Abstract
Click-evoked otoacoustic emissions (CEOAEs) are echo-like waveforms emitted by normal-hearing cochleas in response to a brief transient. CEOAEs are known to be stronger in females than in males. In this experiment, the CEOAEs of homosexual and bisexual females were found to be intermediate to those of heterosexual females and heterosexual males. A parsimonious explanation is that the auditory systems of homosexual and bisexual females, and the brain structures responsible for their sexual orientation, have been partially masculinized by exposure to high levels of androgens prenatally. No difference in CEOAEs was observed between homosexual and heterosexual males.
Evidence continues to accumulate about the biological concomitants of human homosexuality. To date, homosexual males have been reported to have larger suprachiasmatic nuclei of the hypothalamus (1), smaller interstitial nuclei of the anterior hypothalamus (2), larger anterior commissures (3), and thicker isthmuses of the corpus callosum (4) than heterosexual males. (Which, if any, of these concomitants is actually a substrate for homosexual behavior–as distinguished from a structure that simply covaries with homosexual behavior and its structural substrates–is yet to be determined.) Supplementing the findings about differences in brain structure are findings about the heritability of homosexuality. Studies of twins suggest heritability values of about 0.4–0.7 for homosexuality in both males and females (5, 6), and there is evidence for a linkage between certain markers on the X chromosome and sexual orientation in males but not in females (7, 8).
With the exception of the heritability study on twins (6), all of the current evidence for biological concomitants of homosexuality is for males only. Here, we report that the peripheral auditory systems of homosexual females differ from those of heterosexual females, and the nature of the difference is consistent with the idea that homosexual females are exposed to higher levels of androgens prenatally than are heterosexual females.
Otoacoustic emissions (OAEs) are weak sounds produced by elements in the inner ear (refs. 9 and 10; for a review, see ref. 11). These sounds can be measured using a miniature microphone attached to a probe tip inserted into the external ear canal. There are several types of OAE, but only two will be considered in this paper. Click-evoked otoacoustic emissions (CEOAEs) are echo-like waveforms that are emitted in response to a brief transient sound. A click of about one-tenth of a millisecond in duration can produce a CEOAE lasting tens of milliseconds in any person having a normal cochlea and middle-ear system. Because CEOAE waveforms are weak, the responses to many clicks must be averaged for a CEOAE waveform to be obtained. CEOAE waveforms differ considerably across ears, but seem to be stable within an ear (12, 13). Spontaneous otoacoustic emissions (SOAEs) are tonal or narrow-band sounds that are continuously emitted by an ear in the absence of eliciting acoustic stimulation. The number of SOAEs exhibited by an ear can range from zero to several dozen (14), but the pattern of SOAEs in a particular ear seems to be quite stable through life (15, 16). Typically, SOAEs are not heard by their owners (11) and thus are not the basis for problem tinnitus.
OAEs can be diminished, temporarily or permanently, by exposures to intense sounds (17), certain drugs (e.g., refs. 18 and 19), and other manipulations that also produce a concomitant hearing loss (11). The relationships between OAEs and the common behavioral measures of hearing are still being worked out (e.g., refs. 12 and 20), but it is known that the hearing sensitivity of people having several SOAEs is slightly better than that of people having none (21). OAEs have recently come into use for screening newborn infants for hearing loss (22). CEOAEs and SOAEs both have heritabilities of about 0.75 (14, 23).
There are sex and ear differences in OAEs just as in other auditory measures (see ref. 24 for a summary). For example, the CEOAEs from females are stronger than those from males, and the CEOAEs from right ears are stronger than those from left ears (13). Similarly, the SOAEs from females and right ears are more numerous than those from males and left ears (14). This pattern of sex and ear differences is present in infants and children as well as in adults (25, 26), bolstering the idea that characteristic, idiosyncratic OAEs are established at birth and remain (reasonably) constant through life—at least as long as the cochlea remains normal.
