Abstract
Background
Mast cells are known to accumulate at sites of inflammation and upon activation to release their granule content, e.g. histamine, cytokines and proteases. The secretory leukocyte protease inhibitor (SLPI) is produced in the respiratory mucous and plays a role in regulating the activity of the proteases.
Result
We have used the HMC-1 cell line as a model for human mast cells to investigate their effect on SLPI expression and its levels in cell co-culture experiments, in vitro. In comparison with controls, we found a significant reduction in SLPI levels (by 2.35-fold, p < 0.01) in a SLPI-producing, type II-like alveolar cell line, (A549) when co-cultured with HMC-1 cells, but not in an HMC-1-conditioned medium, for 96 hours. By contrast, increased SLPI mRNA expression (by 1.58-fold, p < 0.05) was found under the same experimental conditions. Immunohistochemical analysis revealed mast cell transmigration in co-culture with SLPI-producing A549 cells for 72 and 96 hours.
Conclusion
These results indicate that SLPI-producing cells may assist mast cell migration and that the regulation of SLPI release and/or consumption by mast cells requires interaction between these cell types. Therefore, a "local relationship" between mast cells and airway epithelial cells might be an important step in the inflammatory response.
Keywords: SLPI, mast cells, inflammation, migration
Background
The inflammatory process in the respiratory airways includes the release of several mediators such as chemoattractants, cytokines and proteinases that regulate the adhesion of molecules, and the processes of cell migration, activation and degranulation. The characteristic destruction of tissue in inflammatory diseases is to a large extent mediated by an excess of neutral serine proteinases and matrix metalloproteinases (MMP) [1-4]. The serine proteinases also contribute to the activation of MMPs, which are typically released in a latent form [5]. Therefore, the regulation of proteolytic enzyme activity in the respiratory airways by endogeneous inhibitors is a prerequisite for the maintenance of tissue integrity, and for the repair of tissue damage. Proteinase inhibitors that provide protection against the extracellular activity of serine proteinases include alpha1-antitrypsin (AAT), secretory leukocyte proteinase inhibitor (SLPI) and elafin/skin–derived antileukoproteinase (SKALP). Whereas AAT is produced mainly by the liver and reaches the tissues via passive diffusion [6], SLPI and elafin/SKALP are produced locally [7-12]
SLPI is found in considerable amounts in nasal, bronchial and cervical mucous, in saliva, and in seminal fluid [7,9,13-16]. There is increasing evidence that SLPI has numerous functions that are not related to its protease-inhibitory activity. SLPI is a non-glycosylated, hydrophobic, cationic 12 kDa protein, consisting of two homologous cystein-rich domains of 53 and 54 amino acids [17]. The carboxyl-terminal domain of SLPI manifests inhibitory activities against chymotrypsin, trypsin, granulocyte and pancreatic elastase, cathepsin G and mass cell chymase [18-22], whereas anti-inflammatory, anti-bacterial and anti-fungal activities appear to reside in its amino-terminal domain [23,24]. SLPI is shown to reduce LPS-induced TNFα production in the macrophage cell line [25,26], to suppress the production of prostaglandin E2 and metalloproteinase in monocytes [27], and also to antagonize up-regulation of nuclear transcription factor (NF-κB) activation [28]. Lentsch and co-workers have demonstrated that SLPI attenuates the acute inflammatory response caused by the deposition of IgG immune complexes in the lungs [29]. In addition, Ashcroft and associates found that SLPI might play a crucial role in wound healing [30]. Recently SLPI has also been shown to inhibit HIV-1 replication in cultured human monocytes [23]. The up-regulation of SLPI by bacterial lipopolysaccharides, and cytokines such as TNFα and IL-1β, combined with a broad spectrum antibiotic activity against gram-positive and gram-negative bacteria, suggest it to be a potent anti-microbial "defensin-like" peptide produced by the lungs. States of impaired healing are characterized by excessive proteolysis and often bacterial infection, leading to the hypothesis that SLPI may also have a role in this process.
Historically, SLPI was first purified from secretions of patients with chronic, obstructive pulmonary disease and cystic fibrosis [18], and it was suggested that SLPI being a major anti-elastase inhibitor of the bronchi, is an important molecule for protecting the respiratory epithelium [13,15]. In contrast to α1-antitrypsin, SLPI blocks elastin-bound elastase in the alveolar walls, which might also protect against the development of emphysema [31]. The interaction between SLPI and elastase is reversible, probably facilitating the transfer of neutrophil elastase to α1-antitrypsin [32]. It is important to point out that neutrophil elastase has been found to increase SLPI mRNA expression in lung epithelial cells in vitro, but this increase in SLPI expression was accompanied by a decrease in SLPI protein release [33]. The local induction of SLPI might be important to break the cycle of inflammation. However the mechanisms involved in the regulation of SLPI expression and release still remain to be elucidated. It has been shown that SLPI is up-regulated by pro-inflammatory stimuli including LPS, TNFα, IL-6 and IL-1β, in vitro [25,26,34,35]. Corticosteroids have also been found to enhance SLPI mRNA levels in airway epithelial cells leading to the suggestion that antiinflammatory effects of corticosteroids may be related to the stimulated SLPI levels [36]. The demonstration that neutrophil defensins increase SLPI release from the bronchial epithelial cells supports the idea that leukocytes play a prominent role in the regulation of SLPI production [37].
Contradictory results have been presented concerning the levels of SLPI during allergic rhinitis in antigen-challenged atopic subjects. For example, lower SLPI levels were found in the bronchial secretions of asthma patients [38]. Other studies indicated that levels of SLPI are also lower in nasal secretion of allergic rhinitis patients compared to healthy controls. After allergen challenge SLPI seems to decrease in the nasal secretions in atopic subjects, which probably indicates mucosal damage [39]. However, the question why SLPI levels is decreased during certain allergic reactions, still remains to be answered.
