Abstract
Cryptochrome is a group of flavin-type blue light receptors that regulate plant growth and development. The function of Arabidopsis cryptochrome 2 in the early photomorphogenesis of seedlings was studied by using transgenic plants overexpressing CRY2 protein, and cry2 mutant plants accumulating no CRY2 protein. It is found that cryptochrome 2 mediates blue light-dependent inhibition of hypocotyl elongation and stimulation of cotyledon opening under low intensities of blue light. In contrast to CRY1, the expression of CRY2 is rapidly down-regulated by blue light in a light-intensity dependent manner, which provides a molecular mechanism to explain at least in part that cryptochrome 2 functions primarily under low light during the early development of seedlings.
Plants respond to their surrounding solar radiation and adjust their growth and development accordingly. Etiolated seedlings of dicotyledonous plants develop elongated hypocotyls and small unopened cotyledons. Exposure to light results in inhibition of hypocotyl elongation, stimulation of cotyledon opening and expansion, and eventually the establishment of photoautotrophic growth (1–3). These photomorphogenic responses to light are mediated by at least two different photosensory receptor systems: phytochromes, the red/far-red light receptors; and cryptochromes, the blue/UV-A light receptors (1). The molecular mechanisms of neither photosensory receptor system in higher plants is understood, although phytochrome has been studied extensively and some of the different biological functions corresponding to the different members of the phytochrome gene family have been elucidated (1, 4–6). Recently, an Arabidopsis blue light receptor, cryptochrome 1, has been described (7–9). CRY1 is a 75-kDa flavoprotein encoded by the CRY1 gene (also referred to as HY4) in Arabidopsis, which mediates blue light-induced inhibition of hypocotyl elongation (7–10). The amino-terminal region of CRY1 shares sequence similarity with type I DNA photolyase, a microbial blue/UV-A light-dependent DNA repairing flavoenzyme (11). CRY1 is a soluble protein expressed at similar levels in dark- and light-grown Arabidopsis seedlings (12). hy4 (or cry1) mutants impaired in CRY1 exhibit decreased sensitivity to blue light (7, 10), whereas transgenic plants overexpressing CRY1 show increased photosensitivity (9, 12). These observations indicate that the sensitivity of plants to blue light is dependent on the cellular concentration of the photoreceptor. Here we report experiments showing the function of CRY2 in the inhibition of hypocotyl elongation and stimulation of cotyledon opening under low intensities of blue light. We also demonstrate that the expression of CRY2, in contrast to CRY1, is rapidly down-regulated by blue light, which is probably associated with a protein degradation mechanism. The finding that the rapid decline of CRY2 level occurs under high intensities of blue light may explain why CRY2 functions mainly under low intensities of blue light in the early photomorphogenesis of Arabidopsis seedlings.
MATERIALS AND METHODS
CRY2 Gene and CRY2 Protein.
The DNA sequence of the CRY2 gene has been published in an electronic form (13). Adopting from the traditional nomenclature used for phytochromes (14), symbols for the wild-type gene, mutant gene, holoprotein, and apoprotein of cryptochrome 1/cryptochrome 2 will be designated as CRY1/CRY2, cry1/cry2, cry1/cry2, and CRY1/CRY2, respectively. It should be pointed out that Arabidopsis mutants hy4 and fha, which were isolated previously (10, 15), have been shown recently to be mutations of the CRY1 gene (referred to as HY4 gene previously) (7) and the CRY2 gene (16), respectively; these mutant alleles will be continuously referred to as hy4 and fha.
The carboxyl-terminal region of CRY2 including 135 residues, referred to as CRY2C, was cloned to the vector pET-16b as a translation fusion to a His-tag (Novagen). The His-CRY2C expressed in E. coli was purified by using Ni-affinity chromatography and used to prepare polyclonal antibodies (anti-CRY2) in rabbits as described (12). Immunoblots were analyzed by using enhanced chemiluminescence method (Amersham; refs. 9 and 17). An immunoblot may be probed with different antibodies by stripping the bound antibodies with 0.2 M glycine, pH 2.5 (3 × 8 min), rinsing with PBST (12) and reprobing with a different antibody. The intensity of signals from different blots are not directly comparable.
Plant Materials.
