Abstract
In sheep, the uterus produces luteolytic pulses of prostaglandin F2α (PGF) on Days 15 to 16 of estrous cycle to regress the corpus luteum (CL). These PGF pulses are produced by the endometrial lumenal epithelium (LE) and superficial ductal glandular epithelium (sGE) in response to binding of pituitary and/or luteal oxytocin to oxytocin receptors (OTR) and liberation of arachidonic acid, the precursor of PGF. Cyclooxygenase-one (COX-1) and COX-2 are rate-limiting enzymes in PGF synthesis, and COX-2 is the major form expressed in ovine endometrium. During pregnancy recognition, interferon tau (IFNτ), produced by the conceptus trophectoderm, acts in a paracrine manner to suppress development of the endometrial epithelial luteolytic mechanism by inhibiting transcription of estrogen receptor α (ERα) (directly) and OTR (indirectly) genes. Conflicting studies indicate that IFNτ increases, decreases or has no effect on COX-2 expression in bovine and ovine endometrial cells. In Study One, COX-2 mRNA and protein were detected solely in endometrial LE and sGE of both cyclic and pregnant ewes. During the estrous cycle, COX-2 expression increased from Days 10 to 12 and then decreased to Day 16. During early pregnancy, COX-2 expression increased from Days 10 to 12 and remained higher than in cyclic ewes. In Study Two, intrauterine infusion of recombinant ovine IFNτ in cyclic ewes from Days 11 to 16 post-estrus did not affect COX-2 expression in the endometrial epithelium. These results clearly indicate that IFNτ has no effect on expression of the COX-2 gene in the ovine endometrium. Therefore, antiluteolytic effects of IFNτ are to inhibit ERα and OTR gene transcription, thereby preventing endometrial production of luteolytic pulses of PGF. Indeed, expression of COX-2 in the endometrial epithelia as well as conceptus is likely to have a beneficial regulatory role in implantation and development of the conceptus.
Background
In ruminants (sheep, cattle and goats), endometrial prostaglandins (PGs) play a major role in regulation of the estrous cycle, pregnancy, and parturition. The estrous cycle of sheep is dependent on the uterus as the source of the luteolysin, prostaglandin F2α (PGF) [see [1,2] for review]. On Days 15 and 16 of the estrous cycle, the corpus luteum (CL) is regressed by luteolytic pulses of PGF [3,4], which are produced by the lumenal epithelium (LE) and superficial ductal glandular epithelium (sGE) of the uterine endometrium [5,6]. The coordinated effects of progesterone, estrogen and oxytocin govern the production of luteolytic PGF pulses by the endometrial epithelium [7,8]. Oxytocin, secreted from the posterior pituitary and CL, binds to oxytocin receptors (OTR) in the endometrium and elicits pulsatile release of PGF from the endometrium [9]. Oxytocin receptor (OTR) mRNA and protein levels increase in endometrial LE and sGE immediately before and during luteolysis (Days 14–16) [10-12]. Estrogen affects the timing, magnitude and pattern of PGF response to oxytocin [13] by acting through estrogen receptor alpha (ERα) to increase OTR gene expression [14-16]. Progesterone initially suppresses ERα and OTR expression in the endometrium, but exposure of the endometrium to progesterone for 8–10 days down-regulates expression of the PR [17]. Consequently, loss of expression of PR in endometrial LE and sGE after Day 11 [18] ends the progesterone block to ERα and OTR formation. Thus, ERα is first detected on Days 11 and 13, which is followed by expression of OTR on Day 14. Increases in the abundance of estrogens from ovarian follicles and ERα promote OTR formation resulting in the pulsatile pattern of PGF release that results in luteolysis [7,13]. Oxytocin binding to the OTR results in cell signaling culminating in the liberation of arachidonic acid, the precursor of PGF.
Prostaglandins are generated via the cyclooxygenase (COX) pathway and COX is the rate-limiting enzyme for conversion of arachidonic acid into prostaglandin H2 (PGH2), the common substrate for various PG synthases [18,20]. COX exists in two isoforms that are encoded by two separate genes, Cox-1 and Cox-2, which are also known as prostaglandin endoperoxide H synthases (PGHS)-1 and PGHS-2 [19,20]. These enzymes are responsible for the conversion of arachidonic acid into PGH2, which is the precursor of various PGs including PGE2 (PGE) and PGF2α (PGF). Although COX-1 is a constitutively expressed enzyme in a variety of cell types, COX-2 is the inducible enzyme that plays a role in various pathological and physiological conditions in animal tissues. Although COX-1-deficient female mice are fertile, they have specific defects in parturition, whereas COX-2-deficient female mice are infertile with abnormalities in ovulation, fertilization, implantation and decidualization [21-23]. The requirement of COX-2 for normal blastocyst implantation and decidualization in mice is due to the role of COX-2-derived PGs in regulation of vascular endothelial growth factor (VEGF) and angiopoietin signaling that influence uterine vascular permeability and angiogenesis [24,25].