Allergic inflammation, including rhinitis, asthma, anaphylaxis and urticaria are all disorders associated with mast cell activation [40]. Mast cells are multifunctional cells capable of secreting a wide variety of cytokines, chemokines and growth factors [41,42]. The mediators released by mast cells can independently, and in synergy with macrophage- and T-cell-derived cytokines, induce much of the inflammatory pathology and serve to stimulate a complex immune response [43,44]. Mast cells are the primary initiating cell of IgE-mediated hypersensitivity. Allergen binding to, or the cross-linking of IgE on the surface of mast cells, which is bound to the high affinity IgE-receptor, leads to the rapid release of inflammatory mediators that further provoke a profound immunological and inflammatory process. There are indications that SLPI can inhibit IgE-mediated histamine release from rodent and human nasal mucosa mast cells [45,46]. SLPI may also counterbalance the proteolytic activities caused by protease leakage from the cells [47]. Mast cell and leukocyte serine proteinases are shown to be elevated in the airways of asthmatic patients [40,48]. Individuals with reduced anti-proteinase activity as a result of AAT deficiency, have an increased propensity to develop asthma [49,50]. Together, these findings indicate that proteinase-antiproteinase imbalance in the airways contributes to the pathophysiological responses in the airways. Because SLPI provides a potent, broad-spectrum inhibitory activity against mast cell and leukocyte serine proteinases, this protein is suggested to be an effective protector against antigen-induced inflammatory responses in the airways. The purpose of this study is to further elucidate how local SLPI levels may be influenced during mast cell interaction with epithelial cells. A co-culture model was used, in which we studied mast cells HMC-1 affect on SLPI levels released from the type II alveolar cell line (A549) derived from human lung carcinoma.
Results
Mast cell characterisation
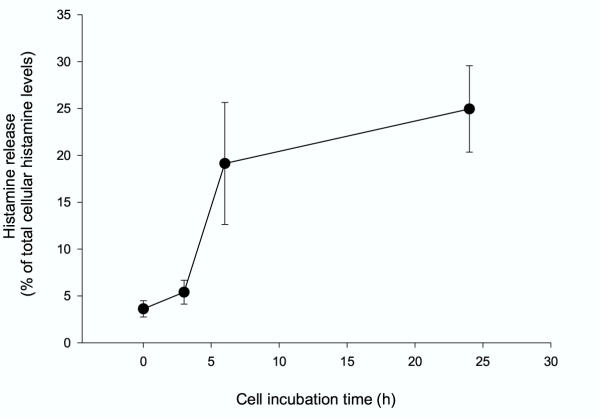
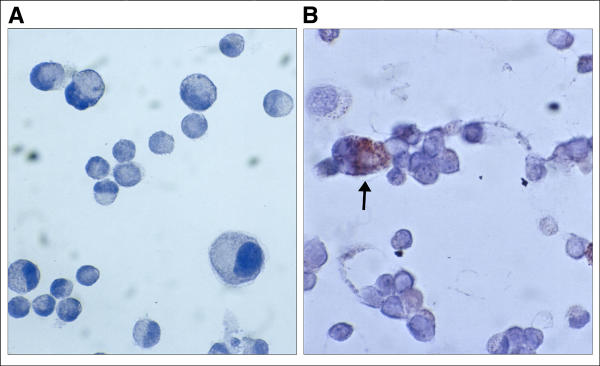
Most studies performed on the mechanisms of the mast cell degranulation are based on the release of histamine. Histamine is electrostatically linked to the protein heparin complex in a manner, which allows it to be released very easily. As shown in figure 1, a spontaneous histamine release from HMC-1 cells consistently increases with incubation time (up to 24 h) from 4% to 25% relative to the total cellular histamine content. Continues cell incubation (up to 96 h) showed no further changes in histamine release (data not shown). In addition, the supernatants from the HMC-1 cells alone did not contain SLPI after cell culture for 24 and 48 h, and only trace amounts of SLPI are detected after 72 and 96 h of incubation (Table 1). The ability of HMC-1 cells to express SLPI was also confirmed by immunohistochemical analysis (Fig. 2A and 2B).
Figure 1.
Histamine release from HMC-1 cells. Histamine release was measured at different time points: 0, 3, 6 and 24 h and calculated as a percent of the total cellular histamine content. Each point represents mean ± S.D. of five or six separate experiments. Histamine release increases from 4 % to 25 % over time (p < 0.05).
Table 1.
Time dependent SLPI release from the mast cells, HMC-1, and type II epithelium cells, A549, cultured alone
| Incubation time | HMC-1 cells | A549 cells | ||||
| (h) | SLPI (pg/ml) | SLPI (pg/ml) | ||||
| Mean | SD | Mean | SD | |||
| 24 | - | ± | - | 6.7 | ± | 0.26 |
| 48 | - | ± | - | 14.5 | ± | 0.9 |
| 72 | 0.17 | ± | 0.07 | 14.4 | ± | 1.5 |
| 96 | 0.2 | ± | 0.06 | 38.75 | ± | 0.98 |
*) mean and standard deviation of 2 experiments
Figure 2.
Localisation of SLPI in HMC-1-mast cells. A, the control slides were incubated with specific antiserum previously adsorbed with SLPI (1/500) and no positive staining for HMC-1 cells was found (original magnification × 500). B, HMC-1 cells stained with polyclonal goat-anti-SLPI antibody (1/500) show immunoreactivity for SLPI (original magnification × 500).
