A. Proc Natl Acad Sci USA. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burr M, Gilbert J F, Holliday R M, Elwood P C, Fehily A M, Rogers S, Sweetnam P M, Deadman N M. Lancet. 1989;334:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 6.de Logeril M, Renaud S, Mamelle N, Salen P, Martin J-L, Monjaud I, Guidollet J, Touboul P, Delaye J. Lancet. 1994;143:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 7.Siscovick D S, Raghunathan T E, King I, Weinmann S, Wicklund K G, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi L H. J Am Med Assoc. 1996;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 8.Kang J X, Leaf A. Proc Natl Acad Sci USA. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang J X, Xiao Y-F, Leaf A. Proc Natl Acad Sci USA. 1995;92:3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y-F, Kang J X, Morgan J P, Leaf A. Proc Natl Acad Sci USA. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Y-F, Gomez A M, Morgan J P, Lederer W J, Leaf A. Proc Natl Acad Sci USA. 1997;94:4182–4187. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vreugdenhill M, Bruehl C, Voskuyl R A, Kang J K, Leaf A, Wadman W J. Proc Natl Acad Sci USA. 1996;93:12559–12563. doi: 10.1073/pnas.93.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannon S C, Strittmatter S M. Neuron. 1993;10:317–326. doi: 10.1016/0896-6273(93)90321-h. [DOI] [PubMed] [Google Scholar]
- 14.Wang S Y, Wang G K. Biophys J. 1997;72:1633–1640. doi: 10.1016/S0006-3495(97)78809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Y-F, McArdle J J. J Hypertens. 1994;12:783–790. [PubMed] [Google Scholar]
- 17.Bean B P, Cohen C J, Tsein R W. J Gen Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence J H, Yue D T, Rose W C, Marban E. J Physiol (London) 1991;443:629–650. doi: 10.1113/jphysiol.1991.sp018855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman L. Biophys J. 1995;69:2369–2377. doi: 10.1016/S0006-3495(95)80106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White M M, Chen I, Kleinfield R, Kallen R G, Barchi R I. Mol Pharmacol. 1991;39:604–608. [PubMed] [Google Scholar]
- 21.Catterall W A. Physiol Rev. 1992;72:S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- 22.Catterall W A. Ann NY Acad Sci. 1994;707:1–19. doi: 10.1111/j.1749-6632.1993.tb38038.x. [DOI] [PubMed] [Google Scholar]
- 23.Krafte D S, Davison K, Dugrenier N, Estep K, Josef K, Barchi R L, Kallen R J, Silver P J, Ezrin A M. Eur J Pharmacol. 1994;266:245–254. doi: 10.1016/0922-4106(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 24.Noda M. Ann NY Acad Sci. 1994;707:20–37. doi: 10.1111/j.1749-6632.1993.tb38039.x. [DOI] [PubMed] [Google Scholar]
- 25.Bendahhou S, Cummins T R, Agnew W S. Am J Physiol. 1997;272:C592–C600. doi: 10.1152/ajpcell.1997.272.2.C592. [DOI] [PubMed] [Google Scholar]
- 26.Kang J X, Leaf A. Proc Natl Acad Sci USA. 1996;93:3542–3546. doi: 10.1073/pnas.93.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stühmer W, Conti F, Suzuki H, Wang X, Noda M, Yahagi N, Kubo H, Numa S. Nature (London) 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- 28.Bennett P B, Valenzuela C, Chen L-Q, Kallen R G. Circ Res. 1995;77:584–592. doi: 10.1161/01.res.77.3.584. [DOI] [PubMed] [Google Scholar]
- 29.Eaholtz G, Scheuer T, Catterall W A. Neuron. 1994;12:1041–1048. doi: 10.1016/0896-6273(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 30.Wright S N, Wang S-Y, Kallen R G, Wang G K. Biophys J. 1997;73:779–788. doi: 10.1016/S0006-3495(97)78110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakayama H, Taki M, Striessnig J, Glossman H, Catterall W A, Kanaoka Y. Proc Natl Acad Sci USA. 1991;88:9203–9207. doi: 10.1073/pnas.88.20.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hallaq H, Smith T W, Leaf A. Proc Natl Acad Sci USA. 1992;89:1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klausner R D, Kleinfeld A M, Hoover R I, Karnovsky M J. J Biol Chem. 1980;255:1286–1295. [PubMed] [Google Scholar]