Abstract
The regulatory influences of glycogen synthase kinase-3β (GSK3β) and lithium on the activity of cyclic AMP response element binding protein (CREB) were examined in human neuroblastoma SH-SY5Y cells. Activation of Akt (protein kinase B) with serum-increased phospho-serine-9-GSK3β (the inactive form of the enzyme), inhibited GSK3β activity, and increased CREB DNA binding activity. Inhibition of GSK3β by another paradigm, treatment with the selective inhibitor lithium, also increased CREB DNA binding activity. The inhibitory regulation of CREB DNA binding activity by GSK3β also was evident in differentiated SH-SY5Y cells, indicating that this regulatory interaction is maintained in non-proliferating cells. These results demonstrate that inhibition of GSK3β by serine-9 phosphorylation or directly by lithium increases CREB activation. Conversely, overexpression of active GSK3β to 3.5-fold the normal levels completely blocked increases in CREB DNA binding activity induced by epidermal growth factor, insulin-like growth factor-1, forskolin, and cyclic AMP. The inhibitory effects due to overexpressed GSK3β were reversed by treatment with lithium and with another GSK3β inhibitor, sodium valproate. Overall, these results demonstrate that GSK3β inhibits, and lithium enhances, CREB activation.
Keywords: CREB, glycogen synthase kinase-3β, lithium, valproate
Glycogen synthase kinase-3β (GSK3β), an enzyme first characterized by its ability to phosphorylate and inhibit glycogen synthase (Embi et al. 1980; Rylatt et al. 1980), is now recognized as a key component of several intracellular signaling systems that, among other actions, regulates the activity of multiple critical transcription factors (Plyte et al. 1992; Kim and Kimmel 2000; Grimes and Jope 2001). GSK3β is perhaps best known as a component of the cell survival-promoting signaling pathway involving phosphatidylinositol 3-kinase (PI3K) and the kinase Akt (also known as protein kinase B) (Datta et al. 1999), and as an intermediate in the Wnt signaling cascade (Ferkey and Kimelman 2000). The PI3K/Akt signaling pathway is activated by many growth factors (Datta et al. 1999), including epidermal growth factor (EGF) and insulin-like growth factor-1 (IGF-1). Activated Akt phosphorylates serine-9 of GSK3β, which inhibits its kinase activity (Cross et al. 1995). Recent studies have revealed that this inhibitory control of GSK3β is an important component in the promotion of cell survival, and that hyperactive GSK3β contributes to cell death (Pap and Cooper 1998; Bijur et al. 2000; Hetman et al. 2000; Li et al. 2001a). The proapoptotic action of GSK3β may be attributable, in part, to the regulation by GSK3β of the activities of an array of transcription factors (reviewed in Grimes and Jope 2001), including β-catenin (Rubinfeld et al. 1996; Yost et al. 1996), AP-1 (Boyle et al. 1991), nuclear factor kappa-B (NF-κB) (Bournat et al. 2000), heat shock factor-1 (HSF-1) (Chu et al. 1996; Bijur and Jope 2000), and others, that control the expression of numerous genes and play prominent roles in the determination of cell fate.
One of the transcription factors that may be regulated by GSK3β is the 43 kDa phosphoprotein cyclic AMP response element binding protein (CREB). The activity of CREB is regulated by complex phosphorylation mechanisms that are not completely characterized. Phosphorylation of CREB at serine-133 is required for recruitment of the coactivator CREB-binding protein (CBP) and transcriptional activity (Gonzalez and Montminy 1989; Chrivia et al. 1993). Numerous signaling events can activate CREB through phosphorylation of serine-133, including activation of adenylyl cyclase, calcium mobilization, and growth factor stimulation (Gonzalez and Montminy 1989; Sheng et al. 1991; Chrivia et al. 1993; Ginty et al. 1994). Activation of CREB contributes to many vital processes, including cell survival (Walton and Dragunow 2000). For example, CREB null mice expressing functionally inactive CREB die immediately after birth (Rudolph et al. 1998), PC12 cells overexpressing CREB have decreased susceptibility to okadaic acid-induced apoptosis (Walton et al. 1996), and apoptosis is facilitated in human melanoma cells expressing dominant-negative CREB upon exposure to UV radiation (Yang et al. 1996) or thapsigargin (Jean et al. 1998). Additionally, multiple reports suggest that CREB promotes cell survival by up-regulating the expression of antiapoptotic proteins, such as bcl-2 (Ji et al. 1996; Wilson et al. 1996; Pugazhenthi et al. 1999; Riccio et al. 1999). These and other findings indicate that the regulation of CREB activity is critical for cell survival and other functions (Walton and Dragunow 2000).
Phosphorylation of CREB at serine-133 creates a consensus site for phosphorylation by GSK3β at serine-129 (Fiol et al. 1987; Fiol et al. 1994; Wang et al. 1994; Bullock and Habener 1998). Two studies have addressed the functional consequences of this hierarchical phosphorylation of CREB by GSK3β, but the two reached opposite conclusions. Fiol et al. (1994) reported that activation of CREB in response to cyclic AMP was potentiated in F9 cells overexpressing wild-type GSK3β, and was impaired in PC12 cells expressing CREB with a mutation in the GSK3β phosphorylation site, suggesting that GSK3β facilitated activation of CREB. In contrast, Bullock and Habener (1998) found that phosphorylation of CREB by GSK3β attenuated protein kinase A-induced CREB DNA binding activity. Thus, although there is a consensus that CREB is phosphorylated by GSK3β, the functional outcome of this modification remains to be clarified. Therefore, this study investigated the regulatory effects of GSK3β on CREB in human neuroblastoma SH-SY5Y cells. Using several experimental paradigms, the results show that GSK3β negatively regulates CREB DNA binding activity, and that lithium and sodium valproate, inhibitors of GSK3β, facilitate CREB activation.
Materials and methods
Cell culture
Human neuroblastoma SH-SY5Y cells were grown on Corning 100 mm tissue culture dishes (Corning, NY, USA) in continuous culture RPMI 1640 medium (Cellgro, Herndon, VA, USA) containing 10% horse serum (Life Technologies, Gaithersburg, MD, USA), 5% fetal clone II (Hyclone, Logan, UT, USA), 2 mm L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin (Life Technologies). SH-SY5Y cells were differentiated to a neuronal phenotype as described previously (Sayas et al. 1999) by maintaining cells in Neurobasal medium supplemented with B-27 (Life Technologies) for 3.5 days. On the third day, B-27 supplement was withdrawn, and cells were maintained in Neurobasal medium without B-27 for 24 h. Stably transfected SH-SY5Y cells overexpressing HA-tagged GSK3β were described previously (Bijur et al. 2000), and were maintained in medium containing 100 µg/mL G418 (Geneticin; Alexis Biochemicals, San Diego, CA, USA). Cells were maintained in humidified, 37°C chambers with 5% CO2. For all experiments, cells were plated at a density of 105 cells per 100 mm dish, serum was withdrawn after 24 h (unless stated otherwise), and cells were harvested ~24 h later, following treatments described in the Resultssection. Cyclic AMP levels were measured using a kit according to the supplier’s instructions (Amersham, Arlington Heights, IL, USA).
Immunoblotting and immunoprecipitation
Cells were washed twice with phosphate-buffered saline and were lysed with 500 µL of lysis buffer (20 mm Tris, pH 7.5, 150 mm NaCl, 2 mm EDTA, 2 mm EGTA, 0.2% NP-40, 1 mm sodium orthovanadate, 100 µm phenylmethanesulfonyl fluoride, 1 nM okadaic acid, and 10 µg/mL each of leupeptin, aprotinin, and pepstatin). The lysates were collected in microcentrifuge tubes, sonicated for 10 s, and centrifuged at 20 800 g for 15 min. Protein concentrations in the supernatants were determined using the bicinchoninic acid (BCA) method (Pierce, Rockford, IL, USA). The lysates were stored at −80°C until used for immunoblotting.
Cell lysates were mixed with Laemmli sample buffer [2% sodium dodecyl sulfate (SDS)] and placed in a boiling water bath for 10 min. Proteins were separated using 8% SDS—polyacrylamide gel electrophoresis (PAGE), and the proteins were transferred to nitrocellulose. Blots were probed with antibodies to Akt, phospho-serine-473-Akt, CREB, phospho-serine-133-CREB (Cell Signaling Technology, Beverly, MA, USA), GSK3β (Pharmingen/Transduction Laboratories, San Diego, CA, USA), or CBP (Santa Cruz Biotechnology, Santa Cruz, CA, USA) followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA, USA). Immunoblots were developed using horseradish peroxidase-conjugated secondary antibodies, detected with enhanced chemiluminescence (Amersham-Pharmacia, Piscataway, NJ, USA), and analyzed with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA, USA).