Abrogation of luteolytic pulses of PGF from the uterus in ruminants is due to effects of conceptus (embryo and associated membranes) signaling [4,26]. Interferon tau (IFNτ), a novel Type I IFN [27], produced by mononuclear trophectoderm cells of the ovine conceptus between Days 11 to 20–25, acts in a paracrine manner on the endometrium to inhibit production of luteolytic pulses of PGF [16,26] by preventing transcription of ERα and OTR genes in LE and sGE [28,29]. Consequently, OTR are absent from endometrial LE and sGE, and OT-induced luteolytic pulses of PGF are abrogated to maintain CL integrity and function. Several in vitro studies using bovine endometrial cells led to reports that IFNτ either increases [30,31] or decreases [31-34] expression of COX-2 in bovine endometrial cells. However, Charpigny et al. [5] reported that COX-2 protein in the endometrium was first detectable on Day 12, still expressed on Day 17, and then progressively decreased to Day 25 of pregnancy in sheep, whereas it was only transiently expressed in LE and sGE between Days 12 and 15 of the estrous cycle. In contrast, COX-1 was constitutively expressed in the endometrium of both cyclie and early pregnancy ewes and was not affected by the conceptus [5].
Given the conflicting evidence for IFNτ effects on COX-2 gene expression in the endometrium, objectives of the presented studies were to: (1) determine the effects of the estrous cycle and pregnancy on COX-2 mRNA and protein expression in the ovine endometrium; and (2) determine the in vivo effects of IFNτ on COX-2 expression in the ovine endometrium.
Methods
Animals and experimental design
Mature ewes of primarily Suffolk breeding were observed daily for estrous behavior using vasectomized rams. All ewes exhibited at least two estrous cycles of normal duration (16–18 days). At estrus (Day 0), ewes were assigned randomly to cyclic or pregnant status. Ewes assigned to pregnant status were bred to intact rams at estrus. All experiments and surgical procedures were in accordance with the Guide for Care and Use of Agriculture Animals and approved by the University Laboratory Animal Care and Use Committee of Texas A&M University.
In Study One, ewes were hysterectomized (n = 5 ewes/day) on Days 10, 12, 14 or 16 of the estrous cycle and Days 10, 12, 14, 16, or 18 of pregnancy. On Days 10 to 16 post-mating, pregnancy was confirmed by the presence of an apparently normal conceptus in the uterine flushing. At hysterectomy, cross-sections (~0.5 cm) of the uterine horn were fixed in fresh 4% paraformaldehyde in PBS (pH 7.2) for 24 h, dehydrated in 70% (v/v) ethanol for 24 h and then embedded in Paraplast-Plus (Oxford Labware, St. Louis, MO). The remaining endometrial tissues were physically dissected from myometrium, frozen in liquid nitrogen, and stored at -80°C for RNA extraction.
In Study Two, 10 cyclic ewes were ovariectomized and fitted with intrauterine catheters on Day 5 of the estrous cycle (Day 0 = estrus) as described previously [35]. Ewes (n = 5 ewes/treatment) received daily intramuscular injections of 50 mg progesterone from Days 5 to 16, and then daily intrauterine infusions of either 200 μg control serum proteins (CX; ovine serum proteins) or recombinant ovine IFNτ (IFN; 2 × 107 antiviral units per day) [36] from Days 11 to 16. Preparation of control serum proteins and roIFNτ for intrauterine injection was performed as described previously [35]. The selected dose of roIFNτ mimics pregnancy recognition in terms of ERα, OTR and PG production in response to OT [16,17,28,29] as well as induction and increases in IFNτ-stimulated gene expression in the endometrium [35]. All ewes were hysterectomized on Day 17, and cross-sections from the mid-region of each uterine horn fixed in 4% paraformaldehyde and embedded in paraffin. The endometrium was physically dissected from the remainder of the uterine horns, frozen in liquid nitrogen, and stored at -80°C for RNA extraction.
RNA isolation and analyses
Total cellular RNA was isolated from frozen endometrial tissue using Trizol reagent (Gibco-BRL, Bethesda, MD) according to the manufacturer's recommendations. The quantity of RNA was assessed spectrophotometrically, and integrity of RNA examined by gel electrophoresis in a denaturing 1% agarose gel.