One line of evidence suggests how the sex differences in OAEs might be implemented. Females from opposite-sex dizygotic (OSDZ) twin pairs have both CEOAEs and SOAEs that are more like those of males than those of other females (13, 27). We have proposed that both this outcome and the existing sex difference in OAEs can be explained by assuming that the exposure of a fetus to high levels of androgens diminishes its OAEs. Male fetuses naturally produce high levels of androgens at specific points in prenatal development (e.g., ref. 28) and thus are responsible for diminishing their own OAEs. OSDZ females may be exposed to higher-than-normal levels of androgens in the amniotic fluids because of diffusion out of their male co-twins; such a mechanism is known to operate in other mammals (29, 30). That is, our proposal was that the auditory systems of OSDZ females have been partially masculinized by virtue of exposure to androgens produced by their male co-twins. If this explanation were correct, and if the mechanisms producing homosexuality also depend upon prenatal androgen levels, then it was possible that OAEs might differ in heterosexuals and homosexuals.
METHODS
Subjects were recruited by contacting local gay organizations, by advertising in gay publications, in the university newspaper, and on public bulletin boards, as well as by word of mouth. All ads stipulated that subjects would be paid $30 for about 2 h of work. Potential subjects were informed in advance about the essence of the experiment and that there would be a required questionnaire containing items about the subject’s sexual experiences and orientation, among other topics. They were also warned against the use of various common drugs and against exposure to intense sounds in the 24 h preceding the test session. Informed consent was obtained upon the subject’s arrival at the laboratory, and hearing was tested using a screening audiometer (Maico, model MA 40). Subjects having hearing worse than 20 dB hearing level (HL) at any octave frequency between 250 and 8000 Hz were dismissed from the experiment and paid for time served. Seventeen females and 24 males failed the hearing screening out of a total of 291 subjects. Subjects having nasal congestion or revealing noncompliance with the drug or noise restrictions were rescheduled. The average ages (and standard deviations) were 21.7 (2.4), 23.6 (5.3), 21.5 (2.1), 22.7 (3.5), 24.0 (4.1), and 22.7 (3.7) for the female heterosexuals, homosexuals, and bisexuals and male bisexuals, homosexuals, and heterosexuals, respectively.
Sexual orientation was determined by consistency of response on several questionnaire items. One item asked directly whether the person was heterosexual, homosexual, or bisexual. Two others were the Kinsey items on sexual fantasies and experiences modeled on ref. 31. The rare uncertainties about classification were resolved by consulting additional items asking about ongoing or previous relationships. All decisions about subjects’ sexual orientation were made in ignorance of the subjects’ emissions data.
For odd-numbered subjects, OAEs were measured before the questionnaire was completed, and for even-numbered subjects this order was reversed. Also, for the odd-numbered subjects, OAEs were measured in the right ear before the left, and this order was reversed for the even-numbered subjects. In addition to CEOAEs, measures of SOAEs were also obtained and will be reported elsewhere.
CEOAE waveforms were collected using an Etymotics ER-10A insert microphone followed by an ER10–72 pre-amplifier and a custom-built low-noise amplifier/filter combination. The latter provided about 30 dB of gain and high-pass filtered the waveform at about 400 Hz to remove extraneous body noises. The output of the amplifier/filter went to an analog-to-digital converter (National Instruments A2100) located in a Macintosh Quadra 950 computer, where the CEOAE waveform was digitized (with 16-bit resolution) at a sampling rate of 48,000 sample points per second. The ER-10A microphone has a soft foam eartip that allows reasonably secure placement in the concha of the external ear. Passing through the tip was a tube that connected to the Etymotic ER-2 miniature earphone used to present the clicks that elicited the CEOAE waveform. The subject lay on a small cot in a darkened, soundproofed room. To avoid initializing effects (19, 32), the subject was allowed to lie quietly for at least 15 min prior to data collection, with the probe tip in place.