To measure levels of phospho-serine-9-GSK3β, cell lysates were incubated with 2 µg of phospho-serine-9-GSK3β polyclonal antibody (Biosource, Camarillo, CA, USA) overnight at 4°C and incubated with 60 µL of protein A sepharose beads (Sigma, St. Louis, MO, USA) for 1 h at 4°C with gentle agitation. Immune complexes were washed and incubated in a boiling water bath, proteins were separated by SDS—PAGE, and immunoblots were obtained using a GSK3β monoclonal antibody (Pharmigen/Transduction Laboratories).
For coimmunoprecipitation experiments, cell lysates were incubated with 2 µg of CBP monoclonal antibody (Pharmingen) overnight at 4°C. Samples were incubated with 60 µL of protein G sepharose beads (Amersham-Pharmacia) for 1 h at 4°C with gentle agitation. The immune complexes were washed three times with lysis buffer. Samples in Laemmli buffer were placed in a boiling water bath, proteins were separated by SDS—PAGE, and samples were immunoblotted for phospho-serine-133-CREB or CBP.
Electrophoretic mobility shift assay (EMSA)
Cells were washed twice with 4 mL of phosphate-buffered saline and lysed with 500 µL of NP-40 lysis buffer (10 mm Tris-Cl, pH 7.4, 3 mm MgCl2, 10 mm NaCl, and 0.5% NP-40). Cell lysates were centrifuged at 4000 g for 5 min at 4°C. The pellet was resuspended in 50 µL of buffer (20 mm HEPES, pH 7.9, 20% glycerol, 0.3 m NaCl, 1.5 mm MgCl2, 0.2 mm EDTA, 1 mm dithiothreitol, 0.1 mm β-glycerophosphate, 0.5 mm vanadate, 1 mm phenylmethanesulfonyl fluoride, and 1 βg/mL each of pepstatin A, leupeptin, and aprotinin). After a 30-min extraction on ice, samples were centrifuged at 16 000 g for 15 min at 4°C. The supernatant containing nuclear extracts was transferred to a sterile microcentrifuge tube, and protein concentrations were determined by the method of Bradford (1976).
A 17-base-pair double-stranded oligonucleotide containing the consensus sequence for CREB 5′-TCGAGCTGACGTCAGAG-3′ was used for EMSAs (Integrated DNA Technologies, Coralville, IA, USA). Early growth response-1 (EGR-1) EMSAs were carried out exactly as described previously (Grimes and Jope 1999). Double-stranded oligonucleotide (200 pmol) was radiolabeled by incubating for 1 h at 37°C in 20 µL containing Reaction Buffer (Amersham-Pharmacia), DNA polymerase I (Klenow Enzyme; Amersham-Pharmacia), and 100 µCi of [α-32P]dCTP (Amersham, Arlington Heights, IL, USA). Following incubation, samples were diluted to 50 µL with sterile TE buffer (10 mm Tris-HCl, pH 8.0 and 1 mm EDTA), and free label was removed by centrifugation at 3000 r.p.m. for 2 min through ProbeQuant G-50 microcolumns (Amersham-Pharmacia).
DNA binding was measured by incubating nuclear extracts (10 µg protein) in 20 µL of binding buffer containing 20 mm HEPES (pH 7.0), 4% glycerol, 500 mm KCl, 1 mm MgCl2, 0.5 mm dithiothreitol, 1 mg of poly(dI-dC), and ~ 12,000 cpm of radio-labeled oligonucleotide for 30 min at 4°C. For supershift experiments, nuclear extracts were incubated with an antibody (0.5 µg) to CREB (Cell Signaling Technology), Fos (Calbiochem, La Jolla, CA, USA), or EGR-1 (Santa Cruz Biotechnology) for 30 min prior to incubation with binding buffer. Reaction mixtures were electrophoresed on 6% non-denaturing polyacrylamide gels in 0.25 × TBE (22.3 mm Tris, 22.3 mm boric acid, and 0.5 mm EDTA) for 1.5 h at 150 V. The gels were then vacuum-dried, exposed to a phosphorscreen overnight, and quantitated using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA, USA). All experiments were carried out at least three times, and statistical significance was determined by analysis of variance.
GSK3β activity assay
The activity of GSK3β was measured essentially as described previously (Lesort et al. 1999). Cells were lysed in immunoprecipitation lysis buffer (20 mm Tris, pH 7.5, 0.2% NP-40, 150 mm NaCl, 2 mm EDTA, 2 mm EGTA, 1 mm sodium orthovanadate, 100 mm phenylmethanesulfonyl fluoride, 1 nM okadaic acid, and 10 µg/mL each of leupeptin, aprotinin, and pepstatin). The lysates were sonicated in microcentrifuge tubes for 10 s on ice and centrifuged at 20 800 g for 15 min. Lysates were precleared with 40 µL of protein G-Sepharose beads (Amersham- Pharamcia) for 15 min at 4°C, protein concentrations were determined, and 100 µg of protein (1 µg/µL) was incubated with 1.5 µg of monoclonal GSK3β antibody (Pharmingen/Transduction Laboratories) for 2 h at 4°C with gentle agitation. Lysates were then incubated with 60 µL protein G-sepharose for 1 h at 4°C. The immobilized immune complexes were washed twice with immunoprecipitation lysis buffer and twice with kinase buffer (20 mm Tris, pH 7.5, 5 mm MgCl2, and 1 mm dithiothreitol). Kinase activity was measured by mixing immunoprecipitated GSK3β with 25 µL of kinase buffer containing 20 mm Tris, pH 7.5, 5 mm MgCl2, 1 mm dithiothreitol, 250 mm ATP, 1.4 µCi [γ-32P]ATP (Amersham, Arlington Heights, IL, USA), varying concentrations of lithium (when indicated), and 0.1 µg/µL recombinant tau protein (provided by Dr Gail V. W. Johnson, University of Alabama at Birmingham). The samples were incubated at 30°C for 15 min and 25 µL of Laemmli sample buffer (2% SDS) was added to each sample to stop the reaction. Samples were placed in a boiling water bath for 10 min and proteins were separated in 8% SDS—PAGE. The gels were vacuum-dried, exposed to a phosphor-screen overnight, and quantitated using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA, USA). The efficiency of GSK3β immunoprecipitation was determined by immunoblotting for GSK3β.
Results
Reduction of GSK3β activity increases CREB DNA binding activity
To determine if GSK3β has an inhibitory influence on CREB DNA binding activity, two treatment protocols were used to reduce the activity of GSK3β in wild-type SH-SY5Y cells. First, GSK3β activity was reduced indirectly by using serum to stimulate the activation of Akt, which inhibits GSK3β activity by phosphorylating serine-9 of GSK3β (Cross et al. 1995). Second, GSK3β activity was reduced directly by treating cells with lithium, a selective inhibitor of GSK3β (Klein and Melton 1996; Stambolic et al. 1996; Davies et al. 2000).