Transgenic Arabidopsis plants overexpressing CRY2 were prepared by using the tissue culture method as described (12, 18). The CRY2 cDNA was modified to have no native 5′ untranslated region of CRY2; the sequence of the first two codons was replaced with sequence that encodes a His-tag of 21 residues [MG(H)10SSGHIEGRH]; and the transgene was under the control of a cauliflower mosaic virus 35S promoter. A similar modification has been shown to have no effect on the function of CRY1 in vivo (12). More than five independent transgenic lines overexpressing CRY2 were analyzed and found to have a similar phenotype; results shown were from one of these lines (H2–9), which has a single copy of the 35S:His-CRY2 transgene inserted in the genome as detected by PCR and Southern blot analyses. Arabidopsis cry2–1 and cry2–2 mutants were isolated from a fast-neutron mutagenized population of Columbia ecotype, both are null mutations resulting from large deletions (16).
Seeds were sown on soil, kept in the dark at 4°C for 4 days, germinated under white light for 4 hr, and grown under blue light with different fluence rates as indicated for 4 days before measurement. Lengths of hypocotyls were measured as described (12), each data point represents the mean of >20 seedlings, and the SDs are shown. The cotyledon opening was measured as the percentage of seedlings with opened cotyledons from a population of >50 seedlings. Seedlings with two cotyledons opened to an angle of >45° were scored as “opened” (the results are shown without SD). For cotyledon opening responses, similar results were obtained from at least three experiments with slightly different fluence rates, although the result from only one experiment is shown. Lights and filters used are essentially as described (7).
RESULTS
The Amino Acid Sequence of CRY2 Shares Similarities to That of CRY1.
We have identified the gene encoding the apoprotein of the second cryptochrome in Arabidopsis, cryptochrome 2, by cross-hybridization by using CRY1 (HY4) cDNA as the probe (13). A recently cloned Arabidopsis gene PHH1 was found to be very similar (99.3% identical in amino acid sequence) to the CRY2 sequence (19). CRY2 and CRY1 are 51% identical in amino acid sequence (Fig. 1). The sequence similarity is mainly concentrated in the amino-terminal region of ≈490 residues where CRY1 and CRY2 are 58% identical. This region of CRY2 is 30% identical to Escherichia coli DNA photolyase, a type I photolyase (20) (Fig. 1), similar to the 28% identity observed between photolyase and CRY1 (7). The C-terminal regions of CRY2 (120 residues) is very different from the corresponding region of CRY1 (186 residues)—there is only 14% sequence identity that is largely attributed to the presence of three small conserved motifs: DQM/QVPS, PEED/EEE, and STAESSSS (Fig. 1). The homology between photolyase and cryptochrome is highest in the region involving flavin-binding: 10 of 13 residues of the E. coli photolyase involved in flavin-binding (21) are conserved in both CRY1 and CRY2, whereas only one of seven photolyase residues involved in binding the second chromophore (21) are conserved in the two cryptochromes (Fig. 1). Similar to that found for CRY1 (8), the recombinant Arabidopsis CRY2 protein expressed and purified from insect Sf9 cells (8) contains flavin adenine dinucleotide and has no DNA photolyase activity (not shown). These results are consistent with the presence of the highly conserved flavin-binding domain in CRY2 (Fig. 1), and the absence of a tryptophan residue at the position corresponding to W277 of the E. coli photolyase that is critical for DNA binding and catalytic activity (22, 23) (Fig. 1).
Figure 1.
Amino acid sequence comparison of Arabidopsis CRY2, CRY1, and E. coli DNA photolyase (PHR). The optimal alignment was obtained by using the MACAW program. Identical residues in all three sequences are boxed; identical residues conserved in CRY2 and CRY1 are underlined; residues of the E. coli photolyase involving in the binding of folate (•), flavin (▪), and the DNA substrate (▴), are indicated.
The Expression of CRY2 Is Negatively Regulated by Blue Light.