Clicks were presented at a nominal rate of 10 clicks per second (each interclick interval was actually sampled at random from the range 90–110 ms to avoid periodicity in the stimulus). Beginning 4 ms after each click, 40 ms of the echo-like response to the click was collected and summed with the responses from the previous clicks in that sequence. Data collection was postponed by 4 ms to avoid the acoustic ringing in the ear canal and the middle-ear system produced by the click. The strongest click was about 75 dB peSPL, and the others were 6 dB weaker successively. Click levels are specified here as peak-equivalent sound-pressure level (SPL re 20 μPa) or peSPL. This is the SPL of a continuous 1000-Hz tone whose maximum acoustic amplitude is equal to the maximum acoustic amplitude of the 104-μs electrical pulse at the output of the ER-2 earphone as measured in a 2-cc coupler. The level of the click was calibrated for each ear individually by displaying on an oscilloscope the output of the ER-10A microphone while presenting a series of clicks and adjusting their amplitude.
The noise level of the subject was monitored continually, and when it exceeded a predetermined noise criterion, the presentation of the next scheduled click was postponed until the noise level had dropped below the criterion value. Thus, while the nominal presentation rate was 10 clicks per second, the actual rate was slower depending upon the noisiness of the subject. The criterion value was established for each ear of each subject individually by collecting a series of 21.3-ms samples (1024 points) of the ambient noise in the ear canal (one such sample every 100 ms for 20 sec) in the absence of the click stimuli. The median and SD of the rms values of these samples were determined, and the noise criterion was set at 0.25 SD above the median. Before a click was to be presented, the rms output of the microphone system was again determined for a 21.3-ms sample, and the scheduled click was presented only if that rms value was below that subject’s noise criterion. A similar procedure was used to reject individual responses to the clicks. At each click level, a series of clicks was presented for 20 sec at 10 clicks per second, without regard to the subject’s noise criterion. The highest peak amplitude during the 40-ms interval following each click was recorded, and the median and SD of these peak amplitudes were determined. During the data-collection process, a response to a click was discarded if its highest peak amplitude exceeded the median plus 0.25 SD as determined during that 20-sec calibration period. Clicks were presented at each level until 250 responses satisfying the collection criteria were accumulated.
To characterize the averaged CEOAE responses, a 21.3-ms segment was taken from each averaged CEOAE waveform beginning 2 ms into the waveform and then filtered from 1–5 kHz. An rms value was calculated in volts, and the result was converted to dB SPL. These SPL values were averaged across subjects to summarize the strength of response in each group. Note that this is not the difference waveform commonly used to characterize the nonlinear component of the CEOAE (see ref. 11). [High-pass filtering the CEOAE waveform at 500 Hz instead of 1000 Hz led to higher variability for all subject groups, in accord with the comparatively high noise level observed at that lower frequency (20).]
Discarded from all analyses were the data from four heterosexual females, two homosexual females, one bisexual male, five homosexual males, and one heterosexual male because of peculiarities in their CEOAE waveforms. Criteria for exclusion included a response weaker than about 1 dB SPL at the highest click level, low cross-correlations between the CEOAE waveforms obtained with different click levels, and rapidly declining cross-correlations as the 21.3-ms analysis window was delayed beyond 6 ms. The latter effect is indicative of simple acoustical ringing in the closed external ear canal rather than a CEOAE response. A more stringent hearing screening test might have eliminated some of these subjects before data collection. For technical reasons, data at one click level in one ear were missing for one heterosexual female and two homosexual males, for whom estimated values were generated using both the available data for those subjects and the average data of the other subjects in their group.
RESULTS
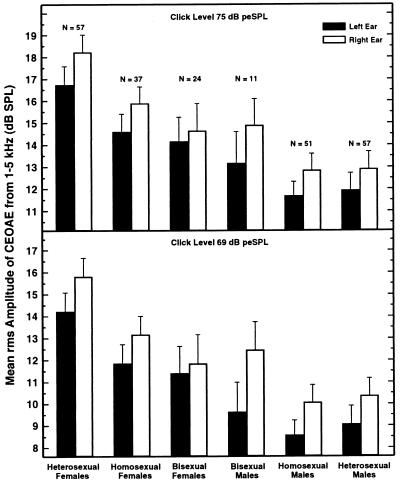
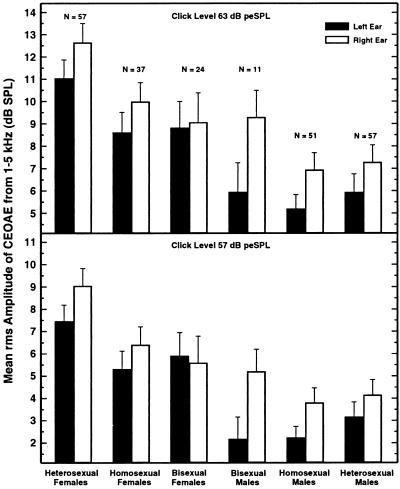
Data are reported for 237 subjects. Shown in Fig. 1 are the mean SPLs of the averaged CEOAE waveforms for the six groups of subjects for two of the four click levels used. The results for the other two click levels are shown in Fig. 2. The pattern of the data was generally similar at all click levels, with CEOAE magnitude declining by approximately 3 dB for each 6-dB decrease in click level. (Note the change in range of ordinate values across the panels of the two figures.)