Cell co-culture experiments
Epithelium cells, A549, cultured alone for 24, 48, 72 and 96 h increased the SLPI release from 6.7 ± 0.26 to 38.8 ± 0.98 pg/ml (Table 1). Next, we studied the effect of mast cells on SLPI levels by using a transmigration model in which mast cells HMC-1 migrated across the topside of the transwell filter towards the A549 cells. The results obtained with this model show that, during the first 48 h of cell co-culture, mast cells did not migrate into the lung epithelium cell culture (Fig. 3A). However, as shown in figure 3B, cell co-culture for 72 h resulted in a slight mast cell transmigration as indicated by the presence of immunoreactive HMC-1 cells among the A549 cells. This was, furthermore, confirmed by the co-culture of these cells for 96 h after which a large number of immunoreactive mast cells was detected among the A549 cells (Fig. 3C). It should be pointed out that HMC-1 cells showed no ability to transmigrate through a "blank" transwells. Moreover, cells which had not migrated into the polyester membrane inserts displayed immunoreactivity to tryptase for all incubation times, from 24 to 96 h, showing that under the chosen experimental conditions mast cells preserved their capacity to differentiate (not shown). We further examined the effect of mast cell trans-migration on the amount of SLPI released by the A549 cells into cell culture media. Only a slight decrease in SLPI levels was observed during 72 h of cell co-culture compared to the SLPI-producing cells alone (Fig. 4). However, when cells were co-cultured for 96 h a significant decrease in SLPI levels was found. As shown in figure 3, SLPI levels decline by 2.35-fold (p < 0.01) in the presence of mast cells relative to the A549 cells alone.
Figure 3.
Mast cell HMC-1 migration towards the lung type II epithelium cells, A549. Specimens stained with a mouse anti-human mast cell 229; kidney juxtaglomerular CE diluted 1/5000 show immunoreactivity for HMC-1 mast cells. A, HMC-1 are not detected among the lung carcinoma cells after 24 h of cell co-culture. B and C, Solitary cells and a large number of cells can be detected after 72 and 96 hours of cell co-culture, respectively. Original magnification × 200. Mast cell immunoreactivity indicated by arrow.
Figure 4.
Effects of mast cells HMC-1 on the ability of the A549 cells to release SLPI. The SLPI release from the A549 cells was decreased in co-cultured with HMC-1 cells, compared to the A549 cells alone (p < 0.006). The most pronounced fall in SLPI production was observed after 96 hours of cell co-culture (**p < 0.01). Each point represents mean of six or five separate experiments.
Effects of conditioned media on SLPI levels
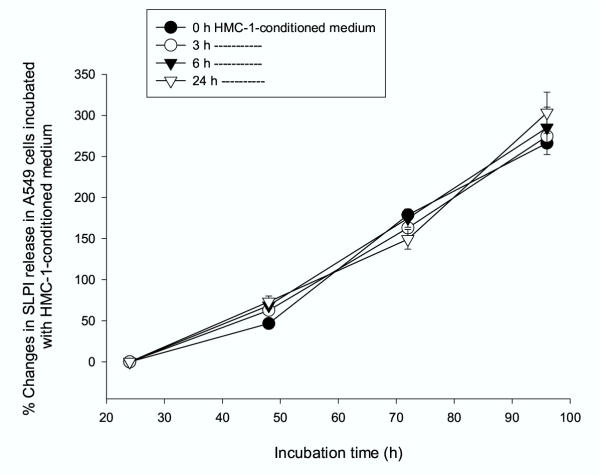
In the next series of experiments we aimed to investigate if concentration of SLPI can be influenced by the incubation of A549 cells with HMC-1-conditioned medium for various time periods (from 24 to 96 h). In this case, a medium collected from mast cells at different time points was added to the A549 cells and allowed to act for 24, 48, 72 and 96 h. As shown in Figure 5, the culture of epithelium cells in the mast cell-media caused no changes in SLPI levels.
Figure 5.
Effects of HMC-1-conditioned medium on SLPI levels in A549 cells. Medium from HMC-1 cells cultured for 0, 3, 6, 8 and 24 h was used to incubate A549 cells for various time periods (from 24 to 96 h). The mast cell-media caused no changes in SLPI levels. Each point represents mean of three independent experiments
SLPI mRNA expression by A549 cells
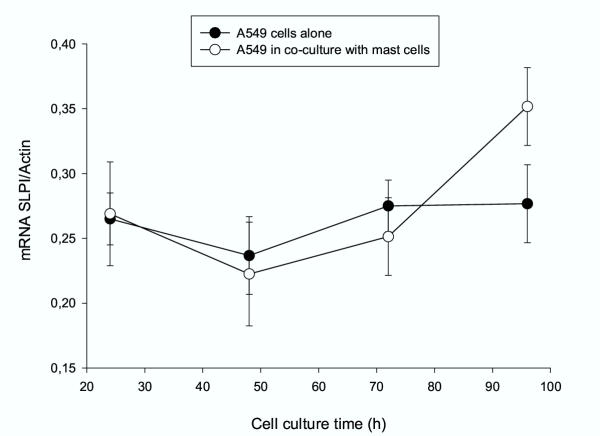
To determine whether the decrease of SLPI levels in co-culture with mast cells involves changes in SLPI mRNA expression in A549 cells, we monitored the SLPI mRNA/β-Actin ratio in A549 cells alone and in co-culture over the period of 24 to 96 h. As shown in figure 6, the levels of SLPI mRNA expression were relatively unchanged when A549 cells were cultured alone. By contrast, increased SLPI mRNA levels were observed in co-culture experiments after 96 h (1.58-fold, p < 0.05) compared to 24 or 48 h. Under the same experimental conditions, we also examined whether addition of conditioned medium from HMC-1 cells effects SLPI mRNA expression in same way. Consistent with our earlier observations (Fig. 5) showing that A549 cells incubated with conditioned medium from mast cells did not change SLPI protein levels, we found that under these experimental conditions SLPI mRNA levels are not changed either (data not shown).
Figure 6.
SLPI mRNA expression by lung epithelium, A549, cells. Increased SLPI mRNA levels in co-culture experiments were observed after 96 h (* p < 0.05), compared to 24 and 48 hours. No change in SLPI mRNA levels was found when lung carcinoma cells were cultured alone.
Discussion
Mast cells are widely distributed within the connective tissue, with a preferential localization adjacent to the small blood vessels. They play a central role in inflammatory and allergic reactions, and are involved in tissue remodelling during wound healing [40]. The mast cell responses involve the ingestion and killing of adherent substances, unlike that of traditional phagocytic cells. Concomitant with this endocytic activity, inflammatory mediators are released by the mast cells.