Analysis of Arabidopsis extracts with polyclonal antibodies prepared against the carboxyl-terminal domain of CRY2 (see Materials and Methods) indicated that CRY2 was a soluble protein expressed in all the tissues examined including hypocotyls, cotyledons, stems, rosette leaves, roots, and flowers (data not shown). Further analysis indicated that the expression of CRY2 is negatively regulated by blue light (Fig. 2). Fig. 2A showed that the abundance of CRY2 protein declined dramatically when seedlings were exposed to blue light, whereas the relative abundance of CRY1 is unchanged (Fig. 2 A and B). It is interesting that CRY2 expression is similarly affected by UV-A and green light, which comprise part of the broad action spectrum of CRY1 (8, 24) and possibly other cryptochromes (25). However, it is unlikely that CRY1 is involved in the regulation of CRY2 expression, because the lack of CRY1 protein in hy4 mutant plants had no apparent effect on the expression of CRY2 (Fig. 3 A and B) (16). The change in CRY2 protein levels in response to blue light is rapid, reaching steady state within 1 hr after exposure of etiolated seedlings to blue light (Fig. 2A) (T.M. and C.L., unpublished data). Similar changes of CRY2 protein abundance in response to blue light was also found in red light-grown seedlings when exposed to blue light (Fig. 2C), which further demonstrated the wavelength specificity of the regulation of CRY2 expression. The blue light-induced down-regulation of CRY2 is reminiscent of the red light-induced down-regulation of the red/far-red photoreceptor phytochrome A, which involves both transcriptional regulation and ubiquitin-mediated posttranslational regulation (4, 26). However, the blue light-induced decrease of CRY2 protein is not explained by changes in the RNA level (Fig. 2D). As shown in Fig. 2D, there is little change in the CRY2 mRNA level when the red-light-grown plants were exposed to blue light for one hour (Fig. 2D), although a rapid decline in the CRY2 protein level occurred in these plants (Fig. 2C). Therefore, neither a decrease in transcription activity nor changes in RNA stability is responsible for the blue-light-induced decline of CRY2 expression. Whether CRY2 negatively autoregulates its own expression and whether the blue light-regulation of CRY2 expression is associated with a protein degradation mechanism is currently under investigation.
Figure 2.
Light-induced down-regulation of CRY2 expression. (A, B) Immunoblots showing CRY2 (A) or CRY1 (B) protein from 4-day-old etiolated Arabidopsis seedlings exposed to red light (R; 24 μmol s−1 m−2), green light (G; 21 μmol⋅s−1⋅m−2), UV-A light (U; 22 μmol⋅s−1⋅m−2), blue light (B; 20 μmol⋅s−1⋅m−2), or kept in the dark (D) for the indicated time before harvesting. Similar amounts of protein for each sample were fractionated in 10% SDS/PAGE gels, and blotted to nitrocellulose membranes; the immunoblots were probed with anti-CRY2 (A) or anti-CRY1 (B) antibodies. (C) An immunoblot showing CRY2 protein level in wild-type seedlings grown under continuous red light (20 μmol⋅s−1⋅m−2) with (lane B) or without (lane R) blue light (5 μmol⋅s−1⋅m−2) treatment for 1 hr before harvesting. (D) A northern blot showing CRY2 mRNA level (CRY2) in wild-type seedlings grown under red light (20 μmol⋅s−1⋅m−2) with (lane B) or without (lane R) blue light (20 μmol⋅s−1⋅m−2) treatment for 1 hr before harvesting. The same blot was stripped by using boiled 0.05 × standard saline citrate, 0.01 M EDTA, 0.1% SDS for three times and reprobed with the cDNA of 18S rRNA.
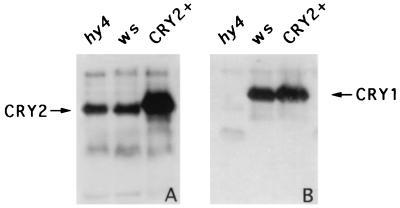
Figure 3.
Immunoblots showing the overexpression of CRY2 in the transgenic plants. Samples were prepared from Arabidopsis seedlings of hy4 mutant (hy4), ws wild-type (ws), and transgenic plants overexpressing CRY2 (CRY2+). The immunoblot was probed with anti-CRY2 antibody (A), stripped, and reprobed with the anti-CRY1 antibody (B).
cry2 Mediates a Blue Light-Induced Hypocotyl Inhibition Response Under Low Light.