Figure 1.
The rms amplitude in the averaged CEOAE waveforms for the two highest click levels tested (75 and 69 dB peSPL), averaged across all subjects in each group. The analysis window was from 6 to 27.3 ms following the presentation of the click. Responses to 250 clicks were collected for each click level. The error bars indicate one standard error.
Figure 2.
The rms amplitude in the averaged CEOAE waveforms for the two lowest click levels tested (63 and 57 dB peSPL). Otherwise, details are the same as for Fig. 1.
Immediately evident in the data of Figs. 1 and 2 are sex and ear differences, both of which were expected from past research. The CEOAEs of heterosexual females were substantially stronger than those of heterosexual males, just as in a previous experiment in which subjects were sorted only by phenotypical sex, not by sexual orientation (13). Also in accord with past experiments, CEOAEs were clearly stronger in the right ear than in the left, and this was true for all subject groups–except at the weakest click level where the bisexual females displayed a 0.3-dB reversal (Fig. 2 Lower).
Far more interesting than these confirmatory findings is the evidence in Figs. 1 and 2 for differences in CEOAEs in homosexuals and heterosexuals. The pattern of results was different for the two sexes. For females, the mean CEOAE amplitudes were similar for the homosexuals and bisexuals, but both were smaller than the mean amplitude for the heterosexuals. That is, it seems that CEOAE magnitude is related to sexual orientation in females. For males, the mean CEOAE amplitudes were no different for the homosexuals and heterosexuals. There was an indication that some male bisexuals might have slightly stronger CEOAEs than the male heterosexuals and homosexuals, but the number of male bisexuals was small, and this difference did not achieve statistical significance.
A three-factor analysis of variance was conducted on these data; the factors were subject type (6 levels) × ear of test (2 levels) × SPL of the click (4 levels), with repeated measures on the latter two factors. The three main effects were all statistically significant. For subject type, F(5,231) = 7.94, P < 0.0001; for ear of test, F(1,231) = 33.23, P < 0.0001; for SPL of the click, F(3,693) = 3116.6, P < 0.0001.
In regard to the findings of prime interest here, means comparisons revealed that the CEOAEs were significantly stronger in heterosexual females than in homosexual females, F(1,231) = 4.84, P < 0.03, and significantly stronger in heterosexual than bisexual females, F(1,231) = 5.50, P < 0.02. Confirming the expectation obtained from visual inspection of the data, the difference between heterosexual and homosexual males was not significant, F(1,231) = 0.19, P = 0.66, nor was the difference between heterosexual and bisexual males, F(1,231) = 0.34, P = 0.56. Finally, the CEOAEs of heterosexual females were significantly stronger than those of heterosexual males, F(1,231) = 26.8, P < 0.0001, in accord with a previous experiment (13).
In the three-factor ANOVA, the interaction between SPL of click and subject type was significant, F(15,693) = 2.28, P < 0.02, because the slopes of the functions relating mean CEOAE amplitude to click level were slightly more steep than the nominal value of 0.5 for the homosexual females and bisexual males, and slightly less steep for the bisexual females and heterosexual males. Also, the interaction between SPL of click and ear of test was significant, F(3,693) = 3.74, P < 0.03, because, across subject groups, the ear difference was slightly smaller at the highest and lowest click levels (where it was about 1.2 dB) than at the intermediate click levels (where it was about 1.4 dB). The interaction between subject type and ear of test was not significant, F(5,231) = 1.16, P = 0.33, nor was the three-way interaction, F(15,693) = 1.24, P = 0.27. [All statistics were done using SuperANOVA (Abacus Concepts, Berkeley, CA, 1989). The P values given are the Greenhouse–Geisser values.]