Mast cells constitute a heterogeneous group of cells containing several proteases, i.e. tryptase, carboxypeptidase, cathepsin G and chymase [51]. Mainly there are two kinds of mast cells, those in the connective tissue (MCTC) and those in the mucosa (MCT). They differ in protease content, MCTC cells contain tryptase and chymase, whereas MCT cells contain only tryptase. The MCTC cells are predominant in the nasal mucosa while MCT cells are more prevalent in the peripheral lung tissue. Mast cells have also been found to interact with different types of cells, including fibroblasts, endothelial cells, lymphocytes, macrophages, neutrophils, eosinophils, nerve cells and cancer cells [40,43,52,53]. However, the adhesive mechanisms initiating cell-cell interaction and the consequences of this are not well understood.
The present study was designed to investigate the adherence of mast cells, HMC-1, to the cell line A549, which represents epithelial cells within the respiratory tract, and to investigate the effects of this cell-cell interaction on SLPI levels.
The mast cell line HMC-1 is known to express a number of β1- and α-integrins as well as other receptors which permit the binding of these cells to the extracellular matrix compounds [53-55]. The factors that stimulate mast cell migration still remain largely undefined, although recent reports have implicated the transforming growth factor-β family (TGFβ) as the potential candidate for acting as mast cell chemotaxin, recruiting mast cells into inflammatory reactions [56]. In accordance with other studies, we found that HMC-1 mast cells growing in suspension adhere efficiently and spread on top of cell monolayers, in our case on top of A549 cells. After 72 h of HMC-1/A549 co-culture, solitary HMC-1 cells were detected among the A549 cells, while after 96 h a large number of mast cells was found to be adhering to the A549 cells. The maintenance of HMC-1 cell maturity was verified by the tryptase immunoreactivity each of the periods of cell culture.
The role of mast cells as primary effector cells in IgE-dependent, immediate hypersensitivity is well established [57]. The discovery that mast cells can release a wide variety of immune mediators, including proteases, cytokines, chemokines and growth factors, suggests an additional role of mast cells in modulating late-phase reactions and other chronic inflammatory processes [40,42]. Here we also demonstrate by immunocytochemistry that HMC-1 mast cells are SLPI-positive, and trace amounts of SLPI were found in the cell culture supernatants collected after 72 and 96 h of culture. Although the amount of SLPI released by few activated mast cells had no importance for the present study, this observation still extends earlier findings showing that SLPI and other chymase inhibitors, i.e. α1-antichymotrypsin and α1-antitrypsin are present in stimulated mast cells and protect the microenvironment against chymase activity [47]. Previously it has been shown that chymase degranulates mast cells, induces histamine release and an increase in SLPI concentration [58]. Neutrophil elastase was also found to increase SLPI transcript levels in primary and transformed human airway epithelial cells in a time- and dose-dependent manner [34,36]. These observations suggest that the sensitive regulation of anti-proteases, such as SLPI, in relation to local levels of proteases, may play an important role in minimising tissue destruction.
With these results as a background we have further investigated the effects of mast cells on the capacity of epithelial-presenting cells, A549, to express and release SLPI. In our experimental model, SLPI-producing epithelial cells were cultured for determined time points in conditioned media obtained from mast cells or in co-culture with mast cells. By using this approach, we were able to show that under cell co-culture conditions SLPI levels in cell culture media are gradually decreased, although the expression of mRNA SLPI increases. A significant diminition in SLPI levels (by 2.35-fold) was observed after 96 h of cell co-culture, although the SLPI mRNA was up-regulated (by 1.58-fold). In contrast, the A549 cell culture in the mast cell conditioned medium for the time periods chosen had no influence either on SLPI protein levels or on SLPI mRNA expression. By measuring histamine release and tryptase activity in media from mast cells cultured alone for up to 96 h, we were able to show that these cells are not activated. Histamine levels plateaued after 24 h of cell culture which explains why the conditioned media collected from mast cells and added to the SLPI producing cells (A549) had no effect on SLPI levels.
Based on these findings one can conclude that mast cells exert an inhibitory effect on SLPI levels only when they are in close contact with SLPI-producing, A549, cells. Since our primary goal was to find out if mast cell interaction with epithelial cells by itself can induce expression of SLPI and reduce its levels in media, we specifically did not investigate mast cell exogenous activation under these conditions. This indirectly shows that SLPI is either consumed (for example degraded or in complex with enzymes) or its release is inhibited. Studies on this point are in progress in our laboratory.
Recently, van Wetering and co-workers have shown that neutrophil elastase increases SLPI mRNA expression, while it decreases SLPI protein release, in vitro [37]. On the other hand, studies by Hill and co-workers, have indicated that SLPI concentration does not decrease until the elastase activity of the samples is in excess of 50 nM [48]. The relationship between SLPI and elastase is therefore found not to be a simple linear, indicating that a certain amount of elastase and/or other enzymes is needed in order to induce epithelial damage or interfere with epithelial cell metabolism resulting in a decreased SLPI secretion [12,32,34].
SLPI levels were found to be decreased in nasal secretion after antigen challenge in vivo [39] as well as in bronchial alveolar lavage obtained from asthmatics compared to healthy subjects [38]. In the airways of allergic patients, mast cells are found in the close proximity of airway epithelial cells, which may indicate that mast cells and epithelial cells influence each other's properties. Together, previous findings, that SLPI levels are lower in inflammatory loci in airways and data from our experimental model, that co-culture of mast cells with SLPI-producing epithelial-like, A549, cells, results in decreased SLPI levels in cell co-culture media suggest that decrease in local antiprotease activity might sustain mucosal damage in reactions in which mast cells are participating.