Steady-state levels of COX-2 mRNA were assessed by semi-quantitative RT-PCR analysis as described previously [37]. Briefly, cDNA was synthesized from total cellular RNA (5 μg) isolated from endometrial tissues using random (Life Technologies, Gaithersburg, MD) oligo(dT) primers and SuperScript II Reverse Transcriptase (Life Technologies). Newly synthesized cDNA was acid-ethanol precipitated, resuspended in 20 μl of water, and stored at -20°C. The cDNAs were diluted (1:10) with water before use in PCR. COX-2 PCR primers used (GenBank accession no. U68486) [38] were forward (bp 486–505) 5'-CAGAGCTCTTCCTCCTGTGC-3' and reverse (bp 762–780) 5'-CAAAAGGCGACGGTTATGC-3'. Using the ovine β-actin mRNA sequence (GenBank accession no. U39357), the β-actin primers were forward (bp 274–295) 5'-CATCCTGACCCTCAAGTACCC-3' and reverse (bp 694–674) 5'-GTGGTGGTGAAGCTGTAGCC-3'. The COX-2 PCR reaction consisted of 95°C for 30 sec, 58°C for 30 sec, and 72°C for 30 sec. The β-actin PCR consisted of 95°C for 30 sec, 55°C for 1 min, and 72°C for 1 min. The optimal number of PCR cycles for COX-2 and β-actin was determined to be 32 and 26, respectively, using methods described previously [37]. In negative control reactions, RT cDNA was substituted by inclusion of uterine total RNA or water. The PCR reactions were performed using AmpliTaq DNA polymerase (PE Applied Biosystems Div., Foster City, CA) and Optimized Buffer C (Invitrogen, Carlsbad, CA) for β-actin and COX-2 according to the manufacturer's recommendations. Following PCR, 20 μl of each reaction was analyzed by agarose gel electrophoresis, and PCR products were visualized using ethidium bromide. The relative amount of DNA present was quantified by measuring the intensity of light emitted from correctly sized bands under ultraviolet light using an AlphaImager (Alpha Innotech Corp., San Leandro, CA). The β-actin values were used as a covariate in statistical analyses to correct for differences in the amount of cDNA template between samples. Data are presented as relative units (RU).
In situ hybridization analysis
COX-2 mRNA was localized in uterine tissue sections (5 μm) by in situ hybridization analysis as described previously [35]. Briefly, deparaffinized, rehydrated, and deproteinated uterine tissue sections were hybridized with radiolabeled antisense or sense cRNA probes generated from a linearized bovine COX-2 partial cDNA (GenBank AF004944) [29] using in vitro transcription with [α-35S] UTP. After hybridization, washing and ribonuclease A digestion, slides were then dipped in NTB-2 liquid photographic emulsion (Kodak, Rochester, NY) and exposed at 4°C for two weeks. Slides were developed in Kodak D-19 developer, counterstained with Harris modified hematoxylin (Fisher Scientific, Fairlawn, NJ) and dehydrated through a graded series of alcohol to xylene. Coverslips were then affixed with Permount (Fisher). Images of representative fields in brightfield and darkfield illumination were recorded using a Nikon Eclipse 1000 photomicroscope (Nikon Instruments Inc., Lewisville, TX) fitted with a Nikon DXM1200 digital camera.
Immunohistochemistry
Expression of immunoreactive COX-2 was detected in uterine tissue cross-sections (5 μm) using anti-human PGHS-2 (COX-2) rabbit polyclonal IgG antibody (PG27, Oxford Biomedical Research, Inc., Oxford, MI) and a Super ABC Rabbit IgG Kit (Biomeda, Foster City, CA) as described previously [35]. Negative controls were performed in which the primary antibody was substituted with the same concentration of purified normal rabbit IgG from Sigma Chemical Co. (St Louis, MO). Sections were deparaffinized, rehydrated, subjected to boiling citrate buffer antigen retrieval [18,35], and then incubated with anti-COX-2 IgG or rabbit IgG. Immunoreactive protein was visualized using diaminobenzidine tetrahydrochloride (Sigma) as the chromogen and then dehydrated and coverslipped over Permount (Fisher Scientific, Pittsburg, PA).
As described previously [18], relative staining intensity for immunoreactive COX-2 protein expression was assessed visually in uterine sections (n = 2 per horn) from each ewe by two independent observers and scored as follows: absent (-; i.e., no staining above IgG control), weak (+), moderate (++), or strong (+++). The scores from the two observers were averaged. If histologically discernable, intercaruncular endometrial tissues, including LE, stroma, and GE, caruncular endometrial tissues, including LE and stroma, and myometrium were scored. Images of representative fields were recorded using a Nikon Eclipse 1000 photomicroscope (Nikon Instruments Inc., Lewisville, TX) fitted with a Nikon DXM1200 digital camera.
Statistical analyses
Integrated light intensity measurement data from RT-PCR analyses were subjected to least-squares analysis of variance (LS-ANOVA) using the General Linear Models (GLM) procedures of the Statistical Analysis System version 8.1 for Windows (SAS Institute, Cary, NC) [38]. The intensity of light emitted from β-actin PCR products was used as the covariate. The least square means (LSM) and standard errors (SE) illustrated in graphs were derived from this analysis. Data is presented as relative units (RU).