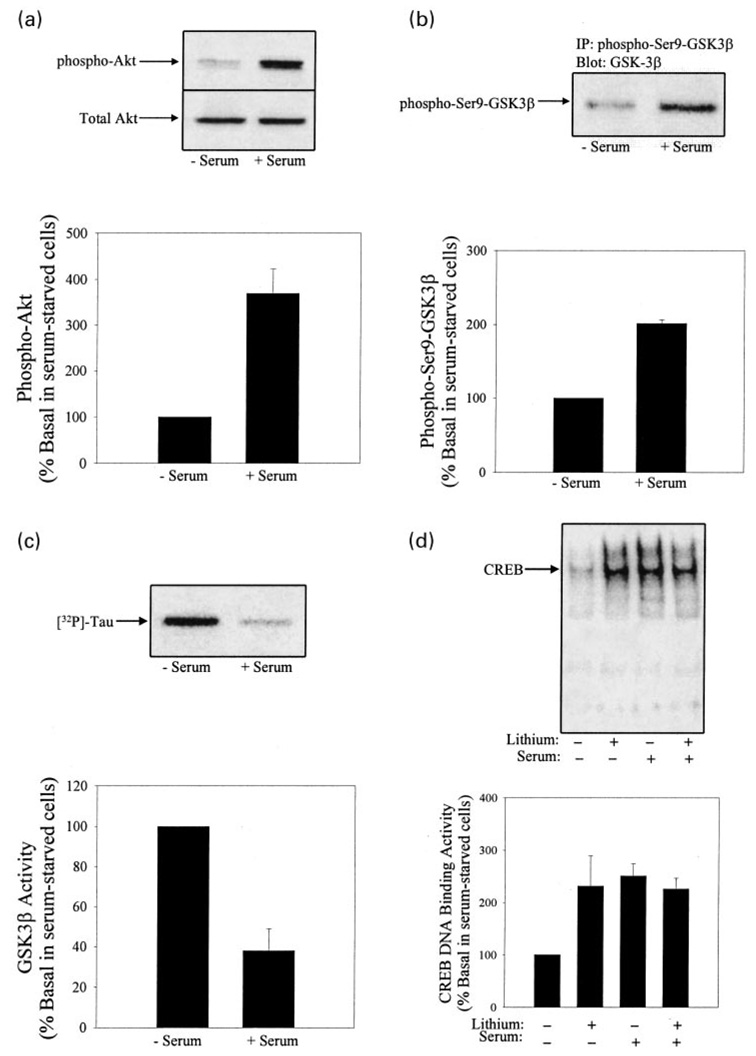
The activation state of Akt was modulated by maintaining cells in growth media containing serum to compare with results obtained in serum-withdrawn (24 h) cells. The phosphorylated, active form of Akt was 3.7-fold higher in cells maintained in medium containing serum compared with cells that were serum-starved for 24 h, although total Akt levels remained unchanged (Fig. 1a). The greater amount of active, phosphorylated Akt in cells in serum-containing medium was associated with more of the inhibited phospho-serine-9 form of GSK3β, which was 200 ± 12% of the level in serum-starved cells (Fig. 1b), and a level of GSK3β activity that was 38 ± 10% of the activity in serum-starved cells (Fig. 1c). In association with the decreased activity of GSK3β in cells maintained in serum, CREB DNA binding activity was 253 ± 23% of that in serum-starved cells (Fig. 1d). Thus, in cells maintained in serum, compared with serum-starved cells, Akt activity was higher, GSK3β activity was lower, and CREB DNA binding activity was higher. These results are consistent with the hypothesis that GSK3β is an inhibitory modulator of CREB DNA binding activity.
Fig. 1.
Serum decreases GSK3β activity and increases CREB DNA binding activity in SH-SY5Y cells. SH-SY5Y cells were serum-starved for 24 h unless stated otherwise. (a) Phospho-serine-473- Akt and total levels of Akt were measured in lysates from cells incubated without or with serum by immunoblot analysis. Quantitative values are expressed as the percent of phosphorylated Akt levels in serum-starved cells. Mean ± SEM, n = 4. (b) Phosphorylated serine-9-GSK3β levels were measured in lysates from cells incubated without or with serum by immunoprecipitating phosphoserine-9-GSK3β and immunoblotting GSK3β. Quantitative values are expressed as the percent of phosphorylated Serine-9-GSK3β in serum-starved cells. Mean ± SEM, n = 3. (c) GSK3β activity was measured by immunoprecipitating GSK3β from cells incubated without or with serum, incubating the immunoprecipitated GSK3β with recombinant human tau and [32P]ATP, and measuring the phosphorylation of tau, as described in Materials and methods. Quantitative values are expressed as the percent of GSK3β activity in serum-starved cells. Mean ± SEM, n = 4. (d) CREB DNA binding activity was measured in nuclear extracts from cells incubated without or with serum and treated without or with lithium (1 mm; 1 h). Quantitative values are expressed as the percent of CREB DNA binding activity in serum-starved cells. Mean ± SEM, n = 4.
To test if the higher GSK3β activity contributed to the lower CREB DNA binding activity in serum-withdrawn cells compared with cells maintained in serum, GSK3β was inhibited directly by treating cells with 1 mm lithium for 1 h. In serum-starved cells, which contained greater GSK3β activity and less of the inactive phospho-serine-9-GSK3β, this treatment with lithium to inhibit GSK3β increased CREB DNA binding activity twofold (Fig. 1d). In contrast, lithium treatment did not have a discernable effect on CREB DNA binding activity in serum-treated cells in which GSK3β was already inhibited by the active Akt, supporting the conclusion that lithium increased CREB DNA binding activity through its inhibitory effect on GSK3β.
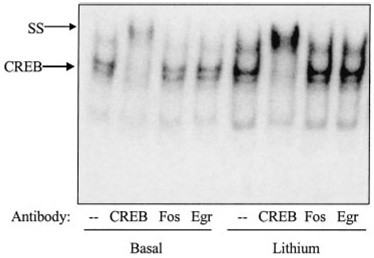
Supershift analyses were used to confirm the identity of the CREB DNA binding activity obtained with the EMSA (Fig. 2). Using cells from which serum was withdrawn for 24 h, an antibody specific for CREB completely super-shifted the CREB DNA binding activity to a slower mobility in nuclear extracts from both untreated cells and from cells in which CREB was activated by treatment with 1 mm lithium for 1 h. In contrast, Fos and EGR-1 antibodies did not affect the CREB DNA binding band intensity or mobility.
Fig. 2.
Supershift analysis of CREB DNA binding activity in SH-SY5Y cells. CREB DNA binding activity was measured by EMSA in nuclear extracts prepared from serum-starved (24 h) SH-SY5Y cells incubated without (basal) or with lithium (1 mm; 1 h). For supershift (ss) analysis, nuclear extracts were incubated with antibody (0.5 µg) to CREB, Fos, or EGR-1 prior to incubation with radiolabeled oligonucleotide.
To examine if the inverse relationship between GSK3β and CREB DNA binding activity was maintained in nonproliferating cells, SH-SY5Y cells were differentiated for 3.5 days, as described previously (Sayas et al. 1999), followed by B27-withdrawal. Inhibition of GSK3β with lithium (1 mm; 1 h) increased CREB DNA binding activity in differentiated SH-SY5Y cells (Fig. 3), but had no effect in cells maintained in B27 supplement, indicating that these responses are not limited to proliferating cells.
Fig. 3.
Lithium stimulates CREB DNA binding activity following B27 supplement-withdrawal in differentiated SH-SY5Y cells. SH-SY5Y cells were differentiated to a neuronal-like phenotype by maintaining cells in Neurobasal medium, supplemented with B27, for 3.5 days. B27 supplement was removed 24 h prior to preparation of nuclear extracts. CREB DNA binding activity was measured by EMSA in nuclear extracts prepared from serum-containing or serum-starved cells treated without or with lithium (1 mm; 1 h).
Taken together, these results indicate that GSK3β has an inhibitory influence on CREB DNA binding activity, and that inhibition of GSK3β either by activation of Akt or with lithium increased CREB DNA binding activity.
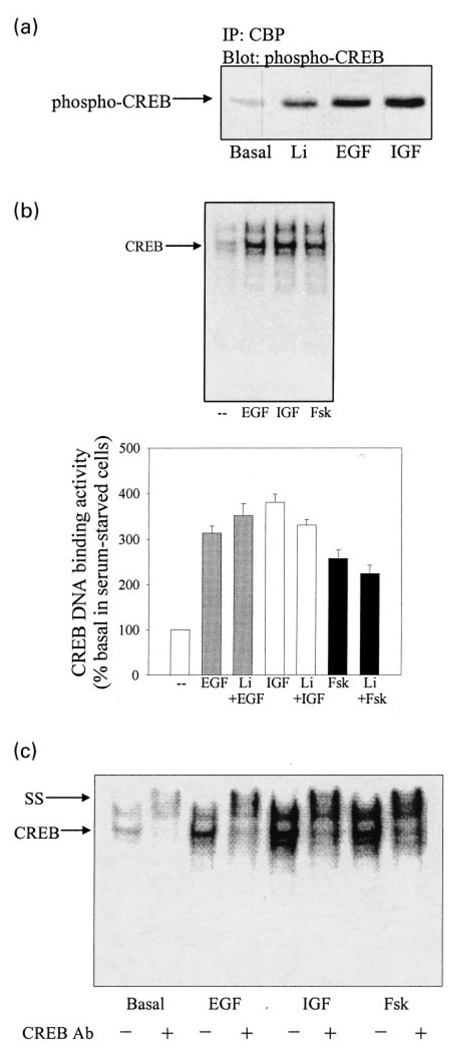
Stimulation of phospho-CREB—CBP complex formation and CREB DNA binding activity
In order to examine the modulatory effects of GSK3β in further experiments, treatments were identified that activate CREB using two assessments, the formation of complexes containing phospho-serine-133-CREB and CBP, and CREB DNA binding activity. Phosphorylation of CREB at serine-133 enables complex formation with CBP, and recruitment of CBP by CREB has been shown to be sufficient for transcriptional activation (Cardinaux et al. 2000). This complex formation was examined by measuring the amount of phospho-serine-133-CREB that coimmunoprecipitated with CBP. In serum-starved wild-type SH-SY5Y cells, treatment with EGF or IGF-1 increased the amount of phospho-serine-133-CREB that coimmunoprecipitated with CBP (Fig. 4a). Additionally, treatment with lithium (10 mm; 30 min) to inhibit GSK3µ increased the coimmunoprecipitation of phospho-serine-133-CREB with CBP (Fig. 4a). Measurements of CREB DNA binding activity demonstrated that treatment with EGF, IGF-1, or forskolin, an activator of adenylyl cyclase, increased CREB DNA binding activity to 314 ± 15%, 381 ± 18%, and 258 ± 19%, respectively, of the basal CREB DNA binding activity (Fig. 4b). Pretreatment with lithium (1 mm; 1 h) did not alter activation of CREB DNA binding activity induced by any of these stimuli (Fig. 4b). Incubation with an antibody specific for CREB supershifted the CREB DNA binding activity to a slower mobility in nuclear extracts from cells treated with each of the stimulants (Fig. 4c).