Conclusion
Airway inflammation, present in asthma, bronchitis, and bronchiectasis, is characterised by the presence of activated inflammatory cells. The proteinases, presumably leaking from the cells during their migration from the blood into the extracellular space, can be detrimental to the connective tissue. Proteinase inhibitors, such as SLPI, produced locally in the airway epithelium, are thought to be important in minimising proteolytic damage during inflammation. On the other hand, proteinases may also play a role in the regulation of the antiproteinase profile at the epithelial site. Thus, the auto-regulatory loop observed, namely that the up-regulation of SLPI mRNA parallels apparent down-regulation of the levels of the SLPI in the mast/epithelial like cell, A549, co-culture model, will need further investigations. An understanding of the mechanisms involved in SLPI protein expression, release and consumption may provide important knowledge regarding the dynamics of the regulation of the protease/antiprotease systems.
Materials and Methods
Cell cultures
The human mast cells HMC-1, established from a patient with mast cell leukaemia, were obtained from Dr J.H. Butterfield, Mayo Clinic, Rochester, MN, USA [59] The cells were cultured in 75 cm2 flasks in Iscove's modified Dulbecco's medium (Gibco BRL, Paisly, UK) supplemented with 10% (v/v) iron-supplemented fetal calf serum (FCS) (Gibco BRL), 1.2 mM α-thioglycerol (Sigma, St Louis, MO, USA), 100 IU/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml amphotericin B and fungizone (Gibco BRL), in humidified air with 5% CO2, at 37°C. At confluence, the cells were centrifuged at 2000 g for 5 min, washed in PBS, re-suspended in cell culture medium and counted in a Bürker chamber. The viability of the HMC-1 cells was ≥ 95%.
SLPI producing a type-II alveolar cell line (A549) derived from human lung carcinoma, was cultured in RMPI-1640 medium supplemented with 10% FCS, 100 IU/ml penicillin, 100 μg/ml streptomycin and 2.5 μg/ml amphotericin B and fungizone at 37°C in an atmosphere of humidified air saturated with 5% CO2. The cells were subcultured every 4–5 days by tripsinization and were used for experiments after reaching confluence.
Human type II lung epithelium cells (A549) and HMC-1 co-culture models
Epithelium cells (A549) were cultured in the lower compartment of the cell co-culture plates (Nunc, Wiesbaden, Germany) to obtain confluent monolayers. A constant amount of HMC-1 cells (106 cells/ml) was seeded on fibronectin-coated polycarbonate transwell filters (pore size 3 μm and 12 mm in diametre). The cells were co-cultured in an Iscove's modified Dulbecco's medium for 24, 48, 72 and 96 h. The cell viability was analysed after 96 h of cell co-culture by a trypan blue staining, and was found to be 90 % and 95% for the mast cells and for the lung epithelium cells, respectively. For the controls, A549 cells and mast cells were cultured separately in co-culture plates under the same experimental conditions as described above. The experiments were repeated six times.
SLPI expression by A549 cells alone and in co-culture models
Total RNA was isolated and quantified from A549 cells cultured alone and in co-culture. The cells were lysed in 1.5 ml lyses buffer (4 M guanidinum thiocyanate, 25 mM sodium citrate (pH 7), containing 0.5% antifoam A and 100 mM 2-mercaptoethanol). Total RNA was extracted using a single-step method based on acid-guanidinum thiocyanate-phenol-chloroform extraction described by Chomczynski and Sacchi [60]. The total RNA yield was quantified at 260 and 280 nm. The transcript levels of SLPI and β-actin were evaluated by slot blot, using a Magnagraph (MSI) nylon membrane according to the manufacturer's instructions. Briefly, 5 μg of total RNA was mixed with 100 μl of dilution buffer containing 7.4% formaldehyde-7 and SSPE (150 mM sodium chloride, 10 nM sodium phosphate and 1 mM EDTA), denatured for 5 min in boiling water, cooled very fast (0°C) for 2 min and loaded onto the nylon membrane. SLPI mRNA was detected with a cocktail of an equimolar mixture of three single stranded oligonucleotide probes (British Biotechnology Products LTD, Oxon, UK). The probes were based on the antisense sequence and modified at the 5' end with digoxigenin. The digoxigenin-labelled β-actin probe was purchased from Roche, catalogue number 1498045. The blots were exposed to Kodak XAR-5 X-ray film (Sigma Chemical, St. Louise, MO). Autoradiographs were analysed using the Fuij film LAS-100, Luminescent Image analyser and Image reader macintosh version 1.0 was used to determine the densitometric units for both SLPI and β-actin. The data represent the mean SLPI/β-actin ratio.
SLPI and histamine quantification assays
SLPI was quantified in the cell supernatants obtained from each experimental condition. Analyses of SLPI were performed by using the quantitative sandwich ELISA kit according to the manufacture recommendations (R&D systems, Inc, USA). The minimum detectable dose of SLPI was less than 25 pg/ml. The mast cell degranulation was verified by the amount of histamine released. Histamine was quantified in the supernatants obtained from the mast cells cultured alone after the various incubation time points. Histamine was measured by a sensitive radioenzyme assay based on the conversion of histamine to [3H] methylhistamine in the presence of the enzyme histamine – N-methyltransferase using S-adenosyl-L-[methyl-3H] methionine as the methyl donor, using a commercial radioimmunoassay (RIA)-kit (Immunotech, KEMILA, Sollentuna, Sweden). Histamine secretion is expressed as a percentage of total cellular content (cell lysate plus spontaneous release) and is corrected for spontaneous release.
Immunohistochemistry
To monitor the differentiation of the mast cells we stained cells for tryptase after the various incubation times of 24, 48, 72 and 96 h. The mast cells were also stained for SLPI in order to eliminate the possibility that these cells themselves produce significant amounts of this protein. To confirm mast cell migration, the lung carcinoma cells, co-cultured with mast cells for various time periods, were immunohistochemically stained with a monoclonal anti-mast cell antibody.