Results
Pregnancy increases COX-2 mRNA and protein in the endometrial epithelium
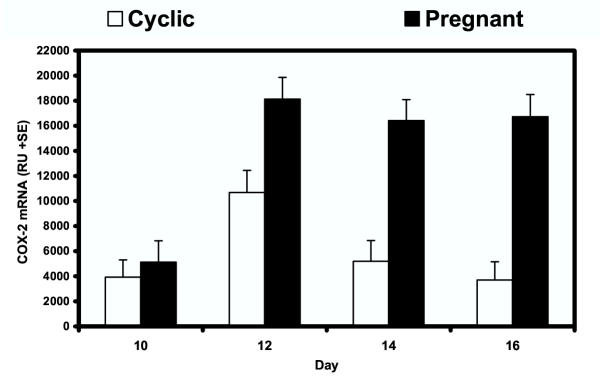
Steady-state levels of COX-2 mRNA in the ovine endometrium were determined by semi-quantative RT-PCR analyses (Fig. 1). In cyclic ewes, COX-2 mRNA levels increased between Days 10 and 12 and then decreased from Day 12 to Day 16 (quadratic effect of day, P < 0.10). In pregnant ewes, endometrial COX-2 mRNA levels were lowest on Day 10, increased approximately 4-fold by Day 12, and remained high thereafter (quadratic, P < 0.10). After Day 10, levels of COX-2 mRNA were greater in the endometrium of pregnant compared to cyclic ewes (day × status, P < 0.01).
Figure 1.
Steady-state levels of COX-2 mRNA expression in endometrium of cyclic and pregnant ewes. Total RNA was isolated from endometrium and analyzed by semi-quantitative RT-PCR. Data are presented as LSM relative units (RU) with SE.
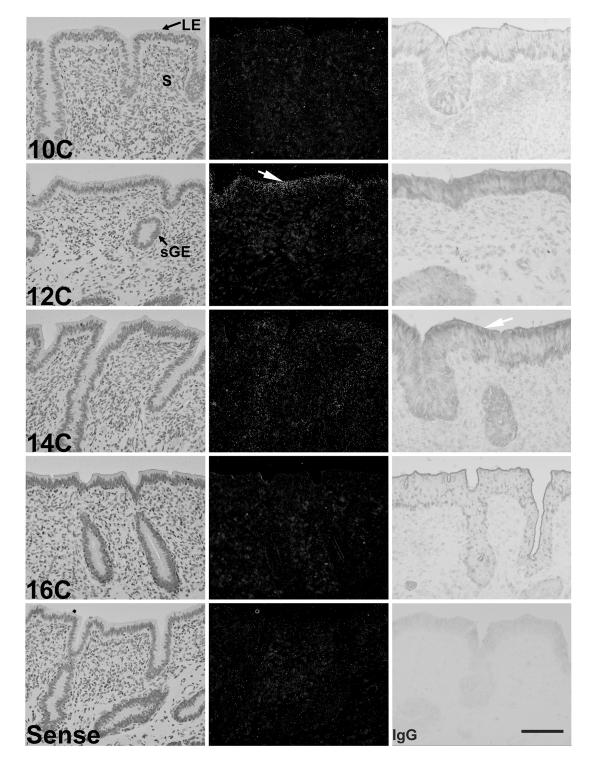
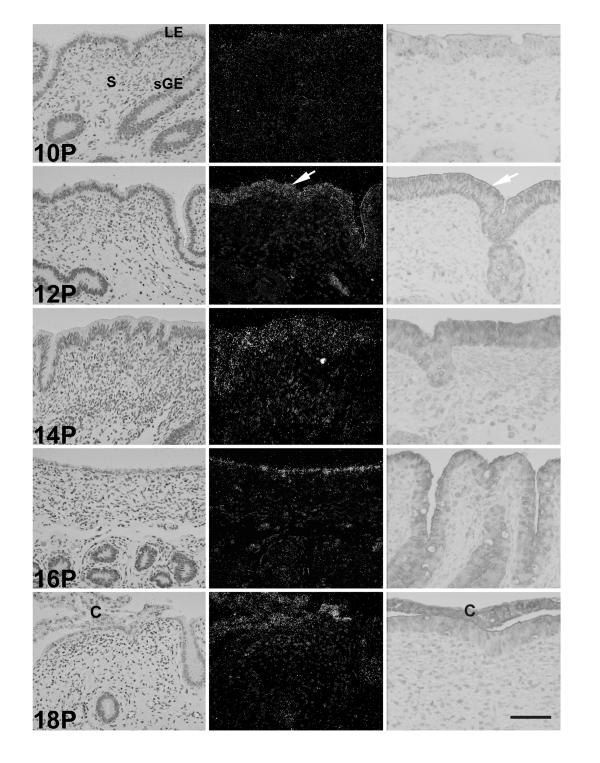
In situ hybridization and immunohistochemical analyses revealed temporal and spatial alterations in COX-2 mRNA and protein expression in the ovine uterus. In cyclic (Fig. 2) and pregnant (Fig. 3) ewes, COX-2 mRNA and protein were detected only in LE and sGE of ovine endometrium. In early pregnant ewes (Fig. 3), COX-2 mRNA and protein abundance in endometrial LE and sGE increased from weak to strong between Days 10 and 12 and remained strong to Day 18. The decline in COX-2 expression after Day 12 in the endometrium of cyclic ewes was not observed in the endometrium of comparable pregnant ewes. On Day 18 of pregnancy, COX-2 mRNA and protein were observed in the conceptus.
Figure 2.