Effect sizes were calculated for various pairs of subject groups by dividing the difference between the two group means of interest by the common standard deviation of the scores in the two groups. These calculations were made across click level and using two-ear averages for the individual subjects. The results are shown in Table 1.
Table 1.
Overall effect sizes between subject types calculated across four click levels and using two-ear averages from each subject
| Groups compared | Effect size |
|---|---|
| Heterosexual females/heterosexual males | 0.71 |
| Heterosexual females/homosexual females | 0.37 |
| Heterosexual females/bisexual females | 0.43 |
| Heterosexual females/homosexual plus bisexual females | 0.40 |
| Homosexual females/homosexual males | 0.52 |
| Bisexual females/bisexual males | 0.18 |
| Heterosexual males/homosexual males | 0.07 |
| Heterosexual males/bisexual males | 0.16 |
| Heterosexual plus homosexual males/bisexual males | 0.20 |
DISCUSSION
Multiple explanations can be generated for the weaker CEOAEs in homosexual and bisexual females than in heterosexual females. As noted, OAEs can be diminished by exposures to intense sounds (17), certain drugs (e.g., refs. 18 and 19), and other manipulations (11). Thus, it may be that something in the life styles of homosexual and bisexual females leads them to be exposed to one or more agents that have reduced their CEOAEs, either temporarily or permanently. The only apparent way definitively to rule out this class of possible explanations is longitudinal research beginning early in life, although there may be some special subpopulations of homosexual females in which such factors are minimized. Under this explanation, the presence of weaker CEOAEs in homosexual and bisexual females is a secondary consequence to the lifestyle adopted by the majority of these women and thus is not conceptually different from the hearing loss developed by rock musicians. Accordingly, the relationship between CEOAE strength and sexual orientation in Figs. 1 and 2 might not be expected to hold across cultures and eras. By implication, any lifestyle factor proposed as the basis for the difference in homosexual females would have to be one not operative for homosexual males. [Note that lifestyle explanations of this sort have difficulty accounting for the basic sex differences in OAEs because the same patterns of sex and ear differences exist in infants, children, and adults (25, 26). Also, the possibility that the basic sex differences in OAEs are simply attributable to size differences in the external and middle ears (24) is contradicted by the finding of a difference in OAEs in heterosexual and homosexual females, who exhibit no obvious size differences. Further, although there were age differences among the subject groups here, they were judged to be too small to explain the CEOAE differences found.]
Another explanation for the data in Figs. 1 and 2 deserves serious consideration because it is both parsimonious and in accord with numerous other facts about OAEs (13, 14), the brain (29, 30), and homosexuality (6). It is an extension of the explanation we offered for the weak OAEs in OSDZ females (13, 21, 27), and it depends heavily on two important facts about OAEs already mentioned: OAEs exist in infants and children, where they show the same patterns of sex and ear differences as in adults (25, 26), and OAEs seem to be highly stable through life (13, 15, 16). Specifically, the present CEOAE data can be interpreted as evidence that prenatal exposure to higher-than-normal levels of androgens in homosexual and bisexual females produced a partial masculinization of both their peripheral auditory systems and some brain structures involved with sexual orientation. Unlike possible explanations that appeal to lifestyle differences, this explanation presumes that CEOAE strength and female sexual orientation are both physiologically based and both affected by similar mechanisms operating during prenatal development. The idea of female homosexuality being related to masculinization of critical brain locations is not new (e.g., ref. 33), but it needed experimental support, such as the present finding of an apparent masculinization of the inner ears of female homosexuals and bisexuals.