To characterize the function of cry2 in vivo, we prepared transgenic Arabidopsis plants overexpressing the photoreceptor (Materials and Methods). The results shown in Fig. 3A demonstrates that Arabidopsis plants expressing CRY2 under the control of the cauliflower mosaic virus 35S promoter accumulated a significantly higher level of CRY2 protein than that of the wild-type plants (Fig. 3A). The expression of CRY1 was not affected by the overexpression of CRY2 (Fig. 3B), indicating again that the expression of these two photoreceptors is independent from each other. The CRY2-overexpressing transgenic plants grown under blue light developed significantly shorter hypocotyls (Fig. 4A) and larger cotyledons (not shown) in comparison to those of wild-type plants. The shorter hypocotyl phenotype of the transgenic plants overexpressing CRY2 is likely to reflect a hypersensitive response due to elevated levels of CRY2 protein. Similar observations have been made previously for CRY1 (9, 12) and for different phytochromes (27, 28). However, it has been found that the hy4 null mutants grown under relative high intensities of blue light had hypocotyls almost as long (80%–90%) as those of the etiolated wild-type seedlings (7, 10, 12), suggesting that cry1 is the predominant photoreceptor mediating hypocotyl inhibition under such conditions. Interestingly, it was consistently observed that the relative lengths of the hypocotyls for the CRY2- and CRY1-overexpressing seedlings differed for plants grown under low light intensity compared with those grown under high light. For example, under blue light with a fluence rate lower than 1 μmol⋅ s−1⋅m−2, transgenic plants overexpressing CRY2 developed shorter hypocotyls than the CRY1-overexpressing plants, whereas under a fluence rate greater than 1 μmol⋅s−1⋅m−2 CRY1-overexpressing plants had shorter hypocotyls (Fig. 4A). These analyses indicated that the endogenous cry2 may function in seedling de-etiolation primarily under low intensities of blue light.
Figure 4.
Comparisons of the blue light-induced hypocotyl inhibition and cotyledon opening for Arabidopsis seedlings of different genotypes. (A) Fluence-rate response of hypocotyl inhibition of ws wild-type (ws), and homozygous lines of transgenic plants overexpressing CRY1 (CRY1+) or CRY2 (CRY2+). (B and C) Fluence-rate response of hypocotyl inhibition (B) and cotyledon opening (C) of Columbia wild-type (Col), cry2–1 mutant (CRY2−), and hy4 mutant (CRY1−).
A test of this hypothesis requires studies of mutant Arabidopsis plants lacking the cry2 photoreceptor. Two such mutant alleles, cry2–1 and cry2–2, have been isolated recently (ref. 16; also see Materials and Methods). These mutants showed similar defects in their blue light responses (Figs. 4 and 5), whereas little phenotypic alternation was observed for the cry2 seedlings grown in the dark or under red light (not shown). As shown in Figs. 4 and 5, seedlings of the cry2 mutant grew much taller than the wild-type seedlings when they were grown under blue light of fluence rates lower than ≈10 μmol⋅s−1⋅m−2 (Fig. 4B, Fig. 5 MB and LB); this difference was greatly reduced under higher light intensities (Fig. 4B and Fig. 5HB). Conversely, the hy4 mutant that, like cry2, exhibited long hypocotyls under low light, also showed substantially longer hypocotyls than wild-type seedlings under a light intensity greater than ≈10 μmol⋅s−1⋅m−2. In addition to the phenotype associated with a loss in light-induced inhibition of hypocotyl growth, cry2 mutant plants also showed a defect in blue light-induced cotyledon opening. Under low intensities of blue light, cotyledons of the cry2 mutant opened later than those of the wild-type plants (Fig. 4C and Fig. 5LB). Once again, and even more striking than in the case of light-induced inhibition of hypocotyl growth, negligible phenotypic differences for cotyledon opening were observed between the mutant and wild-type seedlings under high light intensities (in this case, greater than ≈1 μmol⋅s−1⋅m−2). However, it should be pointed out that no dramatic decrease in the cotyledon size was observed for the cry2 mutant seedlings grown under different light conditions, although the transgenic seedlings overexpressing CRY2 protein generally have cotyledons larger than that of wild-type seedlings grown under blue light.
Figure 5.
Phenotypic comparisons of 4-day-old Arabidopsis seedlings of Columbia wild-type, cry2, and hy4 seedlings grown under high intensity blue light (HB, 50 μmol⋅s−1⋅m−2), medium intensity blue light (MB, 5.5 μmol⋅s−1⋅m−2), and low intensity blue light (LB, 0.6 μmol⋅s−1⋅m−2). Seedlings were taken from the populations used in Fig. 4B.