Fig. 4.
EGF, IGF-1, and forskolin stimulate CREB DNA binding activity in serum-starved SH-SY5Y cells. (a) The association of phospho-serine-133-CREB with CBP was measured by immunoprecipitating CBP from serum-starved (24 h) wild-type SH-SY5Y cells treated without or with lithium (10 mm; 30 min), EGF (50 ng/mL; 30 min), or IGF-1 (50 ng/mL; 30 min) and immunoblotting phospho-serine-133-CREB, as described in Materials and methods. (b) CREB DNA binding activity was measured by EMSA in nuclear extracts prepared from SH-SY5Y cells incubated without serum (24 h) and treated without (basal) or with EGF (50 ng/mL; 1 h), IGF-1 (50 ng/mL; 1 h), or forskolin (Fsk; 10 µm; 1 h) following pre-incubation without or with lithium (Li; 1 mm; 1 h). Quantitative values are expressed as the percent of basal CREB DNA binding activity in serum-starved cells. Mean ± SEM, n = 4–6. (c) Supershift (ss) analysis of CREB DNA binding activity. Nuclear extracts prepared from serum-starved (24 h) cells treated without (basal) or with EGF (50 ng/mL; 1 h), IGF-1 (50 ng/mL; 1 h), or forskolin (10 µm; 1 h) were incubated with antibody to CREB (0.5 µg).
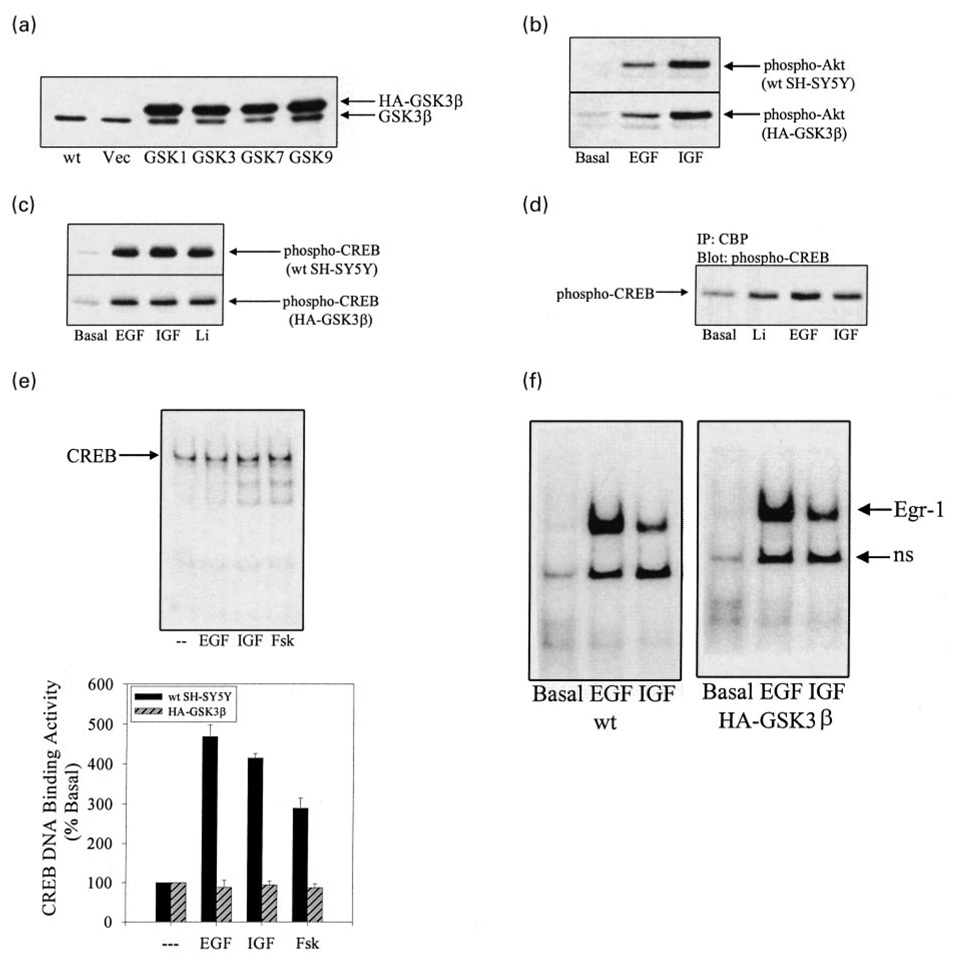
Increased GSK3β activity inhibits stimulation of CREB DNA binding activity
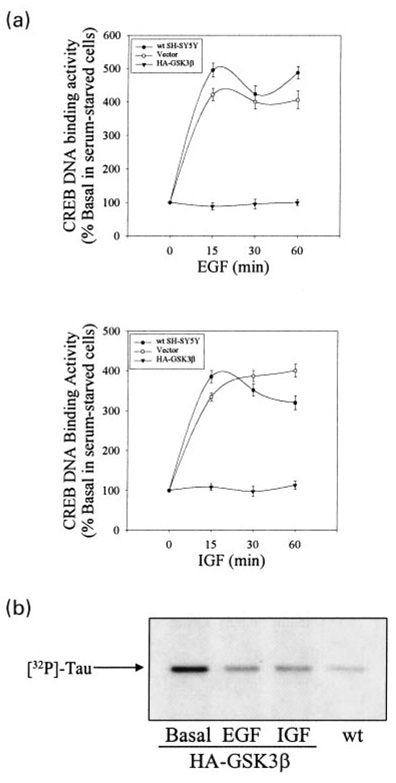
To complement the experiments that showed that inhibition of GSK3β was associated with increased CREB DNA binding activity, experiments were carried out to determine if increased GSK3β activity inhibits the activation of CREB. Since no specific activators of GSK3β are known, these experiments used stable lines of SH-SY5Y cells over-expressing GSK3β (Bijur et al. 2000). These GSK3β-transfected SH-SY5Y stable cell lines have been shown to have three- to fourfold higher levels (Fig. 5a) and activities (Bijur et al. 2000) of GSK3β than wild-type SH-SY5Y cells. Initial signaling induced by EGF and IGF-1, as deduced from measurements of stimulant-induced increases in Akt phosphorylation, were identical in control and GSK3β-overexpressing cells (Fig. 5b). Additionally, treatment with EGF, IGF-1, or lithium increased the level of phospho-serine-133-CREB in both wild-type and GSK3β-overexpressing cells (Fig. 5c) and increased the coimmunoprecipitation of phospho-serine-133-CREB with CBP in GSK3β-overexpressing cells (Fig. 5d). This is in accordance with the findings of Bullock and Habener (1998) that the inhibitory action of GSK3β should not influence the stimulation of CREB, but should inhibit CREB DNA binding activity. This was found to be the case, as in cells overexpressing GSK3β, EGF-, IGF-1-, and forskolin-stimulated CREB DNA binding activity was almost completely blocked (Fig. 5e). In order to ensure that the impaired responses in cells overexpressing GSK3β were not clone-specific, three additional SH-SY5Y cell lines overexpressing GSK3β were tested. In each of these cell lines, stimulation of CREB DNA binding activity by EGF, IGF-1, or forskolin was blocked (Fig. 5e). In contrast, EGF-and IGF-1-stimulated DNA binding activity of a transcription factor not regulated by GSK3β, early growth response-1 (EGR-1, also called zif268, Krox24, NGF1A) (Fig. 5f), was unaffected by the overexpression of GSK3β. These results further support the conclusion that GSK3β is an inhibitory modulator of CREB.