In situ hybridization and immunohistochemical analysis of COX-2 expression in the endometrium of cyclic ewes. Cross-sections of ovine endometrium were hybridized with radiolabeled antisense or sense bovine COX-2 cRNA probes. Hybridized sections were digested with ribonuclease A, and protected transcripts were visualized by liquid emulsion autoradiography. Developed slides were counterstained lightly with hematoxylin, and photomicrographs were taken under bright-field (left) or dark-field illumination (middle). Immunoreactive COX-2 protein was detected using rabbit anti-human COX-2 polyclonal IgG (right). The negative IgG control was performed by substituting irrelevant rabbit IgG for primary antibody. The white arrow denotes areas of specific COX-2 mRNA or immunoreactive protein expression. Legend: C, cyclic; LE, lumenal epithelium; S, stroma; sGE, superficial ductal glandular epithelium. Bar = 20 μm.
Figure 3.
In situ hybridization and immunohistochemical analysis of COX-2 expression in the endometrium of early pregnant ewes. Cross-sections of the ovine endometrium were hybridized with radiolabeled antisense or sense bovine COX-2 cRNA probe. Hybridized sections were digested with ribonuclease A, and protected transcripts were visualized by liquid emulsion autoradiography. Developed slides were counterstained lightly with hematoxylin, and photomicrographs were taken under bright-field or dark-field illumination (left). Immunoreactive COX-2 protein was detected using rabbit anti-human COX-2 polyclonal IgG and a BioStain Super ABC Kit (right). The white arrow denotes areas of specific COX-2 mRNA or immunoreactive protein expression. Legend: C, conceptus; LE, lumenal epithelium; S, stroma; sGE, superficial ductal glandular epithelium; P, pregnant. Bar = 20 μm.
Intrauterine infusion of ovine IFNτ has no effect on COX-2 expression in the endometrium
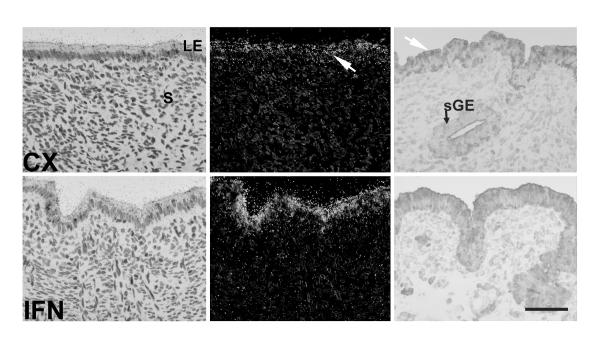
In Study Two, intrauterine infusion of roIFNτ into cyclic ewes did not affect (P > 0.10) steady-state levels of endometrial COX-2 mRNA as determined by semi-quantitative RT-PCR analysis (data not shown). These results were confirmed by in situ hybridization and immunohistochemical analyses of COX-2 mRNA and protein (Fig. 4). COX-2 mRNA and protein were detected predominantly in endometrial LE and were not different in CX as compared to IFNτ infused ewes.
Figure 4.
In situ hybridization and immunohistochemical analysis of COX-2 expression in the ovine endometrium (Study Two). Ewes were ovariectomized on Day 5 of the estrous cycle, treated daily with progesterone, and infused from Days 11 to 15 with either control (CX) proteins or recombinant ovine IFNτ (IFN). On Day 16, ewes were hysterectomized. Cross-sections of the ovine uterus were hybridized with radiolabeled antisense or sense bovine COX-2 cRNA probe. Hybridized sections were digested with ribonuclease A, and protected transcripts were visualized by liquid emulsion autoradiography. Developed slides were counterstained lightly with hematoxylin, and photomicrographs were taken under bright-field or dark-field illumination (left). Immunoreactive COX-2 protein was detected using rabbit anti-human COX-2 polyclonal IgG (right). The negative IgG control was performed by substituting irrelevant rabbit IgG for primary antibodies. The white arrow denotes areas of specific COX-2 mRNA or immunoreactive protein expression. Legend: CX, control; IFN, interferon tau; LE, lumenal epithelium; S, stroma; sGE, superficial ductal glandular epithelium. Bar = 20 μm.
Discussion
In the present study, COX-2 mRNA and protein were detected in endometrial LE and sGE from cyclic ewes. Overall, levels of COX-2 mRNA increased between Days 10 and 12 post-estrus and then decreased. These results conflict with reports that COX-2 protein was expressed maximally in ovine endometrium between Days 10 and 16 of the estrous cycle [40] and that COX-2 mRNA did not fluctuate across the estrous cycle [41]. However, Charpigny et al. [5] found that COX-2 was transiently expressed in the ovine endometrial LE and sGE between Days 12 and 15 of the estrous cycle and then decreased in abundance. The differences in the present study may stem from breed of ewe used in the studies to sensitivity of the biochemical techniques and estrus detection. In bovine studies, COX-2 mRNA and protein were expressed at low and high levels on Days 1–12 and 13–21, respectively, of the estrous cycle [42]. Nevertheless, COX-2 is the predominant enzyme expressed in ovine and bovine uteri [42] and is expressed in LE and sGE of the endometrium, which are responsible for production of luteolytic pulses of PGF2α [6].