To block unspecific staining, the slides were incubated with 5% or 10% of normal horse serum for 20 minutes at room temperature. Polyclonal goat-anti-SLPI antibody (produced at our laboratory) was used as primary antibody at dilutions of 1/500 and 1/1000, a monoclonal mouse anit-tryptase antibody, Mab 1254 (DAKO, Glostrup, Denmark) diluted 1/1000 was added and allowed to react for 90 min at room temperature. To identify migrated mast cells, a mouse anti-human mast cell 229; kidney juxtaglomerular CE (Swant, Switzerland) diluted 1/5000 was applied under the conditions described above. Control slides were incubated with the buffer or non-immunised mouse IgG (negative control), instead of primary antibody. The slides were washed and the second labelled antibody was applied and left for 30 min at room temperature. The slides were incubated with biotinylated rabbit anti-goat IgG antibody (5 mg/ml buffer) and then incubated with avidin DH biotinylated horseradish peroxidase (ABC) complexes. After this, the slides were washed again and stained with 0.06% 3,3'-diamino-benzidine-tetrahydrochloride (DAB) for 20 min and mounted. In addition, the control slides were incubated with specific antiserum previously adsorbed with SLPI.
Human type II lung epithelium cells, A549, cultured in HMC-1-conditioned medium
Lung epithelium, A549, cells and mast cells were cultured and prepared as described above. A549 cells were re-seeded into the six-well plates and grown till confluence. The supernatants collected from the mast cells after different periods of time (0, 3, 9 and 24 h) were added to the A549 cells for a further incubation of 24, 48, 72 and 96 h. A549 cell controls were cultured in a cell growth medium for the same length of time. SLPI levels were measured in the supernatants collected from the HMC-1 cells before and after addition to the lung carcinoma cells. These experiments were repeated four times.
Statistics
Regression coefficients were calculated for each SLPI release curve from each of the experiments. The hypothesis i.e. the differences in regression coefficient were tested with a non-parametric Wilcoxon's paired rank sum test. The Mann-Whitney U-test was calculated on the results of 24, 48, 72 and 96 h of cell culture. Results are expressed as the mean ± SD of at least four to six independent experiments. P values exceeding 0.05 were considered not significant.
Abbreviations
SLPI, secretory leukocyte protease inhibitor; MMP, matrix metalloproteinases; AAT, alpha1-antitrypsin; IL, interleukin; TNFα, tumor necrosis factor; FCS, fetal calf serum; ELISA, enzyme-linked immunosorbent assays.
Authors' contributions
HC and NM, carried out the cell culture experiments and immunohistochemistry and drafted the manuscript, SJ, performed the statistical analysis and presentation of the data, described and interpreted data, UW, participated in study design, data evaluation and coordination.
Acknowledgments
Acknowledgements
This study was supported by grants from the Swedish Medical Research Council (3910), the foundations of Alfred Österlund and the Medical Foundations of University Hospital Malmö and the Medical Faculty.
Contributor Information
Camilla Hollander, Email: sabina.janciauskiene@medforsk.mas.lu.se.
Max Nyström, Email: sabina.janciauskiene@medforsk.mas.lu.se.
Sabina Janciauskiene, Email: sabina.janciauskiene@medforsk.mas.lu.se.
Ulla Westin, Email: sabina.janciauskiene@medforsk.mas.lu.se.
References
- Janoff A. Proteases and lung injury. A state-of-the-art minireview. Chest. 1983;83:54S–58S. [PubMed] [Google Scholar]
- Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207–16. doi: 10.1146/annurev.me.36.020185.001231. [DOI] [PubMed] [Google Scholar]
- Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3:409–21. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002;121:156S–159S. doi: 10.1378/chest.121.5_suppl.156S. [DOI] [PubMed] [Google Scholar]
- Finlay GA, Russell KJ, McMahon KJ, D'Arcy M, Masterson EJB, FitzGerald MX, O'Connor CM. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax. 1997;52:502–6. doi: 10.1136/thx.52.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell RW, Jeppsson JO, Laurell CB, Brennan SO, Owen MC, Vaughan L, Boswell DR. Structure and variation of human alpha 1-antitrypsin. Nature. 1982;298:329–34. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Fryksmark U, Ohlsson K, Polling A, Tegner H. Distribution of antileukoprotease in upper respiratory mucosa. Ann Otol Rhinol Laryngol. 1982;91:268–71. doi: 10.1177/000348948209100308. [DOI] [PubMed] [Google Scholar]
- Mooren HW, Kramps JA, Franken C, Meijer CJ, Dijkman JA. Localisation of a low-molecular-weight bronchial protease inhibitor in the peripheral human lung. Thorax. 1983;38:180–3. doi: 10.1136/thx.38.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson M, Fryksmark U, Polling A, Tegner H, Ohlsson K. Localization of antileukoprotease in the parotid and the submandibular salivary glands. Acta Otolaryngol. 1984;98:147–51. doi: 10.3109/00016488409107547. [DOI] [PubMed] [Google Scholar]
- Kuijpers AL, Alkemade HA, Schalkwijk J, van de Kerkhof PC. Topographic relation between skin-derived antileukoproteinase (SKALP) and leukocyte elastase in a case of annular pustular psoriasis. Acta Derm Venereol. 1995;75:110–3. doi: 10.2340/0001555575110113. [DOI] [PubMed] [Google Scholar]
- Mihaila A, Tremblay GM. Human alveolar macrophages express elafin and secretory leukocyte protease inhibitor. Z Naturforsch [C] 2001;56:291–7. doi: 10.1515/znc-2001-3-420. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Masuda T, Shimura S, Fushimi T, Shirato K. Secretion and gene expression of secretory leukocyte protease inhibitor by human airway submucosal glands. Am J Physiol Lung Cell Mol Physiol. 2001;280:L79–87. doi: 10.1152/ajplung.2001.280.1.L79. [DOI] [PubMed] [Google Scholar]