If the offered interpretation is correct, the overall pattern of CEOAE strength across subject type in Figs. 1 and 2 is the result of a graded progression of exposure to androgens prenatally. Namely, heterosexual females have the strongest CEOAEs because they are exposed to the lowest levels of androgens prenatally. Homosexual and bisexual females, and bisexual, homosexual, and heterosexual males are exposed to successively higher levels, in that order, and their CEOAEs are correspondingly reduced. The emphasis here on androgen exposure is not meant to deny the importance of both androgens and estrogens to both sexes for normal fetal development, including normal development of the cochlea.
We have previously shown that the CEOAEs of opposite-sex dizygotic females are weaker than those of both same-sex dizygotic females and non-twin females (13), and here we have shown that the CEOAEs of homosexual and bisexual females are weaker than those of heterosexual females. If we are correct that the weaker CEOAEs in these two subpopulations of females are attributable to exposure to high levels of androgens prenatally, then one might expect the prevalence of homosexuality to be higher in OSDZ females than in non-OSDZ females. However, the one study known to us on this topic found no elevation in the prevalence of homosexuality in OSDZ females (J. M. Bailey, personal communication). This contradiction admittedly may indicate that our interpretation is wrong, and similar mechanisms are not involved in the two instances. Alternatively, the contradiction may simply reflect a difference in patterns of prenatal androgen exposure. It is intuitive that different brain sites would be masculinized at slightly different times in prenatal development, meaning that androgen levels must be adequately high in each time period for all sites to be masculinized fully. Further, some brain sites might require lower levels of androgens than others to be masculinized fully. Accordingly, it is possible that both OSDZ and homosexual females are exposed to higher-than-normal androgen levels prenatally but that the timing and/or magnitude of the excess exposure is such that there is more masculinization of the auditory systems of the OSDZ females than of the brain structures responsible for their sexual orientation, whereas both sites are affected in the homosexual females. [OSDZ females have been found to be more masculine than same-sex female twins in some behaviors (34) but not in others (35).]
In the literature on possible biological origins of homosexuality, it is frequently suggested that different processes may underlie homosexuality in males and females (e.g., refs. 7, 8, and 33), and the present CEOAE data do seem to support that view. There were significant differences in CEOAEs for the females but not for the males [contradicting the idea that homosexual males have brains that are globally female-like (e.g., G. Dörner, summarized in ref. 33)]. However, when it is appreciated that different timing or concentration of androgen exposure may be necessary for full masculinization in different brain locations, the possibility is raised that the auditory systems of homosexual males might be fully masculinized even though the brain structures responsible for sexual orientation are not. That is, logically, male homosexuality still might be attributable to a deficiency in prenatal androgen exposure at some critical brain site. Indeed, while not statistically significant here, the CEOAEs of the bisexual males suggest that at least some of them may have been exposed to lower-than-normal concentrations of androgens prenatally, leaving their auditory systems—and the brain structures responsible for their sexual orientation—less than fully masculinized.
If, within sexes, homosexuality had multiple ontogenetic origins, that fact might be revealed by greater variability in the data from the homosexual groups than in those from the heterosexual groups. In fact, the difference was in the opposite direction. Although the standard deviations for CEOAE amplitude were generally larger for females than for males, they were invariably the same or smaller for homosexual (or bisexual) females than for heterosexual females, and invariably smaller for homosexual (or bisexual) males than for heterosexual males.
Some clear predictions emerge from the present findings. The incidence of homosexuality is reported to be elevated in females whose mothers took diethylstilbestrol during their pregnancy to prevent miscarriage (36) and in females afflicted with congenital adrenal hyperplasia in infancy (37). Under the prenatal-androgen interpretation offered here, these females should have OAEs that are weaker than those in control females. This implication is currently under test in females with congenital adrenal hyperplasia. Also, the findings in Figs. 1 and 2, in conjunction with past work on emissions in twins (13, 23) and the relationship between emissions and hearing sensitivity (21), suggest that hearing sensitivity should range from best to worst as follows: monozygotic female twins, same-sex dizygotic female twins, heterosexual females, homosexual and bisexual females, (male bisexuals?), OSDZ females, and homosexual and heterosexual males (whether twins or not). Unfortunately, the difference in hearing sensitivity between males and females (for unselected subjects) is only about 3 dB (38), so it will be difficult to test this psychophysical implication from emissions research. Other psychoacoustical tasks do exhibit a larger sex difference than hearing sensitivity (24, 39), but the relationship to emissions is currently unknown.