Fig. 5.
Stimulation of CREB DNA binding activity is impaired in SH-SY5Y cells overexpressing GSK3β. (a) Cell lysates from wildtype SH-SY5Y cells (wt), vector-transfected SH-SY5Y cells (Vec), and four cell lines stably overexpressing HA-GSK3β (1, 3, 7, and 9) were immunoblotted for GSK3β. (b) Serum-starved (24 h) wild-type SH-SY5Y cells and GSK3β-overexpressing cells were treated without (basal) or with EGF (50 ng/mL; 1 h) or IGF-1 (50 ng/mL; 1 h) and lysates were immunoblotted for phospho-serine-473-Akt. (c) Serum-starved (24 h) wild-type SH-SY5Y cells and GSK3β-overexpressing cells were treated without (basal) or with EGF (50 ng/mL; 1 h), IGF-1 (50 ng/mL; 1 h), or lithium (1 mm; 1 h) and lysates were immunoblotted for phospho-serine-133-CREB. (d) The association of phospho-serine-133-CREB with CBP was measured by immunoprecipitating CBP from serum-starved (24 h) GSK3β-overexpressing SH-SY5Y cells treated without or with lithium (20 mm; 30 min), EGF (50 ng/mL; 30 min), or IGF-1 (50 ng/mL; 30 min) and immunoblotting phospho-serine-133-CREB, as described in the Materials and methods. (e) A representative CREB EMSA is shown obtained with GSK3β-overexpressing cells and quantitative data is shown of CREB DNA binding activity in nuclear extracts prepared from wild-type and GSK3β-overexpressing cells (cell lines 1, 3, 7, and 9) incubated without serum (24 h) and treated without (basal) or with EGF (50 ng/mL; 1 h), IGF-1 (50 ng/mL; 1 h), or forskolin (Fsk; 10µm; 1 h). Quantitative values are expressed as the percent of basal CREB DNA binding activity in serum-starved cells. Mean ± SEM, n = 4–6. (f) EGR-1 DNA binding activity was measured by EMSA in nuclear extracts prepared from wild-type and GSK3β-overexpressing cells incubated without serum (24 h) and treated without (basal) or with EGF (50 ng/mL; 1 h) or IGF-1 (50 ng/mL; 1 h). ns signifies non-specific binding.
To confirm that the GSK3β overexpression-induced inhibition of growth factor-stimulation of CREB was not specific to one time point of treatment, the time courses of IGF-1- and EGF-stimulated CREB DNA binding activity were measured in wild-type, vector-transfected, and GSK3β-overexpressing cells. Treatment of wild-type and vector-transfected SH-SY5Y cells with EGF or IGF-1 resulted in robust, time-dependent increases in CREB DNA binding activity, which increased three- to fivefold within 15 min of treatment and remained significantly elevated for at least 60 min (Fig. 6a). In contrast, in SH-SY5Y cells overexpressing GSK3β, stimulation of CREB DNA binding activity in response to EGF or IGF-1 was completely blocked throughout 60 min of treatment. To further confirm that the impaired activation of CREB DNA binding activity could be due to inadequate inhibition of GSK3β following growth factor treatments, GSK3β activity was measured in GSK3β-overexpressing SH-SY5Y cells before and after treatments with EGF or IGF-1. EGF and IGF-1 treatment reduced GSK3β activity in GSK3β-overexpressing cells, but the activity of GSK3β remained well above the activity levels in control cells (Fig. 6b) This Finding is consistent with the conclusion that GSK3β negatively regulates CREB activity.
Fig. 6.
Growth factor stimulation of CREB DNA binding activity is blocked at multiple time points in SH-SY5Y cells overexpressing GSK3β. (a) CREB DNA binding activity was measured in nuclear extracts prepared from wild-type, vector-transfected, and HAGSK3β-overexpressing SH-SY5Y cells incubated without serum (24 h) and treated without or with EGF (50 ng/mL) or IGF-1 (50 ng/ mL). Quantitative values are expressed as the percent of basal CREB DNA binding activity in serum-starved cells. Mean ± SEM, n = 3–4. (b) GSK3β activity was measured by immunoprecipitating GSK3β, incubating the immunoprecipitated GSK3β with recombinant human tau and [32P]ATP, and measuring the phosphorylation of tau, as described in Materials and methods, in wild-type (wt) cells, or in HA-GSK3β-overexpressing cells (HA-GSK3β) incubated without serum (24 h) and treated without (basal) or with EGF (50 ng/mL; 1 h) or IGF-1 (50 ng/mL; 1 h).
Inhibitors of GSK3β restore stimulation of CREB DNA binding activity in cells overexpressing GSK3β
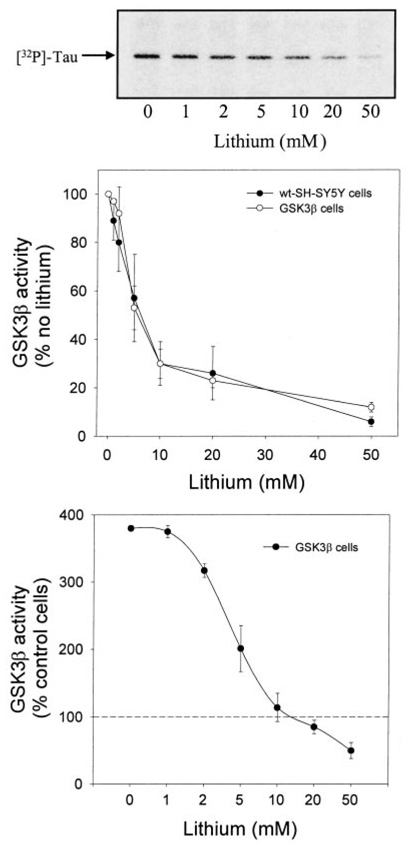
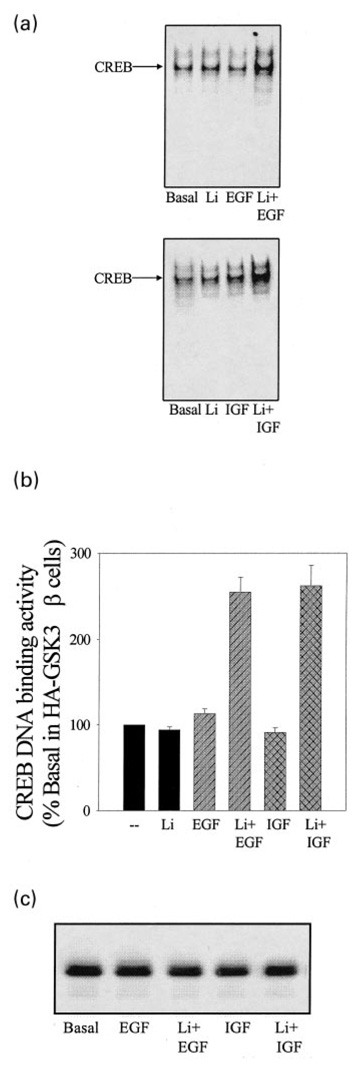
To test further if increased GSK3β activity caused the attenuation of growth factor-stimulated CREB activity in GSK3β-overexpressing cells, experiments were carried out with lithium to inhibit GSK3β. To determine the concentration of lithium necessary to reduce the GSK3β activity to below that present in wild-type SH-SY5Y cells, the lithium concentration-dependent inhibition of GSK3β activity was measured in GSK3β immunoprecipitates from GSK3β-overexpressing SH-SY5Y cells. As reported previously (Klein and Melton 1996; Stambolic et al. 1996), lithium directly inhibited GSK3β activity in these in vitro assays, and the percentage inhibition of GSK3β caused by incubation with 1–50 mm lithium was equivalent using wild-type SH-SY5Y cells or GSK3β-overexpressing cells (Fig. 7). The lithium concentration-dependent inhibition of GSK3β revealed that in GSK3β-overexpressing cells 20 mm lithium was needed to reduce the GSK3β activity to a level below that in wild-type untreated cells (i.e. the overexpressed GSK3β activity needed to be inhibited by approximately 75% to achieve an activity equivalent to that in wild-type cells). Based on this result, a concentration of 20 mm lithium was used to inhibit GSK3β activity in cells overexpressing GSK3β, although it is possible that the intracellular lithium concentration is less than 20 mm at the short incubation times used. Measurements of CREB DNA binding activity in cells overexpressing GSK3β revealed that stimulation by EGF and IGF-1 was restored by pretreatment with lithium (Figs 8a and b). These treatments had no effect on the nuclear level of CREB (Fig. 8c). These findings indicate that inhibition of GSK3β activity is necessary for optimal CREB DNA binding activity.