- Ohlsson K, Tegner H. Inhibition of elastase from granulocytes by the low molecular weight bronchial protease inhibitor. Scand J Clin Lab Invest. 1976;36:437–45. doi: 10.3109/00365517609054461. [DOI] [PubMed] [Google Scholar]
- Ohlsson K, Bjartell A, Lilja H. Secretory leucocyte protease inhibitor in the male genital tract: PSA-induced proteolytic processing in human semen and tissue localization. J Androl. 1995;16:64–74. [PubMed] [Google Scholar]
- Tegner H. Quantitation of human granulocyte protease inhibitors in non-purulent bronchial lavage fluids. Acta Otolaryngol. 1978;85:282–9. doi: 10.3109/00016487809111936. [DOI] [PubMed] [Google Scholar]
- Fritz H. Human mucus proteinase inhibitor (human MPI). Human seminal inhibitor I (HUSI-I), antileukoprotease (ALP), secretory leukocyte protease inhibitor (SLPI) Biol Chem Hoppe Seyler. 1988;369:79–82. [PubMed] [Google Scholar]
- Thompson RC, Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986;83:6692–6. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser K, Reichert R, Schwarz S, Werle E. [Isolation and characterisation of a protease inhibitor from human bronchial secretion] Hoppe Seylers Z Physiol Chem. 1972;353:221–6. [PubMed] [Google Scholar]
- Eisenberg SP, Hale KK, Heimdal P, Thompson RC. Location of the protease-inhibitory region of secretory leukocyte protease inhibitor. J Biol Chem. 1990;265:7976–81. [PubMed] [Google Scholar]
- Renesto P, Balloy V, Kamimura T, Masuda K, Imaizumi A, Chignard M. Inhibition by recombinant SLPI and half-SLPI (Asn55-Ala107) of elastase and cathepsin G activities: consequence for neutrophil-platelet cooperation. Br J Pharmacol. 1993;108:1100–6. doi: 10.1111/j.1476-5381.1993.tb13511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Kamimura T, Kanesaki M, Ishii K, Imaizumi A, Sugiyama T, Suzuki Y, Ohtsuka E. Efficient production of the C-terminal domain of secretory leukoprotease inhibitor as a thrombin-cleavable fusion protein in Escherichia coli. Protein Eng. 1996;9:101–6. doi: 10.1093/protein/9.1.101. [DOI] [PubMed] [Google Scholar]
- Pemberton AD, Huntley JF, Miller HR. Differential inhibition of mast cell chymases by secretory leukocyte protease inhibitor. Biochim Biophys Acta. 1998;1379:29–34. doi: 10.1016/S0304-4165(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Shugars DC, Sauls DL, Weinberg JB. Secretory leukocyte protease inhibitor blocks infectivity of primary monocytes and mononuclear cells with both monocytotropic and lymphocytotropic strains of human immunodeficiency virus type I. Oral Dis. 1997;3:S70–2. doi: 10.1111/j.1601-0825.1997.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Tomee JF, Koeter GH, Hiemstra PS, Kauffman HF. Secretory leukoprotease inhibitor: a native antimicrobial protein presenting a new therapeutic option? Thorax. 1998;53:114–6. doi: 10.1136/thx.53.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin FY, Nathan C, Radzioch D, Ding A. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–26. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- Jin F, Nathan CF, Radzioch D, Ding A. Lipopolysaccharide-related stimuli induce expression of the secretory leukocyte protease inhibitor, a macrophage-derived lipopolysaccharide inhibitor. Infect Immun. 1998;66:2447–52. doi: 10.1128/iai.66.6.2447-2452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, De Witt DL, McNeely TB, Wahl SM, Wahl LM. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteases. J Clin Invest. 1997;99:894–900. doi: 10.1172/JCI119254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentsch AB, Jordan JA, Czermak BJ, Diehl KM, Younkin EM, Sarma V, Ward PA. Inhibition of NF-kappaB activation and augmentation of IkappaBbeta by secretory leukocyte protease inhibitor during lung inflammation. Am J Pathol. 1999;154:239–47. doi: 10.1016/s0002-9440(10)65270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PA, Lentsch AB. Endogenous regulation of the acute inflammatory response. Mol Cell Biochem. 2002;234–235:225–8. doi: 10.1023/A:1015944709177. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, Hale-Donze H, McGrady G, Song XY, Wahl SM. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–53. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- Bruch M, Bieth JG. Influence of elastin on the inhibition of leucocyte elastase by alpha 1-proteinase inhibitor and bronchial inhibitor. Potent inhibition of elastin-bound elastase by bronchial inhibitor. Biochem J. 1986;238:269–73. doi: 10.1042/bj2380269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K. Interactions between granulocyte proteases and protease inhibitors in the lung. Bull Eur Physiopathol Respir. 1980;16:209–22. doi: 10.1016/b978-0-08-027379-2.50022-3. [DOI] [PubMed] [Google Scholar]
- Abbinante-Nissen JM, Simpson LG, Leikauf GD. Neutrophil elastase increases secretory leukocyte protease inhibitor transcript levels in airway epithelial cells. Am J Physiol. 1993;265:L286–92. doi: 10.1152/ajplung.1993.265.3.L286. [DOI] [PubMed] [Google Scholar]
- Sallenave JM, Shulmann J, Crossley J, Jordana M, Gauldie J. Regulation of secretory leukocyte proteinase inhibitor (SLPI) and elastase-specific inhibitor (ESI/elafin) in human airway epithelial cells by cytokines and neutrophilic enzymes. Am J Respir Cell Mol Biol. 1994;11:733–41. doi: 10.1165/ajrcmb.11.6.7946401. [DOI] [PubMed] [Google Scholar]