It should be emphasized that the effects described here are for groups. The individual variability in CEOAE amplitude is considerable, meaning that CEOAEs presently could not be used as an indicator of sexual orientation in individual people. Nonetheless, the present finding, along with the OSDZ findings (13, 27), do suggest that OAEs deserve serious consideration as supplementary, noninvasive measures for the study of developmental processes in brain regions other than the auditory pathway itself.
Acknowledgments
We thank N. L. Callaway, S. Oropeza, L. S. Ramirez, S. Bratcher, C. Furche, and A. Phan for help in recruiting and scheduling subjects, data collection, and data entry. Especially helpful during the planning stages of the experiment were S. E. Finn and J. M. Bailey. This work was supported by Research Grant NIDCD 00153 from the National Institute on Deafness and Other Communication Disorders.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: OAE, otoacoustic emission; CEOAE, click-evoked otoacoustic emission; SOAE, spontaneous otoacoustic emission; OSDZ, opposite-sex dizygotic (twin); SPL, sound-pressure level; peSPL, peak-equivalent sound-pressure level.
References
- 1.Swaab D F, Hofman M A. Brain Res. 1990;537:141–148. doi: 10.1016/0006-8993(90)90350-k. [DOI] [PubMed] [Google Scholar]
- 2.LeVay S. Science. 1991;253:1034–1037. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- 3.Allen L S, Gorski R A. Proc Natl Acad Sci USA. 1992;89:7199–7202. doi: 10.1073/pnas.89.15.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scamvougeras A, Witelson S F, Bronskill M, Stanchev P, Black S, Cheung G, Steiner M, Buck B. Soc Neurosci Abstr. 1994;20:1425. [Google Scholar]
- 5.Bailey J M, Pillard R C. Arch Gen Psychiatry. 1991;48:1089–1096. doi: 10.1001/archpsyc.1991.01810360053008. [DOI] [PubMed] [Google Scholar]
- 6.Bailey J M, Pillard R C, Neale M C, Agyei Y. Arch Gen Psychiatry. 1993;50:217–223. doi: 10.1001/archpsyc.1993.01820150067007. [DOI] [PubMed] [Google Scholar]
- 7.Hamer D H, Hu S, Magnuson V L, Hu N, Pattatucci A M L. Science. 1993;261:321–327. doi: 10.1126/science.8332896. [DOI] [PubMed] [Google Scholar]
- 8.Hu S, Pattatucci A M L, Patterson C, Li L, Fulker D W, Cherny S S, Kruglyak L, Hamer D H. Nat Genet. 1995;11:248–256. doi: 10.1038/ng1195-248. [DOI] [PubMed] [Google Scholar]
- 9.Kemp D T. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- 10.Kemp D T. Arch Otol Rhinol Laryngol. 1979;224:37–45. doi: 10.1007/BF00455222. [DOI] [PubMed] [Google Scholar]
- 11.Probst R, Lonsbury-Martin B L, Martin G K. J Acoust Soc Am. 1991;89:2027–2067. doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- 12.Prieve B A, Gorga M P, Schmidt A, Neely S, Peters J, Schulte L, Jesteadt W. J Acoust Soc Am. 1993;93:3308–3319. doi: 10.1121/1.405715. [DOI] [PubMed] [Google Scholar]
- 13.McFadden D, Loehlin J C, Pasanen E G. Hear Res. 1996;97:102–119. [PubMed] [Google Scholar]
- 14.Talmadge C L, Long G R, Murphy W J, Tubis A. Hear Res. 1993;71:170–182. doi: 10.1016/0378-5955(93)90032-v. [DOI] [PubMed] [Google Scholar]