The Function of cry2 in De-Etiolation of Seedlings Correlates with the Level of CRY2 Expression.
The results from both the overexpression studies and the mutant studies indicated that cry2 functions primarily under low light intensities in mediating the de-etiolation responses of Arabidopsis seedlings. This sensitivity of cry2 function to light intensity presumably reflects our observation that the amount of CRY2 protein declined rapidly in plants grown under blue light (Fig. 2). To test this hypothesis, we analyzed the effect of blue light intensities on the decrease of CRY2 protein levels in the wild-type and the CRY2-overexpressing transgenic plants. The immunoblot shown in Fig. 6A demonstrated that CRY2 declined to a barely detectable level within 1 hr after exposing the wild-type plants to greater than ≈20 μmol⋅s−1⋅m−2 of blue light. In contrast, the amount of CRY2 remained relatively high in the wild-type plants exposed to a low intensity of blue light (1 μmol⋅s−1⋅m−2), even after 24 hr of light treatment (Fig. 6A). Therefore, our finding that the function of cry2 in seedling development was largely restricted to low light can be explained at least in part by the presence of higher levels of CRY2 protein in seedlings grown under low light. Similarly, the CRY2 protein level in the transgenic plants overexpressing CRY2 also showed a fluence-rate dependent decrease in response to blue light (Fig. 6B); such changes were not found for CRY1 (Fig. 6C). This result also correlates with the fluence-rate dependent nature of the blue-light hypersensitive response found in transgenic plants overexpressing CRY2 (Fig. 4A). Furthermore, because the majority of the CRY2 protein expressed in the transgenic plants is derived from the transgene lacking the native 5′ UTR of CRY2 and under the control of the cauliflower mosaic virus 35S promoter (see Materials and Methods), involvement of both the transcriptional suppression and translational inhibition (associated with the 5′ UTR) for the regulation of CRY2 expression in response to blue light can be excluded.
Figure 6.
Immunoblots showing the effect of blue light intensities on the blue-light induced decrease of CRY2 protein. (A) Protein samples prepared from wild-type seedlings were collected 1 hr or 24 hr after exposure of the 4-day-old etiolated seedlings to blue light with different fluence rates indicated. (B and C) Samples were prepared from CRY2-overexpressing transgenic seedlings grown in the dark (lane D), under red light with (B lanes) or without (lane R) 1 hr blue light treatment at the fluence rates indicated before harvesting. The immunoblot was probed with anti-CRY2 antibody (B), stripped, and reprobed with anti-CRY1 antibody (C).
DISCUSSION
We conclude that the function of cry2 in early photomorphogenesis of seedlings is largely restricted to low light conditions. Under low light intensity, both cry1 and cry2 contribute to the blue light-induced hypocotyl inhibition and cotyledon opening; in this respect the overall sensitivity to light is maximized. Under high light intensities, the blue-light responses of Arabidopsis seedlings are predominantly mediated by cry1, and the contribution of cry2 is negligible at this developmental stage. It is interesting to compare these observations with those pertaining to phytochrome-mediated responses. Here also, light-induced shortening of hypocotyl growth is mediated by more than one photoreceptor, primarily phyA and phyB. As in the case of cry2, phyA is light-labile. An additional complication with the phytochromes is that phyA and phyB respond to different wavelengths, with phyA mediating light-induced shortening of Arabidopsis hypocotyl growth in response to continuous irradiation with far-red light whereas phyB mediates a similar response upon irradiation with red light (4). Present studies suggest that the two Arabidopsis cryptochromes share similar action spectra. However, the possibility remains that they may also respond differently to changes in wavelength. What is clear is that seedling de-etiolation, involving, in the case of Arabidopsis, at least five known (phyA, phyB, phyD, cry1, and cry2) and possibly additional photoreceptors, is a complex process enabling plants to adjust optimally to changes in light quality and quantity.
Acknowledgments
We thank Hien Duong and Elham Zarabian for their help in the measurement of hypocotyl length and cotyledon opening, Dr. Jose Jiarillo for the rRNA probe, and Dr. Elaine Tobin for the critical readings of the manuscript. This work is supported by University of California, Los Angeles (start-up fund to C.L.), and National Institutes of Health (GM51956 to A.R.C., and GM56265 to C.L.). T.M. is partially supported by a predoctoral fellowship (GM08375) from the National Institutes of Health.