Fig. 7.
Lithium concentration-dependently inhibits GSK3β activity. GSK3β activity was measured by immunoprecipitating GSK3β from serum-starved (24 h) wild-type (wt) and GSK3β-overexpressing SH-SY5Y cells, incubating the immunoprecipitated GSK3β with recombinant human tau, [32P]ATP, and lithium, and measuring the phosphorylation of tau, as described in Materials and methods. Shown is a representative autoradiograph from an experiment with GSK3β-overexpressing cells, and quantitative values from both cell types expressed as the percent of GSK3β activity in the respective celltype in the absence of lithium, and values from GSK3β-overexpressing cells also are shown expressed as the percent of GSK3β activity in wild-type cells. Mean ± SEM, n = 3 experiments.
Fig. 8.
Lithium restores growth factor stimulation of CREB DNA binding activity in SH-SY5Y cells overexpressing GSK3β. (a) CREB DNA binding activity was measured by EMSA in nuclear extracts prepared from GSK3β-overexpressing cells incubated without serum (24 h) and treated without or with EGF (50 ng/mL; 1 h), or IGF-1 (50 ng/mL; 1 h) alone or following pre-incubation with lithium (Li; 20 mm; 1 h). (b) Quantitative values are expressed as the percent of basal CREB DNA binding activity in serum-starved GSK3β-overexpressing cells. Mean ± SEM, n = 4–6. (c) Nuclear extracts prepared from GSK3β-overexpressing cells incubated without serum (24 h) and treated without or with EGF (50 ng/mL; 1 h), or IGF-1 (50 ng/mL; 1 h) alone or following pre-incubation with lithium (Li; 20 mm; 1 h) were immunoblotted for total levels of CREB.
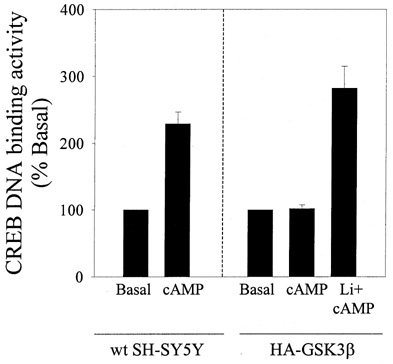
In contrast to the results obtained with EGF and IGF-1, lithium had no effect on the impaired forskolin-induced stimulation of CREB DNA binding activity in cells overexpressing GSK3β (data not shown), indicating that another mechanism accounted for deficient CREB activation induced by forskolin in GSK3β-overexpressing cells. To test if this could result from diminished production of cyclic AMP in these cells, cyclic AMP levels were measured in control and GSK3β-overexpressing cells before and after treatment with 10 µm forskolin. In control SH-SY5Y cells, cyclic AMP levels were increased 4.4-fold by forskolin, from 48 ± 4 to 213 ± 30 nmol/µg protein (n = 7). In contrast, in GSK3β-overexpressing cells the cyclic AMP level was only increased 2.1-fold by forskolin, to 79 ± 1 from a basal level of 38 ± 1 nmol/µg protein (n = 7, measurements made in clones 1, 3, and 7 of GSK3β-overexpressing cells). Thus, deficient forskolin-induced CREB activation in GSK3β-overexpressing cells resulted in part from impaired production of cyclic AMP, rather than solely from an inhibitory effect of GSK3β on CREB. Therefore, to examine if cyclic AMP-induced stimulation of CREB DNA binding activity is inhibited by GSK3β, the cyclic AMP analog, dibutyryl cyclic AMP (diBcAMP), was used in both wildtype and GSK3β-overexpressing SH-SY5Y cells to stimulate CREB DNA binding activity. DiBcAMP stimulated CREB DNA binding activity in wild-type SH-SY5Y cells to 224 ± 16% of the basal activity (Fig. 9). However, diBcAMP stimulation of CREB DNA binding was completely blocked in GSK3β-overexpressing SH-SY5Y cells, but pretreatment of these cells with 20 mm lithium restored diBcAMP stimulation of CREB DNA binding activity (Fig. 9). These findings support the conclusion that GSK3β inhibits cAMP-induced CREB DNA binding activity.
Fig. 9.
Lithium restores cyclic AMP stimulation of CREB DNA binding activity in SH-SY5Y cells overexpressing GSK3β. CREB DNA binding activity was measured by EMSA in nuclear extracts prepared from wild-type (wt) and GSK3β-overexpressing cells incubated without serum (24 h) and treated without (basal) or with diBcAMP (100 µm; 1 h) alone or following pre-incubation with lithium (20 mm; 1 h). Quantitative values are expressed as the percent of basal CREB DNA binding activity in serum-starved cells. Mean ± SEM, n = 2–3.
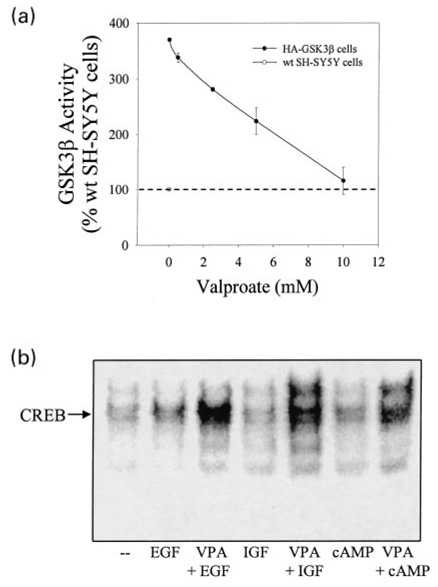
To confirm that the facilitation by lithium of CREB DNA binding activity in GSK3β-overexpressing cells was due to its inhibition of GSK3β, another inhibitor of GSK3β, sodium valproate (Chen et al. 1999) was utilized. In vitro, sodium valproate concentration-dependently inhibited GSK3β activity in GSK3β immunoprecipitates from SH-SY5Y cells overexpressing GSK3β (Fig. 10a). In accordance with the results obtained using lithium, pretreatment of GSK3β-overexpressing cells with sodium valproate restored EGF, IGF-1, and diBcAMP stimulation of CREB DNA binding activity (Fig. 10b). These findings support the conclusion that CREB DNA binding activity is negatively regulated by GSK3β, and demonstrate that this inhibition is reversible by two inhibitors of GSK3β.
Fig. 10.
Valproate concentration-dependently inhibits GSK3β activity and restores growth factor and cyclic AMP stimulation of CREB DNA binding activity in SH-SY5Y cells overexpressing GSK3β. (A) GSK3β activity was measured by immunoprecipitating GSK3β from serum-starved (24 h) GSK3β-overexpressing SH-SY5Y cells, incubating the immunoprecipitated GSK3β with recombinant human tau, [32P]ATP, and valproate, and measuring the phosphorylation of tau, as described in Materials and methods. Quantitative values are expressed as the percent of GSK3β activity in serum-starved wildtype SH-SY5Y cells. Mean ± SEM, n = 3 experiments. (b) CREB DNA binding activity was measured by EMSA in nuclear extracts prepared from GSK3β-overexpressing cells incubated without serum (24 h) and treated without or with EGF (50 ng/mL; 1 h), IGF-1 (50 ng/mL; 1 h), or diBcAMP (100 βM; 1 h) alone or following preincubation with sodium valproate (10 mm; 1 h).
Discussion
CREB is a key transcription factor involved in several critical functions of the brain, including learning, neuronal plasticity, and cell survival (Davis et al. 1996; Deisseroth et al. 1996; Silva et al. 1998; Walton and Dragunow 2000). However, the complex signaling systems that regulate the activity of CREB are incompletely defined. Although CREB is known to be activated by several growth factors that activate the PI3K/Akt signaling pathway, which leads to inhibition of GSK3β, it was unclear if the inhibition of GSK3β by Akt contributes to the regulation of CREB. The results of the present investigation demonstrated with several experimental paradigms that GSK3β is an inhibitory regulator of CREB, that inhibition of GSK3β is necessary for optimal CREB activity induced by growth factors or cyclic AMP, and that inhibition of GSK3β by lithium can facilitate CREB activation.