- 15.Burns E M, Campbell S L, Arehart K H, Keefe D H. Abstr Assoc Res Otolaryngol. 1993;16:98. [Google Scholar]
- 16.Burns E M, Campbell S L, Arehart K H. J Acoust Soc Am. 1994;95:385–394. doi: 10.1121/1.408330. [DOI] [PubMed] [Google Scholar]
- 17.Norton S J, Mott J B, Champlin C A. Hear Res. 1989;38:243–258. doi: 10.1016/0378-5955(89)90069-5. [DOI] [PubMed] [Google Scholar]
- 18.McFadden D, Plattsmier H S. J Acoust Soc Am. 1984;76:443–448. doi: 10.1121/1.391585. [DOI] [PubMed] [Google Scholar]
- 19.McFadden D, Pasanen E G. J Acoust Soc Am. 1994;95:3460–3474. doi: 10.1121/1.410022. [DOI] [PubMed] [Google Scholar]
- 20.Gorga M P, Neely S T, Bergman B M, Beauchaine K L, Kaminski J R, Peters J, Schulte L, Jesteadt W. J Acoust Soc Am. 1993;94:2639–2648. doi: 10.1121/1.407348. [DOI] [PubMed] [Google Scholar]
- 21.McFadden D, Mishra R. Hear Res. 1993;71:208–213. doi: 10.1016/0378-5955(93)90036-z. [DOI] [PubMed] [Google Scholar]
- 22.Norton S J. Am J Otol. 1994;15:4–12. [PubMed] [Google Scholar]
- 23.McFadden D, Loehlin J C. Hear Res. 1995;85:181–198. doi: 10.1016/0378-5955(95)00045-6. [DOI] [PubMed] [Google Scholar]
- 24.McFadden, D. (1998) Dev. Neuropsych., in press.
- 25.Burns E M, Arehart K H, Campbell S L. J Acoust Soc Am. 1992;91:1571–1575. doi: 10.1121/1.402438. [DOI] [PubMed] [Google Scholar]
- 26.Norton, S. J. (1992) J. Acoust. Soc. Am. 91 (Suppl. 1), 2409.
- 27.McFadden D. Proc Natl Acad Sci USA. 1993;90:11900–11904. doi: 10.1073/pnas.90.24.11900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes F I, Boroditsky R S, Winter J S D, Faiman C. J Clin Endocrinol Metab. 1974;38:612–617. doi: 10.1210/jcem-38-4-612. [DOI] [PubMed] [Google Scholar]
- 29.vom Saal F S. J Anim Sci. 1989;67:1824–1840. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- 30.Clark, M. M. & Galef, B. G., Jr. (1998) Dev. Neuropsych., in press.
- 31.Kinsey A C, Pomeroy W B, Martin C E. Sexual Behavior in the Human Male. Philadelphia: Sanders; 1948. p. 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitehead M L. Hear Res. 1991;53:269–280. doi: 10.1016/0378-5955(91)90060-m. [DOI] [PubMed] [Google Scholar]
- 33.Ruse M. Homosexuality. New York: Basil Blackwell; 1988. pp. 84–129. [Google Scholar]
- 34.Resnick S M, Gottesman I I, McGue M. Behav Genet. 1993;23:323–329. doi: 10.1007/BF01067432. [DOI] [PubMed] [Google Scholar]
- 35.Loehlin J C, Martin N G. Behav Genet. 1998;28:21–27. doi: 10.1023/a:1021452630561. [DOI] [PubMed] [Google Scholar]
- 36.Ehrhardt A A, Meyer-Bahlburg H F L, Rosen L R, Feldman J F, Veridiano N P, Zimmerman I, McEwen B S. Arch Sex Behav. 1985;14:57–77. doi: 10.1007/BF01541353. [DOI] [PubMed] [Google Scholar]
- 37.Berenbaum S A, Snyder E. Dev Psych. 1995;31:31–42. [Google Scholar]
- 38.McFadden D. Hear Res. 1993;68:143–151. doi: 10.1016/0378-5955(93)90118-k. [DOI] [PubMed] [Google Scholar]
- 39.Neff D L, Kessler C J, Dethlefs T M. J Acoust Soc Am. 1996;100:2547–2550. doi: 10.1121/1.417364. [DOI] [PubMed] [Google Scholar]