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. U43397).
References
- 1.Kendrick R E, Kronenberg G H M, editors. Photomorphogenesis in Plants. 2nd Ed. Dordrecht, The Netherlands: Kluwer; 1994. [Google Scholar]
- 2.McNellis T W, Deng X-W. Plant Cell. 1995;7:1749–1761. doi: 10.1105/tpc.7.11.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chory J. Plant Cell. 1997;9:1225–1234. doi: 10.1105/tpc.9.7.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quail P H, Boylan M T, Parks B M, Short T W, Xu Y, Wagner D. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- 5.Furuya M, Schafer E. Trends Plant Sci. 1996;1:301–307. [Google Scholar]
- 6.Aukerman M J, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino R M, Sharrock R A. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad M, Cashmore A R. Nature (London) 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 8.Lin C, Robertson D E, Ahmad M, Raibekas A A, Jorns M S, Dutton P L, Cashmore A R. Science. 1995b;269:968–970. doi: 10.1126/science.7638620. [DOI] [PubMed] [Google Scholar]
- 9.Lin C, Ahmad M, Gordon D, Cashmore A R. Proc Natl Acad Sci USA. 1995a;92:8423–8427. doi: 10.1073/pnas.92.18.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koornneef M, Rolff E, Spruit C J P. Z Pflanzenphysiol Bd. 1980;100:147–160. [Google Scholar]
- 11.Sancar A. Biochemistry. 1994;33:2–9. doi: 10.1021/bi00167a001. [DOI] [PubMed] [Google Scholar]
- 12.Lin C, Ahmad M, Cashmore A R. Plant J. 1996a;10:893–902. doi: 10.1046/j.1365-313x.1996.10050893.x. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Ahmad M, Chan J, Cashmore A R. Plant Physiol. 1996b;110:1047. [Google Scholar]
- 14.Quail P H, Briggs W, Chory J, Hangarter R P, Harberd N P, Kendrick R E, Koornneef M, Parks B, Sharrock R A, Schafer E, et al. Plant Cell. 1994;6:468–471. doi: 10.1105/tpc.6.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koornneef M, Hanhart C J, van der Veen J H. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- 16.Guo, H., Yang, H., Mockler, T. & Lin, C. (1998) Science, in press. [DOI] [PubMed]
- 17.Durrant I. Nature (London) 1990;346:297–298. doi: 10.1038/346297a0. [DOI] [PubMed] [Google Scholar]
- 18.Valvekens D, Montagu M V, Lijebettens M V. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffman P D, Batschauer A, Hays J B. Mol Gen Genet. 1996;253:259–265. doi: 10.1007/s004380050321. [DOI] [PubMed] [Google Scholar]
- 20.Sancar G B, Smith F W, Lorence M C, Rupert C S, Sancar A. J Biol Chem. 1984;259:6033–6038. [PubMed] [Google Scholar]
- 21.Park H W, Kim S T, Sancar A, Deisenhofer J. Science. 1995;268:1866–1872. doi: 10.1126/science.7604260. [DOI] [PubMed] [Google Scholar]
- 22.Li Y F, Sancar A. Biochemistry. 1990;29:5698–5706. doi: 10.1021/bi00476a009. [DOI] [PubMed] [Google Scholar]
- 23.Kim S-T, Li Y F, Sancar A. Proc Natl Acad Sci USA. 1992;89:900–904. doi: 10.1073/pnas.89.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young J C, Liscum E, Hangarter R P. Planta. 1992;188:106–114. doi: 10.1007/BF00198946. [DOI] [PubMed] [Google Scholar]
- 25.Liscum E, Briggs W. Plant Cell. 1995;7:473–485. doi: 10.1105/tpc.7.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vierstra R D. In: Photomorphogenesis in Plants. 2nd Ed. Kendrick R E, Kronenberg G H M, editors. Dordrecht, The Netherlands: Kluwer Academic; 1994. pp. 141–162. [Google Scholar]
- 27.Wagner D, Tepperman J M, Quail P H. Plant Cell. 1991;3:1275–1288. doi: 10.1105/tpc.3.12.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagatani A, Kay S, Deak M, Chua N-H, Furuya M. Proc Natl Acad Sci USA. 1991;88:5207–5211. doi: 10.1073/pnas.88.12.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]