- Grobmyer SR, Barie PS, Nathan CF, Fuortes M, Lin E, Lowry SF, Wright CD, Weyant MJ, Hydo L, Reeves F, Shiloh MU, Ding A. Secretory leukocyte protease inhibitor, an inhibitor of neutrophil activation, is elevated in serum in human sepsis and experimental endotoxemia. Crit Care Med. 2000;28:1276–82. doi: 10.1097/00003246-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Abbinante-Nissen JM, Simpson LG, Leikauf GD. Corticosteroids increase secretory leukocyte protease inhibitor transcript levels in airway epithelial cells. Am J Physiol. 1995;268:L601–6. doi: 10.1152/ajplung.1995.268.4.L601. [DOI] [PubMed] [Google Scholar]
- van Wetering S, van der Linden AC, van Sterkenburg MA, de Boer WI, Kuijpers AL, Schalkwijk J, Hiemstra PS. Regulation of SLPI and elafin release from bronchial epithelial cells by neutrophil defensins. Am J Physiol Lung Cell Mol Physiol. 2000;278:L51–8. doi: 10.1152/ajplung.2000.278.1.L51. [DOI] [PubMed] [Google Scholar]
- Ochnio JJ, Abboud RT, Lam S, Johal SS, Smith CJ, Johnson DA. Bronchial leukocyte proteinase inhibitor levels in bronchial washings in asthma patients. Chest. 1988;93:1008–12. doi: 10.1378/chest.93.5.1008. [DOI] [PubMed] [Google Scholar]
- Westin U, Lundberg E, Wihl JA, Ohlsson K. The effect of immediate-hypersensitivity reactions on the level of SLPI, granulocyte elastase, alpha1-antitrypsin, and albumin in nasal secretions, by the method of unilateral antigen challenge. Allergy. 1999;54:857–64. doi: 10.1034/j.1398-9995.1999.00938.x. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy G, Kelley J, Johnson D, Youngberg G, Stone W, Huang SK, Bieber J, Chi DS. The human mast cell: functions in physiology and disease. Front Biosci. 2001;6:D1109–27. doi: 10.2741/krishnas. [DOI] [PubMed] [Google Scholar]
- Bradding P, Holgate ST. The mast cell as a source of cytokines in asthma. Ann N Y Acad Sci. 1996;796:272–81. doi: 10.1111/j.1749-6632.1996.tb32589.x. [DOI] [PubMed] [Google Scholar]
- Bradding P, Holgate ST. Immunopathology and human mast cell cytokines. Crit Rev Oncol Hematol. 1999;31:119–33. doi: 10.1016/S1040-8428(99)00010-4. [DOI] [PubMed] [Google Scholar]
- Anderson DF, Zhang S, Bradding P, McGill JI, Holgate ST, Roche WR. The relative contribution of mast cell subsets to conjunctival TH2-like cytokines. Invest Ophthalmol Vis Sci. 2001;42:995–1001. [PubMed] [Google Scholar]
- Church MK, Cauldfield JP. Mast cells and basophil functions. In: Holgate ST, Church MK, editor. Allery. Gover Medical Publishing; 1993. pp. 5.1–5.12. [Google Scholar]
- Dietze SC, Sommerhoff CP, Fritz H. Inhibition of histamine release from human mast cells ex vivo by natural and synthetic chymase inhibitors. Biol Chem Hoppe Seyler. 1990;371:75–9. [PubMed] [Google Scholar]
- Westin U, Lundberg E, Ohlsson K. IgE-mediated histamine release from nasal mucosa is inhibited by SLPI (secretory leukocyte protease inhibitor) to the level of spontaneous release. Mediators Inflamm. 1998;7:217–20. doi: 10.1080/09629359891162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin U, Polling A, Ljungkrantz I, Ohlsson K. Identification of SLPI (secretory leukocyte protease inhibitor) in human mast cells using immunohistochemistry and in situ hybridisation. Biol Chem. 1999;380:489–93. doi: 10.1515/BC.1999.063. [DOI] [PubMed] [Google Scholar]
- Hill AT, Bayley D, Stockley RA. The interrelationship of sputum inflammatory markers in patients with chronic bronchitis. Am J Respir Crit Care Med. 1999;160:893–8. doi: 10.1164/ajrccm.160.3.9901091. [DOI] [PubMed] [Google Scholar]
- Pierce JA, Jeppsson JO, Laurell CB. alpha-1 Antitrypsin phenotypes determined by isoelectric focusing of the cysteine-antitrypsin mixed disulfide in serum. Anal Biochem. 1976;74:227–41. doi: 10.1016/0003-2697(76)90327-4. [DOI] [PubMed] [Google Scholar]
- Sigsgaard T, Brandslund L, Omland O, Hjort C, Lund ED, Pedersen OF, MR Miller. S and Z alpha1-antitrypsin alleles are risk factors for bronchial hyperresponsiveness in young farmers: an example of gene/environment interaction. Eur Respir J. 2000;16:50–5. doi: 10.1034/j.1399-3003.2000.16a09.x. [DOI] [PubMed] [Google Scholar]
- Chaughey GH. The structure and airway biology of mast cell proteinases. Am J Resoir Cell Mol Biol. 1991;4:387–394. doi: 10.1165/ajrcmb/4.5.387. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Metcalf DD. Contemporary issues in mast cell biology. Allergy and Asthma Proc. 1990;17:59–63. doi: 10.2500/108854196778645074. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Feuerstein B, Ernst N, Brocker EB, Klein CE. Heterotypic cell-cell adhesion of human mast cells to fibroblasts. Arch Dermatol Res. 1997;289:194–203. doi: 10.1007/s004030050180. [DOI] [PubMed] [Google Scholar]
- Kruger-Krasagakes S, Grutzkau A, Baghramian B, Henz BM. Interactions of immature human mast cells with extracellular matrix: expression of specific adhesion receptors and their role in cell binding to matrix proteins. J Invest Dermatol. 1996;106:538–43. doi: 10.1111/1523-1747.ep12343953. [DOI] [PubMed] [Google Scholar]
- Weber S, Babina M, Feller G, Henz BM. Human leukaemic (HMC-1) and normal skin mast cells express beta 2-integrins: characterization of beta 2-integrins and ICAM-1 on HMC-1 cells. Scand J Immunol. 1997;45:471–81. doi: 10.1046/j.1365-3083.1997.d01-420.x. [DOI] [PubMed] [Google Scholar]
- Olsson N, Piek E, ten Dijke P, Nilsson G. Human mast cell migration in response to members of the transforming growth factor-beta family. J Leukoc Biol. 2000;67:350–6. doi: 10.1002/jlb.67.3.350. [DOI] [PubMed] [Google Scholar]
- Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402:B24–30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- Miller HD, Pemberton AD. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology. 2002;04:375–390. doi: 10.1046/j.1365-2567.2002.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]