In pregnant ewes, the present study found that COX-2 mRNA and protein expression increased between Days 10 and 12 and remained high thereafter in the LE and sGE of the endometrium. Charpigny et al. [5] also reported that COX-2 increased on Day 12 of pregnancy and was expressed to Day 17. The present study also detected COX-2 mRNA and protein expression in the Day 18 conceptus. Similarly, Charpigny et al. [5] found that COX-2 was expressed in the ovine conceptus and was developmentally regulated in the trophoblast. The increase in COX-2 expression in ovine endometrium and conceptus is correlated with production of several types of PGs [5,43]. The COX-2-derived PGs produced by the blastocyst are proposed to be involved in blastocyst formation, hatching and elongation [44,45], whereas PGs produced by the endometrium play essential roles in endometrial vascular permeability and implantation [23-25,46,47].
Conclusions
Several reports supported the hypothesis that IFNτ prevents the COX-2 expression in endometrium to block the production of PGF and that IFNτ acts on endometrium to inhibit OT-stimulated COX-2. Using an in vitro model to investigate effects of IFNτ on expression of COX-2, IFNτ was found to decrease COX-2 expression in bovine endometrial cells [32-34]. However, in vivo studies showed that expression of COX-2 is essential for blastocyst implantation and decidualization especially changes in vascular permeability and angiogenesis in mice [23-25]. Thus, available results support the contention that the antiluteolytic effect of IFNτ on endometrial epithelia is not manifest on COX-2 gene expression. Rather, IFNτ inhibits or silences expression of the ERα gene [48], which, in turn, prevents increases in OTR gene expression [49], thereby preventing endometrial production of luteolytic pulses of PGF.
Acknowledgments
Acknowledgements
We thank the NIH for funding this work (Grant HD32534) and Dr. Michael A. Fortier (University of Laval, Quebec, Canada) for kindly providing the bovine COX-2 cDNA.
Contributor Information
Seokwoon Kim, Email: swkim@neo.tamu.edu.
Youngsok Choi, Email: ychoil@bmc.tmc.edu.
Thomas E Spencer, Email: tspencer@ansc.tamu.edu.
Fuller W Bazer, Email: fbazer@cvm.tamu.edu.
References
- McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine mediated event. Physiol Rev. 1999;79:263–323. doi: 10.1152/physrev.1999.79.2.263. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci. 2002;7:d1879–98. doi: 10.2741/spencer. [DOI] [PubMed] [Google Scholar]
- McCracken JA, Carlson JC, Glew ME, Goding JR, Baird DT, Green K, Samuelsson B. Prostaglandin F2α identified as a luteolytic hormone in sheep. Nature New Biol. 1971;238:129–134. doi: 10.1038/newbio238129a0. [DOI] [PubMed] [Google Scholar]
- Hooper SB, Watkins WB, Thorburn GD. Oxytocin, oxytocin-associated neurophysin, and prostaglandin F2 alpha concentrations in the utero-ovarian vein of pregnant and nonpregnant sheep. Endocrinology. 1986;119:2590–2597. doi: 10.1210/endo-119-6-2590. [DOI] [PubMed] [Google Scholar]
- Charpigny G, Reinaud P, Tamby JP, Créminon C, Martal J, Maclouf J, Guillomot M. Expression of cyclooxygenase-1 and -2 in ovine endometrium during the estrous cycle and early pregnancy. Endocrinology. 1997;138:2163–2171. doi: 10.1210/en.138.5.2163. [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Taylor KM, Wiley AA, Ramsey WS, Ott TL, Bazer FW, Spencer TE. Ovine uterine gland knock-out model: effects of gland ablation on the estrous cycle. Biol Reprod. 2000;62:448–456. doi: 10.1095/biolreprod62.2.448. [DOI] [PubMed] [Google Scholar]
- McCracken JA. Hormone receptor control of prostaglandin F2 alpha secretion by the ovine uterus. Adv Prostaglandin Thromboxane Res. 1980;8:1329–1344. [PubMed] [Google Scholar]
- Spencer TE, Ott TL, Bazer FW. tau-Interferon: pregnancy recognition signal in ruminants. Proc Soc Exp Biol Med. 1996;213:215–229. doi: 10.3181/00379727-213-44053. [DOI] [PubMed] [Google Scholar]
- Flint AP, Leat WM, Sheldrick EL, Stewart HJ. Stimulation of phosphoinositide hydrolysis by oxytocin and the mechanism by which oxytocin controls prostaglandin synthesis in the ovine endometrium. Biochem J. 1986;237:797–805. doi: 10.1042/bj2370797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayad VJ, Matthews EL, Wathes DC, Parkinson TJ, Wild ML. Autoradiographic localization of oxytocin receptors in the endometrium during the oestrous cycle of the ewe. J Endocrinol. 1991;130:199–206. doi: 10.1677/joe.0.1300199. [DOI] [PubMed] [Google Scholar]