Phosphorylation of CREB at serine-133 is required for complex formation with CBP, activation of DNA binding, and transcription, since mutation of this residue results in the complete loss of transcriptional activity and the mutated protein can act as a negative regulator of active phosphoserine-133-CREB (Gonzalez and Montminy 1989; Habener et al. 1995). Phosphorylation of CREB at serine-133 also creates the consensus sequence, SXXXS(P), for hierarchical phosphorylation by GSK3β of serine-129, whereas in the absence of phosphorylation at serine-133, CREB is not phosphorylated by GSK3β (Fiol et al. 1994; Wang et al. 1994). Many kinases, including protein kinase A, extracellular- regulated kinases, protein kinase C, Ras-dependent protein kinase, RSK2, and calcium-calmodulin-dependent protein kinase IV, have been shown to activate CREB by phosphorylating serine-133 (Dash et al. 1991; Sheng et al. 1991; Ginty et al. 1994; Xing et al. 1996), thus priming CREB as a substrate for GSK3β-mediated phosphorylation. This raised the question of what effect secondary phosphorylation by GSK3β has on CREB function. One possible function would be for phosphorylation of serine-129 of CREB by GSK3β to synergize with serine-133 phosphorylation to fully activate CREB. Another possibility would be for phosphorylation by GSK3β to act as a signal for termination of CREB activity. Evidence for each of these opposing actions has been reported in the only two previous investigations of the functional consequences of GSK3β-mediated phosphorylation of CREB. The results of Fiol et al. (1994) indicated that GSK3β-mediated phosphorylation of CREB at serine-129 is necessary for CREB function, since expression of CREB with a mutation of serine-129 to alanine in PC12 cells was refractory to stimulation by cyclic AMP, and activation of a Gal4-CREB reporter was greatly induced by cotransfection of GSK3β in F9 cells. The findings of Bullock and Habener (1998) also demonstrated the hierarchical phosphorylation of CREB by GSK3β, but they reported an opposite functional outcome of this phosphorylation. Phosphorylation of CREB by protein kinase A increased the DNA binding of CREB, whereas secondary phosphorylation of primed CREB by GSK3β attenuated protein kinase A stimulation of CREB DNA binding activity, a finding that was attributed to phosphorylation-mediated changes in structure and net charge of CREB (Bullock and Habener 1998). Bullock and Habener (1998) attributed the conflicting results of Fiol et al. (1994) to methodological differences, although this does not entirely explain the stimulatory effects of GSK3β that were observed. The results reported here support the conclusion of Bullock and Habener (1998), that phosphorylation of serine-129 by GSK3β provides inhibitory regulation of CREB DNA binding. This was evident in the present investigation from the increased CREB DNA binding activity that followed treatments that inhibit GSK3β, either indirectly through activation of the PI3K/Akt signaling pathway or directly by lithium, and from the decreased CREB DNA binding activity evident in cells with increased GSK3β activity, an attenuation that was reversed by inhibition of GSK3β. Thus, these results support the conclusion that phosphorylation of CREB by GSK3β causes inactivation of the transcription factor.
These findings indicate that stimulation of growth factor receptors and of receptors coupled to increased production of cyclic AMP each activate two signaling systems that regulate CREB activation. Each of these receptors activates a signaling pathway that stimulates kinases (e.g. RSK2, cyclic AMP-dependent protein kinase) that phosphorylate serine-133 of CREB, causing its activation. Each receptor also activates a signaling pathway that inhibits GSK3β, thus blocking its inhibitory effect on CREB. This is accomplished by growth factor receptors through activation of the PI3K/Akt signaling pathway, in which Akt phosphorylates serine-9 of GSK3β to inhibit its activity. Additionally, recent reports indicate that activation of cyclic AMP-dependent protein kinase can cause inhibition of GSK3β by two mechanisms, by direct phosphorylation and activation of Akt which can inhibit GSK3β (Filippa et al. 1999), and by direct phosphorylation of serine-9 of GSK3β (Fang et al. 2000; Li et al. 2000). Thus, dual pathways are recruited by these receptors, one to stimulate CREB phosphorylation and one to block the inhibitory influence of GSK3β, in order to optimize the activation of CREB.
In 1996, lithium, the primary agent used in the treatment of bipolar disorder (manic-depressive illness), was found to be a direct inhibitor of GSK3β (Klein and Melton 1996; Stambolic et al. 1996). Since lithium inhibits both GSK3β and the closely related enzyme GSK3α (Stambolic et al. 1996), it is important to recognize that many of the effects of lithium that are attributed to its inhibition of GSK3β may very well be due to inhibition of both forms of GSK. The specificity of lithium’s inhibitory effect on GSK3β was further validated by Davies et al. (2000) who reported in an extensive study of 24 different kinases that no other kinase was inhibited more than GSK3β by lithium, although mild inhibition by lithium of casein kinase 2 and mitogenactivated protein kinase-activated protein kinase 2 was noted. The finding that lithium selectively inhibits GSK3β provided a possible mechanism for the therapeutic action of lithium and initiated the now widespread use of lithium as an effective tool to identify specific actions of GK3β. The further discovery that GSK3β is directly inhibited by sodium valproate (Chen et al. 1999) provided a second agent with which the actions of GSK3β could be verified, and added further support to the possibility that inhibition of GSK3β may be therapeutically important in the treatment of bipolar disorder because sodium valproate is now widely used in the treatment of this illness (Li et al. 2001b). Thus, the findings that treatment of cells with lithium or sodium valproate facilitated CREB DNA binding activity support the conclusion that GSK3β is inhibitory. Previous findings of Ozaki and Chuang (1997) are in accordance with this conclusion, since they reported that lithium treatment increased CREB DNA binding activity in cerebellar granule cells, although based on the literature available at that time this effect was correlated to effects of lithium on protein kinase C. However, in a similar study Wang et al. (1999) did not observe increased CREB activation in SH-SY5Y cells treated with lithium. This difference is likely attributable to the use by Wang et al. (1999) of cells maintained in serum-containing media, a condition that maintains a low level of GSK3β activity and a condition in which we also found little effect of lithium. Thus, in the present study it was found that lithium increased CREB DNA binding activity only when GSK3β was not adequately inhibited, i.e. in cells maintained in serum-free media and in cells overexpressing GSK3β. Our findings that sodium valproate, which also inhibits GSK3β, also facilitated CREB activation further supports the conclusion that GSK3β is inhibitory towards CREB activity. This common action of lithium and sodium valproate further suggests that inhibition of GSK3β and the consequential facilitation of CREB activation may be of therapeutic relevance since it identifies an outcome common to the two most prevalently used agents to treat bipolar disorder.
The inhibitory effect of GSK3β on CREB should be considered in context with other actions of GSK3β and potential outcomes on cell function. GSK3β has been reported to negatively regulate, through phosphorylation, the activity of a diverse array of transcription factors, including AP-1 (Boyle et al. 1991), heat shock factor-1 (Chu et al. 1996; He et al. 1998; Bijur and Jope 2000), β-catenin (Rubinfeld et al. 1996; Yost et al. 1996), nuclear factor of activated T-cells (Beals et al. 1997), Myc (Henriksson et al. 1993), and NF-κB (Bournat et al. 2000), as recently reviewed (Grimes and Jope 2001). Thus, the control of GSK3β activity contributes to the regulation of many transcription factors in addition to CREB, and thereby must be a central figure in the regulation of gene expression. From this, it appears likely that GSK3β-induced down-regulation of these diverse transcription factors likely contributes to the multiple modes of apoptosis that are facilitated by GSK3β (Pap and Cooper 1998; Bijur et al. 2000; Hetman et al. 2000), and that the protective effect of lithium against a remarkably broad array of insults is likely to result, in part, from its inhibition of GSK3β (reviewed in Jope 1999; Grimes and Jope 2001). However, preceding cell death, inadequate inhibition of GSK3β likely contributes to neuronal dysfunction, a condition able to be ameliorated by lithium. Additional studies are necessary to further understand the role of CREB and other transcription factors regulated by GSK3β in the apoptotic-facilitating and neuronal function-impairing actions of GSK3β, in mood disorders, and in the neuroprotection conferred by lithium.
Acknowledgements
This research was supported by National Institutes of Health grant MH38752 and a grant from the Alzheimer’s Association. The authors thank Dr Gail V. W. Johnson (University of Alabama at Birmingham) for generously providing purified recombinant tau protein.