- Wathes DC, Hamon M. Lozalization of oestradiol, progesterone and oxytocin receptors in the uterus during the oestrous cycle and early pregnancy of the ewe. J Endocrinol. 1993;138:479–491. doi: 10.1677/joe.0.1380479. [DOI] [PubMed] [Google Scholar]
- Stevenson KR, Riley PR, Stewart HJ, Flint AP, Wathes DC. Localization of oxytocin receptor mRNA in the ovine uterus during the oestrous cycle and early pregnancy. J Mol Endocrinol. 1994;12:93–105. doi: 10.1677/jme.0.0120093. [DOI] [PubMed] [Google Scholar]
- Beard AP, Lamming GE. Oestradiol concentration and the development of the uterine oxytocin receptor and oxytocin-induced PGF2 alpha release in ewes. J Reprod Fertil. 1994;100:469–475. doi: 10.1530/jrf.0.1000469. [DOI] [PubMed] [Google Scholar]
- Hixon JE, Flint AP. Effects of a luteolytic dose of oestradiol benzoate on uterine oxytocin receptor concentrations, phosphoinositide turnover and prostaglandin F-2 alpha secretion in sheep. J Reprod Fertil. 1987;79:457–467. doi: 10.1530/jrf.0.0790457. [DOI] [PubMed] [Google Scholar]
- Burgess KM, Ralph MM, Jenkin G, Thorburn GD. Effect of oxytocin and estradiol on uterine prostaglandin release in nonpregnant and early-pregnant ewes. Biol Reprod. 1990;42:822–833. doi: 10.1095/biolreprod42.5.822. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Becker WC, George P, Mirando MA, Ogle TF, Bazer FW. Ovine interferon-tau inhibits estrogen receptor up-regulation and estrogen-induced luteolysis in cyclic ewes. Endocrinology. 1995;136:4932–4944. doi: 10.1210/en.136.11.4932. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Mirando MA, Mayes JS, Watson GH, Ott TL, Bazer FW. Effects of interferon-τ and progesterone on oestrogen-stimulated expression of receptors for oestrogen, progesterone and oxytocin in the endometrium of ovariectomized ewes. Reprod Fertil Dev. 1996;8:843–853. doi: 10.1071/rd9960843. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod. 1995;53:1527–1544. doi: 10.1095/biolreprod53.6.1527. [DOI] [PubMed] [Google Scholar]
- Smith WL, Garavito RM, Dewitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- Smith WL, Dewitt DL, Garavito RM. Cyclooxygenases; structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase-2 deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Ma WG, Daikoku T, Zhao X, Paria BC, Das SK, Trzaskos JM, Dey SK. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem. 2002;277:29260–29267. doi: 10.1074/jbc.M203996200. [DOI] [PubMed] [Google Scholar]
- Fincher KB, Bazer FW, Hansen PJ, Thatcher WW, Roberts RM. Proteins secreted by the sheep conceptus suppress induction of uterine prostaglandin F-2 alpha release by oestradiol and oxytocin. J Reprod Fertil. 1986;76:425–433. doi: 10.1530/jrf.0.0760425. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Ealy AD, Alexenko AP, Han CS, Ezashi T. Trophoblast interferons. Placenta. 1999;20:259–264. doi: 10.1053/plac.1998.0381. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Ing NH, Ott TL, Mayes JS, Becker WC, Watson GH, Mirando MA, Bazer FW. Intrauterine injection of ovine interferon-tau alters oestrogen receptor and oxytocin receptor expression in the endometrium of cyclic ewes. J Mol Endocrinol. 1995;15:203–220. doi: 10.1677/jme.0.0150203. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Ovine interferon τ suppresses transcription of the estrogen receptor and oxytocin receptor genes in the ovine endometrium. Endocrinology. 1996;137:1144–1147. doi: 10.1210/en.137.3.1144. [DOI] [PubMed] [Google Scholar]
- Asselin E, Lacroix D, Fortier MA. IFN-tau increases PGE2 production and COX-2 gene expression in the bovine endometrium in vitro. Mol Cell Endocrinol. 1997;132:117–126. doi: 10.1016/S0303-7207(97)00128-7. [DOI] [PubMed] [Google Scholar]
- Asselin E, Drolet P, Fortier MA. Cellular mechanisms involved during oxytocin-induced prostaglandin F2α production in endometrial epithelial cells in vitro: role of cyclooxygenase-2. Endocrinology. 1997;138:4798–4805. doi: 10.1210/en.138.11.4798. [DOI] [PubMed] [Google Scholar]
- Parent J, Villeneuve C, Alexenko AP, Ealy AD, Fortier MA. Influence of different isoforms of recombinant trophoblastic interferons on prostaglandin production in cultured bovine endometrial cells. Biol Reprod. 2003;68:1035–1043. doi: 10.1095/biolreprod.102.008250. [DOI] [PubMed] [Google Scholar]