Abbreviations used
- AP-1
activator protein-1
- BCA
bicinchoninic acid
- CBP
CREB-binding protein
- CRE
cyclic AMP response element
- CREB
cyclic AMP response element binding protein
- diBcAMP
dibutyryl cyclic AMP
- EGF
epidermal growth factor
- EGR-1
early growth response-1
- EMSA
electrophoretic mobility shift assay
- GSK3β
glycogen synthase kinase-3β
- HSF-1
heat shock factor-1
- IGF-1
insulin-like growth factor-1
- NF-κB
nuclear factor-kappa B
- PI3K
phosphatidylinositol-3-kinase
- SDS—PAGE
sodium dodecyl sulfate—polyacrylamide gel electrophoresis
References
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1933. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3β in the regulation of HSF-1 activity. J. Neurochem. 2000;75:2401–2408. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- Bijur GN, DeSarno P, Jope RS. Glycogen synthase kinase-3β facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J. Biol. Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- Bournat JC, Brown AMC, Soler AP. Wnt-1 dependent activation of the survival factor NF-kB in PC12 cells. J. Neurosci. Res. 2000;61:21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Smeal T, Defize LH, Angel P, Woodgett JR, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNAbinding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bullock BP, Habener JF. Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA binding affinity, conformation, and increases net charge. Biochemistry. 1998;37:3795–3809. doi: 10.1021/bi970982t. [DOI] [PubMed] [Google Scholar]
- Cardinaux JR, Notis JC, Zhang Q, Vo N, Craig JC, Fass DM, Brennan RG, Goodman RH. Recruitment of CREB binding protein is sufficient for CREB-mediated gene activation. Mol. Cell. Biol. 2000;20:1546–1552. doi: 10.1128/mcb.20.5.1546-1552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J. Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J. Biol. Chem. 1996;271:30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Dash PK, Karl KA, Colicos MA, Prywes P, Kandel ER. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc. Natl Acad. Sci. USA. 1991;88:5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. III. CREB is necessary for presynaptic functional plasticity. Neuron. 1996;17:669–679. doi: 10.1016/s0896-6273(00)80199-3. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus. postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMPdependent protein kinase and phosphorylase kinase. Eur. J. 1980;107:519–527. [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl Acad. Sci. USA. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkey DM, Kimelman D. GSK-3. New thoughts on an old enzyme. Dev. Biol. 2000;225:471–479. doi: 10.1006/dbio.2000.9816. [DOI] [PubMed] [Google Scholar]
- Filippa N, Sable CL, Filloux C, Hemmings B, Van Obberghen E. Mechanism of protein kinase B activation by cyclic AMP-dependent protein kinase. Mol. Cell. Biol. 1999;19:4989–5000. doi: 10.1128/mcb.19.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol CJ, Haseman JH, Wang YH, Roach PJ, Roeske RW, Kowalczuk M, DePaoli-Roach AA. Phosphoserine as a recognition determinant for glycogen synthase kinase-3. phosphorylation of a synthetic peptide based on the G-component of protein phosphatase-1. Arch. Biochem. Biophys. 1987;267:797–802. doi: 10.1016/0003-9861(88)90089-6. [DOI] [PubMed] [Google Scholar]
- Fiol CJ, Williams JS, Chou CH, Wang QM, Roach PJ, Andrisanni OM. A secondary phosphoryation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in control of gene expression. J. Biol. Chem. 1994;269:32187–32193. [PubMed] [Google Scholar]
- Ginty DD, Bonni A, Greenberg ME. Nerve growth factor activates Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77:713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. Cholinergic stimulation of early growth response-1 DNA binding activity requires protein kinase C and mitogen-activated protein kinase kinase activation and is inhibited by sodium valproate in SH-SY5Y cells. J. Neurochem. 1999;73:1384–1392. doi: 10.1046/j.1471-4159.1999.0731384.x. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase-3β in cellular signaling. Prog. Neurobiol. 2001 doi: 10.1016/s0301-0082(01)00011-9. in press. [DOI] [PubMed] [Google Scholar]
- Habener JF, Miller CP, Vallejo M. cAMP-dependent regulation of gene transcription by cAMP response element-binding protein and cAMP response element modulator. Vitam. Horm. 1995;51:1–57. doi: 10.1016/s0083-6729(08)61037-7. [DOI] [PubMed] [Google Scholar]
- He B, Meng YH, Mivechi NF. Glycogen synthase kinase 3β and extracellular signal-regulated kinase inactivate heat shock transcription factor 1 by facilitating the disappearance of transcriptionally active granules after heat shock. Mol. Cell. Biol. 1998;18:6624–6633. doi: 10.1128/mcb.18.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson M, Bakardjiev A, Klein G, Luscher B. Phosphorylation sites mapping the N-terminal domain of c-myc modulate its transforming potential. Oncogene. 1993;8:3199–3209. [PubMed] [Google Scholar]
- Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3β in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 2000;20:2567–2674. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean D, Harbison M, McConkey DJ, Ronai Z, Bar-Eli M. CREB and its associated proteins act as survival factors for human melanoma cells. J. Biol. Chem. 1998;273:24884–24890. doi: 10.1074/jbc.273.38.24884. [DOI] [PubMed] [Google Scholar]
- Ji L, Mochon E, Arcina M, Boxer LM. CREB proteins function as positive regulators of the translocated bcl-2 allele in t(14; 18) lymphomas. J. Biol. Chem. 1996;271:22687–22691. doi: 10.1074/jbc.271.37.22687. [DOI] [PubMed] [Google Scholar]
- Jope RS. Anti-bipolar therapy. mechanism of action of lithium. Mol. Psychiatry. 1999;4:117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr. Opin. Genet. Dev. 2000;10:508–514. doi: 10.1016/s0959-437x(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc. Natl Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesort M, Jope RS, Johnson GVW. Insulin biphasically modulates tau phosphorylation. involvement of glycogen synthase kinase-3β and fyn tyrosine kinase. J. Neurochem. 1999;72:576–584. doi: 10.1046/j.1471-4159.1999.0720576.x. [DOI] [PubMed] [Google Scholar]
- Li X, Bijur GN, Jope RS. Glycogen synthase 3β, mood stabilizers, and neuroprotection. Bipolar Disorders. 2001a doi: 10.1034/j.1399-5618.2002.40201.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ketter TA, Frye MA. Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders? J. Affective Disorders. 2001b doi: 10.1016/s0165-0327(00)00361-x. in press. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Chuang D-M. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J. Neurochem. 1997;69:2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR. Glycogen synthase kinase-3. functions in oncogenesis and development. Biochim. Biophys. Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Miller E, Sable C, Young P, Heidenreich KA, Boxer LM, Reusch JE-B. Insulin-like growth factor-I induces bcl-2 promoter through the transcription factor cAMP-response element binding protein. J. Biol. Chem. 1999;274:27529–27535. doi: 10.1074/jbc.274.39.27529. [DOI] [PubMed] [Google Scholar]
- Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri R, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Tafuri A, Gass P, Hammerling GJ, Arnold B, Schutz G. Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc. Natl Acad. Sci. USA. 1998;95:4481–4486. doi: 10.1073/pnas.95.8.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylatt DB, Aitken A, Bilham T, Condon GD, Embi N, Cohen P. Glycogen synthase from rabbit skeletal muscle. Amino acid sequence at the sites phosphorylated by glycogen synthase kinase-3, and extension of the N-terminal sequence containing the site phosphorylated by phosphorylase kinase. Eur. J. Biochem. 1980;107:529–537. [PubMed] [Google Scholar]
- Sayas CL, Moreno-Flores MT, Avila J, Wandosell F. The neurite retraction induced by lysophosphatidic acid increases Alzheimer’s disease-like Tau phosphorylation. J. Biol. Chem. 1999;274:37046–37052. doi: 10.1074/jbc.274.52.37046. [DOI] [PubMed] [Google Scholar]
- Sheng M, Thomspon MA, Greenberg ME. CREB. A Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Franklan PW, Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Walton MR, Dragunow I. Is CREB a key to neuronal survival; Trends Neurosci. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- Walton M, Sirimanne E, Williams C, Gluckman P, Dragunow M. The role of cyclic AMP-responsive element binding protein (CREB) in hypoxic-ischemic brain damage and repair. Mol. Brain. Res. 1996;43:21–29. doi: 10.1016/s0169-328x(96)00144-1. [DOI] [PubMed] [Google Scholar]
- Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. Glycogen synthase kinase-3β is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J. Biol. Chem. 1994;269:14566–14574. [PubMed] [Google Scholar]
- Wang JF, Asghari V, Rockel C, Young LT. Cyclic AMP responsive element binding protein phosphorylation and DNA binding is decreased by chronic lithium but not valproate treatment of SH-SY5Y neuroblastoma cells. Neuroscience. 1999;91:771–776. doi: 10.1016/s0306-4522(98)00627-7. [DOI] [PubMed] [Google Scholar]
- Wilson BE, Mochon E, Boxer LM. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol. Cell. Biol. 1996;16:5546–5556. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factorregulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]