- Xiao CW, Murphy BD, Sirois J, Goff AK. Down-regulation of oxytocin-induced cyclooxygenase-2 and prostaglandin F synthase expression by interferon-t in bovine endometrial cells. Biol Reprod. 1999;60:656–663. doi: 10.1095/biolreprod60.3.656. [DOI] [PubMed] [Google Scholar]
- Xiao CW, Liu JM, Sirois J, Goff AK. Regulation of cyclooxygenase-2 and prostaglandin F synthase gene expression by steroid hormones and interferon-τ in bovine endometrial cells. Endocrinology. 1998;139:2293–2299. doi: 10.1210/en.139.5.2293. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Stagg AG, Ott TL, Johnson GA, Ramsey WS, Bazer FW. Differential effects of intrauterine and subcutaneous administration of recombinant ovine interferon tau on the endometrium of cyclic ewes. Biol Reprod. 1999;61:464–470. doi: 10.1095/biolreprod61.2.464. [DOI] [PubMed] [Google Scholar]
- Van Heeke G, Ott TL, Strauss A, Ammaturo D, Bazer FW. High yield expression and secretion of the ovine pregnancy recognition hormone interferon-tau by Pichia pastoris. J Interferon Cytokine Res. 1996;16:119–126. doi: 10.1089/jir.1996.16.119. [DOI] [PubMed] [Google Scholar]
- Stewart MD, Johnson GA, Gray CA, Schuler LA, Burghardt RC, Joyce MM, Bazer FW, Spencer TE. Prolactin receptor and UTMP expression in the ovine endometrium during the estrous cycle and pregnancy. Biol Reprod. 2000;62:1779–1789. doi: 10.1095/biolreprod62.6.1779. [DOI] [PubMed] [Google Scholar]
- Zhang V, O'Sullivan M, Hussain H, Roswit WT, Holtzman MJ. Molecular cloning, functional expression, and selective regulation of ovine prostaglandin H synthase-2. Biochem Biophys Res Commun. 1996;227:499–506. doi: 10.1006/bbrc.1996.1536. [DOI] [PubMed] [Google Scholar]
- SAS Institute . SAS User's Guide: Statistics, Ver 6. Cary, NC: Statistical Analysis System Institute; 1990. [Google Scholar]
- Salamonsen LA, Findlay JK. Immunocytochemical localization of prostaglandin synthase in the ovine uterus during the oestrous cycle and in early pregnancy. Reprod Fertil Dev. 1990;2:311–319. doi: 10.1071/rd9900311. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Hampton AL, Clements JA, Findlay JK. Regulation of gene expression and cellular localization of prostaglandin synthase by estrogen and progesterone in the ovine uterus. J Reprod Fertil. 1991;92:393–406. doi: 10.1530/jrf.0.0920393. [DOI] [PubMed] [Google Scholar]
- Arosh JA, Parent J, Chapdelaine P, Sirois J, Fortier MA. Expression of cyclooxygenases 1 and 2 and prostaglandin e synthase in bovine endometrial tissue during the estrous cycle. Biol Reprod. 2002;67:161–169. doi: 10.1095/biolreprod67.1.161. [DOI] [PubMed] [Google Scholar]
- Charpigny G, Reinaud P, Tamby JP, Creminon C, Guillomot M. Cyclooxygenase-2 unlike cyclooxygenase-1 is highly expressed in ovine embryos during the implantation period. Biol Reprod. 1997;57:1032–1040. doi: 10.1095/biolreprod57.5.1032. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Leonov BV, Baskar JF, Fried J. Inhibition of hatching of mouse blastocysts in vitro by prostaglandin antagonists. Biol Reprod. 1978;19:519–533. doi: 10.1095/biolreprod19.3.519. [DOI] [PubMed] [Google Scholar]
- Sayre BL, Lewis GS. Arachidonic acid metabolism during early development of ovine embryos: a possible relationship to shedding of the zona pellucida. Prostaglandins. 1993;45:557–569. doi: 10.1016/0090-6980(93)90019-4. [DOI] [PubMed] [Google Scholar]
- Kennedy TG. Embryonic signals and the initiation of blastocyst implantation. Aust J Biol Sci. 1983;36:531–543. doi: 10.1071/bi9830531. [DOI] [PubMed] [Google Scholar]
- Psychoyos A, Nikas G, Gravanis A. The role of prostaglandins in blastocyst implantation. Hum Reprod. 1995;10:30–42. doi: 10.1093/humrep/10.suppl_2.30. [DOI] [PubMed] [Google Scholar]
- Fleming JGW, Choi Y, Bazer FW, Johnson GA, Spencer TE. Cloning of the ovine estrogen receptor alpha promoter and functional regulation by ovine interferon tau. Endocrinology. 2001;99:1461–1466. doi: 10.1210/endo.142.7.8245. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Fleming JGF, Safe SH, Spencer TE. The ovine oxytocin receptor promoter/enhancer region is responsive to estrogen receptor alpha. Biol Reprod. 2003;68:131 (Abstract 47). [Google Scholar]